Abstract
Expression of metastasis-associated protein 1 (MTA1) gene correlates with the degree of invasion and metastasis in hepatocellular carcinoma (HCC). Expression of MTA1 is induced by hepatitis B virus X protein (HBx); however, little is known about the transcriptional regulation of MTA1 gene expression. Here, we report that the 5′-flanking region of the human MTA1 promoter contains two CpG islands. Transient expression of HBx in Chang liver cells increased the methylation of the CpG island1 from 18 to 49% when measured by bisulfite-modified direct sequencing. Chromatin immunoprecipitation showed that HBx recruited DNA methyltransferase 3a (DNMT3a) and DNMT3b to the CpG island1. In silico analysis of CpG island1 predicted the existence of putative p53-binding sequences. p53 was pulled down by a DNA probe encoding the p53-binding sequences but not by the methylated DNA probe. The mouse MTA1 promoter also contains a CpG island encoding a p53-binding sequence of which p53 binding was decreased in the presence of HBx, and the expression of MTA1 and DNMT3 was increased in the liver of HBx-transgenic mice. Comparison of MTA1 and DNMT3a expression in the human normal liver and HCC specimens produced a significant correlation coefficient >0.5 (r=0.5686, P=0.0001) for DNMT3a, and a marginally significant coefficient (r=0.3162, P=0.0103) for DNMT3b. These data show that HBx induces methylation of CpG island in the MTA1 promoter, which interferes with DNA binding of p53 in the specific DNA region. This result may explain the molecular mechanism responsible for the induction of MTA1 gene expression by HBx.
Keywords: MTA1, p53, HBx, DNA methylation
Introduction
Chronic infection by hepatitis B virus (HBV) is a major cause of the development and progression of hepatocellular carcinoma (HCC).1 Among the viral proteins encoded in the 3.2 kb HBV genome, HBV X (HBx) protein is implicated in hepatocellular carcinogenesis because it leads to modulation of transcriptional expression of many cellular genes involved in oncogenesis, proliferation, inflammation, and immune responses.1, 2 Recently published data support the idea that HBx has critical roles in the invasive and metastatic properties of intrahepatic and extrahepatic metastases of HCC. The balance between adhesion and de-adhesion in cells is modulated by HBx, which may facilitate integrin-mediated cell migration in primary tumor sites.3 HBx increases the invasion potential by upregulating the expression of the membrane-type matrix metalloproteinases and miR-143, which contributes to tumor metastasis.4, 5
The metastasis-associated protein 1 (MTA1) is a strong candidate for a metastasis-promoting gene in liver cancer. The MTA1 expression level is significantly higher in HCC hepatocytes than in nonmalignant hepatocytes.6, 7 We reported previously that the expression of the MTA1 gene is a key transcriptional target of HBx protein, which contributes to angiogenesis in liver cancer by activating hypoxia-inducible factor 1α and vascular endothelial growth factor.8 It was shown recently that HBx recruits p65 to the nuclear factor-κB consensus motif on the relaxed MTA1 gene chromatin.9 HBx controls the expression of the MTA1 gene by suppressing miR-661 and subsequently activates inducible nitric oxide synthase in liver cancer cells.10 However, the mechanisms responsible for the increased expression of MTA1 in HCC remain largely unknown.
Epigenetic modifications are defined as heritable changes in gene expression occurring without alteration of underlying DNA sequences and comprise many layers of complexity, including DNA methylation, histone modifications, and chromatin remodeling.11 DNA methylation is a covalent modification of the cytosine ring at the 5′ position of a CpG dinucleotide and is performed by DNA methyltransferases (DNMTs).12 In HCC, the epigenetic inactivation caused by DNA hypermethylation has been established for several tumor suppressors and adhesion molecules such as p16INK4A, p14ARF, and cadherins.13 For example, hypermethylation around the E-cadherin promoter region is associated with reduced E-cadherin level in HCC.14 Methylation-induced silencing of the M-cadherin gene has significance as a prognostic indicator of poor survival in HCC patients.15 The promoter of tissue factor pathway inhibitor-2, a Kunitz-type serine protease inhibitor that represses cellular invasion, is highly methylated and is repressed in human HCC.16 However, the epigenetic regulation of the MTA1 gene promoter has not been reported.
HBx protein is involved in epigenetic modifications during hepatocarcinogenesis by increasing the recruitment of DNMTs and methyl-binding proteins to the promoters of target genes. HBx induces the transcriptional activation of DNMT1, and subsequent DNA hypermethylation of the promoter of E-cadherin.17 HBx also induces hypermethylation of the promoters of genes such as insulin-like growth factor-binding protein-3 (IGFBP-3), p16INK4A, retinoic acid receptor-β2 (RAR-β2), ankyrin repeat-containing, SH3 domain-containing, and proline-rich region-containing protein 1 (ASPP1), and ASPP2 by recruiting DNMT1, DNMT3a1, and DNMT3a2.18, 19, 20, 21 Here, we report on an important epigenetic regulation of the MTA1 promoter that is mediated by HBx during hepatocarcinogenesis. A CpG island and its methylation patterns of the human MTA1 promoter were characterized in HBx-expressing cell lines and in tissues obtained from patients with HBV-associated HCC. We also found that the HBx-induced methylation of the MTA1 promoter was linked to a decrease in the tumor-suppressor function of p53, which further supports the oncogenic and metastatic potential of HBx in the development and progression of HBV-associated HCC.
Results
HBx increases methylation of the MTA1 promoter
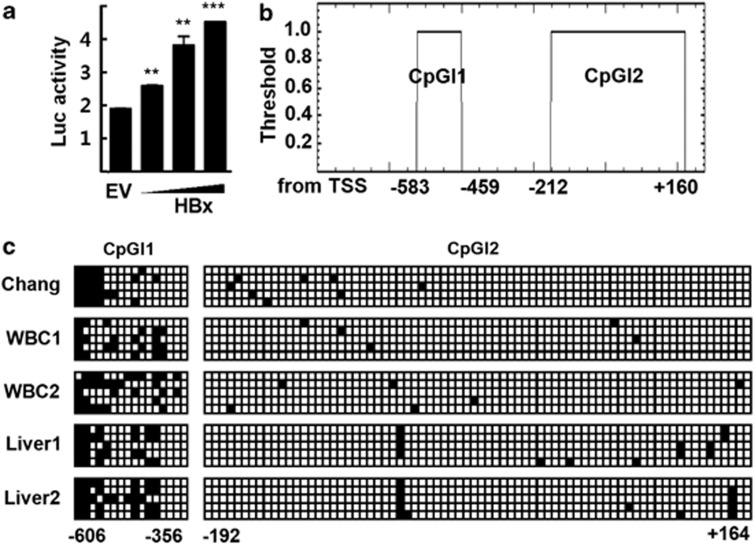
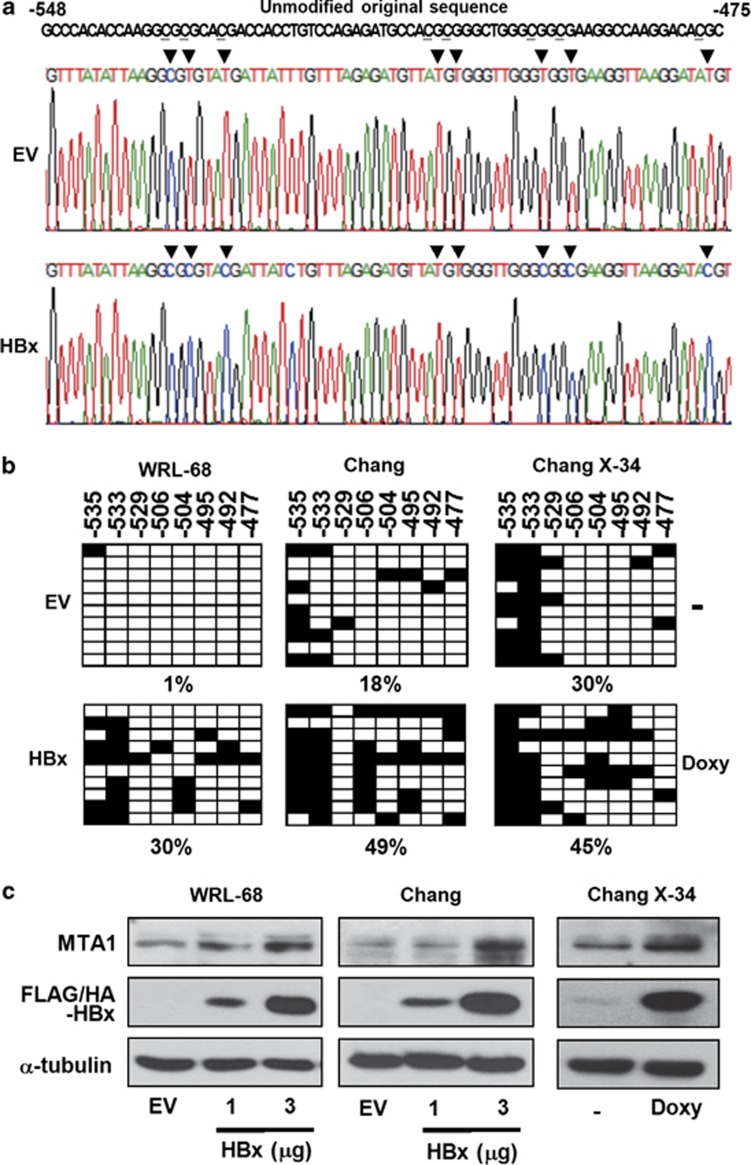
Although HBx is associated with high expression of MTA1 in HCC, the mechanism underlying this regulation of MTA1 expression is largely unknown. To identify the molecular details of HBx-induced MTA1 gene expression, we cloned a potential MTA1 promoter located in the 5′ sequence upstream of the coding region of MTA1 (from −860 to +216) in the human BAC genomic clone encoding chromosome 14. Cotransfection of the reporter containing the putative human MTA1 promoter with the HBx expression vector dose-dependently increased the reporter activity (Figure 1a), suggesting that the 5′ upstream sequence functions as a promoter of MTA1. An analysis using the CpGplot program identified two potential CpG islands that span positions from −583 to −459 and −212 to +160 on the MTA1 promoter (http://www.ebi.ac.uk/emboss/cpgplot) (Figure 1b). To study the methylation status of the regions, we performed bisulfite sequencing. The methylation level of CpG island1 was about 35%, whereas that of CpG island2 was about 0.1% in Chang liver cells, white blood cells, and normal liver tissues obtained from healthy donors (Figure 1c). Surprisingly, exogenously introduced HBx significantly increased methylation level of the CpG island1 (span positions −548 to −475) in WRL-68 and Chang liver cells. Doxycycline increased the methylation level of CpG island1 in Chang X-34 cells whose expression of the HBx gene is under the control of an inducible doxycycline promoter (Figures 2a and b).8 The increases in methylation were accompanied by increases in the expression level of MTA1 (Figure 2c). Expression of HBx was slightly detectable without doxycyclin treatment in Chang X-34, which probably represented leaky expression of HBx, and a relatively high basal expression level of MTA1 and methylation status of CpG island1.22 By contrast, the methylation level of CpG island2 was not changed in the presence of HBx (data not shown).
Figure 1.
Methylation analysis of the MTA1 promoter. (a) HepG2 cells were transfected with the human MTA1 promoter-Luc reporter and increasing amounts of p3XFLAG7.1-HBx or empty vector (EV). Experimental values are expressed as the mean±s.d. (n=3). **P<0.01 and ***P<0.001 vs EV transfected. (b) Putative CpG islands are located at bases −583 to −459 (CpGI1) and −212 to +160 (CpGI2) in the human MTA1 promoter. (c) Sequencing analysis of the putative MTA1 promoter CpG islands after bisulfite modification. Five clones from Chang liver cells (Chang), human white blood cells (WBC1 and WBC2), and normal liver tissues (Liver1 and Liver2) were analyzed for each PCR fragments. Filled squares, methylated; and open squares, unmethylated.
Figure 2.
HBx increases methylation of the human MTA1 promoter CpG island1. (a) Representative sequencing traces from bisulfite-modified direct sequencing. ▾ denotes methylated or unmethylated cytosines. Seven of unmethylated cytosines were converted to uracil in empty vector (EV)-transfected WRL-68 cells, but two in p3XFLAG7.1-HBx-transfected cells. (b) p3XFLAG7.1-HBx or EV was transfected into the indicated cells or treated with vehicle or 2 mg/ml of doxycycline (Doxy) for 24 h. Results from bisulfite sequencing analysis of the MTA1 promoter CpG island1 were shown. Filled squares, methylated; and open squares, unmethylated. Numbers at the bottom indicate the proportion of methylated CpG sites relative to total CpG sites examined. (c) Indicated amount of p3XFLAG7.1-HBx or EV was transfected into cells, or treated with doxycycline. Whole-cell lysates were prepared and protein levels were evaluated by western blotting.
p53 does not bind to the methylated promoter of the MTA1 gene
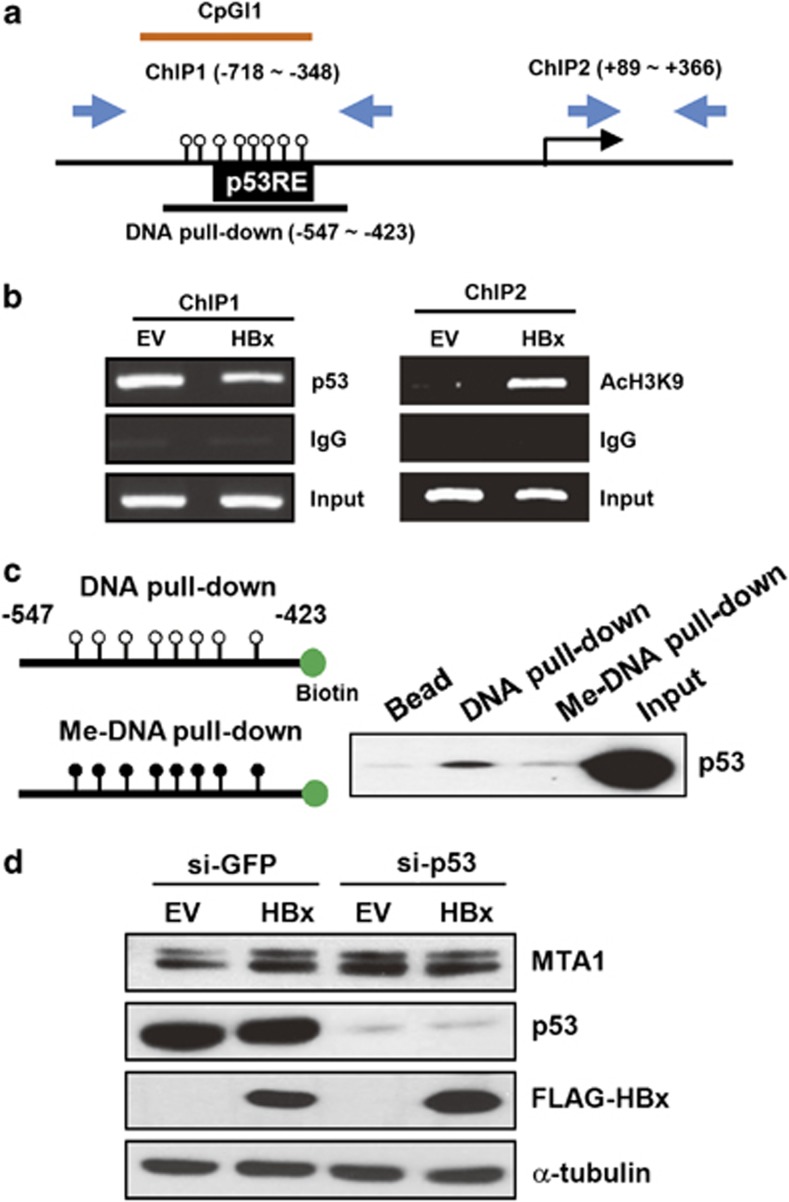
We found that CpG island 1 in the MTA1 promoter contains a p53-binding site that is associated with transcriptional repression of MTA1 (Figure 3a).23 We hypothesized that the HBx-induced methylation of the CpG island interferes with the p53 binding and that this could rescue transcription of MTA1. To prove that p53 binds to this region, we performed chromatin immunoprecipitation (ChIP) analysis with primers that amplify the CpG island 1 and the region near the transcription start site. Introduction of exogenous HBx decreased the binding of p53 to CpG island 1 (ChIP1) and increased acetylation level of the lysin 9 in histone 3 at near the transcription start site (ChIP2; Figure 3b). It has been reported that p53 does not bind to methylated DNA promoters, resulting in the upregulation of genes such as survivin.24 We performed DNA pull-down experiments using nuclear extracts containing p53. A clear binding of p53 was observed with the unmethylated probe, but the binding was reduced with the methylated probe, indicating that p53 binds mainly to the unmethylated MTA1 promoter (Figure 3c). The involvement of p53 in HBx-induced MTA1 was demonstrated further in p53-knocked down WRL-68 cells. Repression of p53 expression by si-RNA markedly increased the protein level of MTA1 (2.9-fold, lane 3). However, coexpression of HBx did not increase MTA1 further (2.6-fold, lane 4), suggesting that MTA1 expression is fully activated by p53 knockdown.
Figure 3.
Methylation of the MTA1 promoter CpG island 1 leads to inhibition of p53 binding. (a) Schematic presentation of the human MTA1 promoter encoding p53-binding sequences (p53RE) and DNA regions for ChIP and pull-down experiments. (b) Chang liver cells were transfected with empty vector (EV) or p3XFLAG7.1-HBx. DNA fragments that immunoprecipitated by anti-p53 (left) and anti-acetyl histone 3 lysine 9 (right) were amplified by PCR using primers specific for ChIP1 and ChIP2. (c) Nuclear extracts obtained from HCT116 cells were incubated with unmethylated or methylated double-stranded biotin end-labeled oligonucleotide probes. Precipitates pulled down by sterptoavidine beads were analyzed for p53 binding by western blotting. (d) WRL-68 cells were transfected with si-GFP or si-p53. After 24 h of transfection, cells were transfected with EV or p3XFLAG7.1-HBx for another 24 h. Expression of the indicated proteins was analyzed by western blotting.
HBX recruits DNMT3a and DNMT3b to the CpG island of the human MTA1 promoter
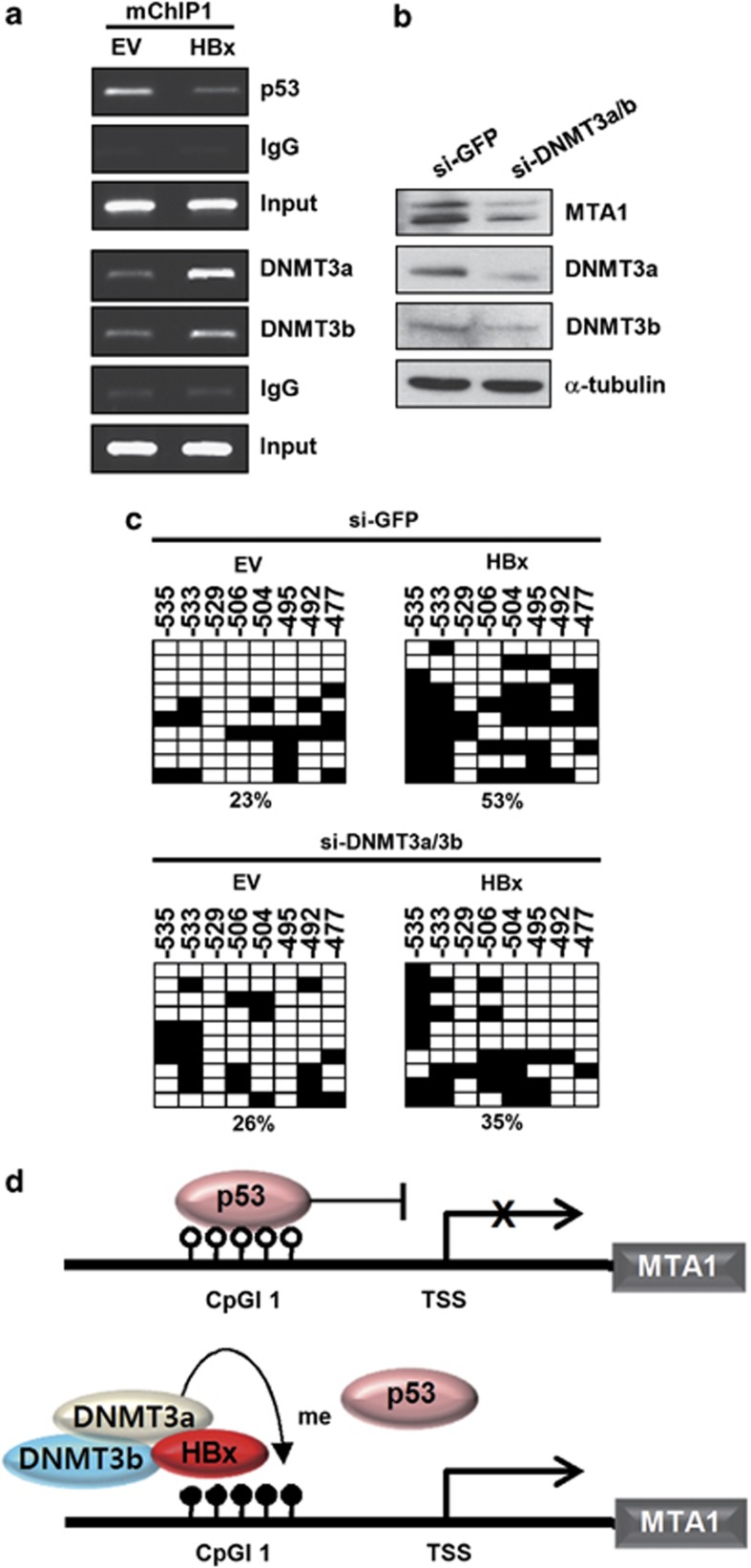
To identify the catalytic enzymes associated with HBx-induced DNA methylation, we examined whether DNMT3a and DNMT3b are recruited in the CpG island of the MTA1 promoter. When HBx was exogenously introduced in Chang cells, expression level of DNMT3a was enhanced as previously shown (data not shown).18, 20 ChIP analysis showed that both DNMT3a and DNMT3b bound to CpG island 1 and that the binding increased in the presence of HBx. Interestingly, HBx itself bound to the same region of the promoter (Figure 4a). Further, DNMT3a and DNMT3b knock down by si-RNA reduced expression levels of MTA1 (Figure 4b). To confirm the involvement of DNMT3a and DNMT3b in the methylation of CpG island1, we monitored the DNA methylation status of CpG island1 after DNMT3a and DNMT3b were knocked down by si-RNA. HBx did not significantly alter the methylation status of CpG island1 when expression of DNMT3a and DNMT3b was repressed (Figure 4c). This result demonstrates that HBx increases the transcription of MTA1 by releasing p53 from CpG island1 by increasing promoter methylation through recruitment of DNMT3a and DNMT3b (Figure 4d).
Figure 4.
HBx enhances recruitment of DNMT3a and DNMT3b on the MTA1 promoter CpG island. (a) Chang liver cells were transfected with empty vector (EV) or p3XFLAG7.1-HBx. DNA fragments that immunoprecipitated were amplified by PCR. (b) Chang liver cells were transfected with si-GFP or mixed si-DNMT3 (si-DNMT3a and si-DNMT3b). After 72 h of transfection, whole-cell lysates were prepared and protein levels were evaluated by western blotting. (c) Chang liver cells were transfected with si-GFP or mixed si-DNMT3 (si-DNMT3a and si-DNMT3b). After 24 h of transfection, cells were transfected with EV or p3XFLAG7.1-HBx for another 24 h. Results from bisulfite sequencing analysis of the MTA1 promoter CpG island were shown. Filled squares, methylated and open squares, unmethylated. (d) Schematic representation for the HBx-induced derepression of MTA1 gene by methylation of the CpG island on the promoter.
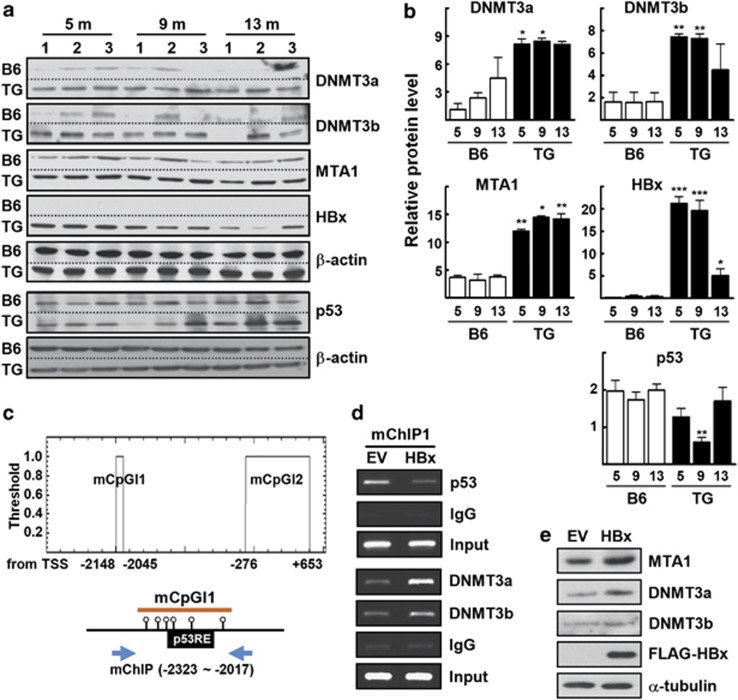
The expression level of DNMT3 increases in the liver of HBx-expressing transgenic mice
Previously, we showed that expression level of MTA1 was elevated markedly in the livers from HBx-transgenic mice compared with wild-type mice.8 Therefore, we next examined whether the expression levels of DNMT3a and DNMT3b are associated with the increased expression of MTA1 in the HBx transgenic mouse livers. In a similar pattern with the MTA1 expression, the levels of DNMT3a and DNMT3b were increased markedly in HBx-transgenic livers, suggesting a potential link between the induction of MTA1 and DNMT3 expression (Figures 5a and b). The level of p53 protein was significantly decreased in 9 month-old HBx TG compared with wild-type control mice. This result may be relevant with the previous observation that HBx downregulates p53 promoter activity.25 Interestingly, a p53-binding sequence was found in one of the two potential CpG islands in the mouse MTA1 promoter (Figure 5c). When HBx was exogenously introduced, DNA binding of p53 in the CpG island1 was reduced, whereas DNA bindings of DNMT3a and DNMT3b were enhanced greatly (Figure 5d). Increases in expression levels of DNMT3a and DNMT3b were also observed in the mouse NIH3T3 cells in the presence of HBx (Figure 5e).
Figure 5.
Increases in expression level of MTA1, DNMT3a, and DNMT3b in the liver of HBx-transgenic mice. (a) Tissue lysates were obtained from the liver of the indicated aged B6 and HBx-TG mice. The tissue lysates were separated on two different polyacrylamide gels, transferred to the same PVDF membranes, and probed with specific antibody by the same procedure. (b) Density of each protein band in (a) was determined using an image analysis system and normalized to that of the corresponding β-actin. Y axis represents the relative protein level to compare with that of 5-month-old WT mice. *P<0.05, **P<0.005, ***P<0.0001 vs age-matched B6 mice (n=3). (c) Putative CpG islands are located at bases −2148 to −2045 (mCpGI1) and −276 to +653 (mCpGI2) in the mouse MTA1 promoter (upper). Schematic presentation of the mCpGI1-encoding p53-binding sequences (p53RE) with DNA region for ChIP (lower). (d) NIH3T3 cells were transfected with empty vector (EV) or p3XFLAG7.1-HBx. DNA fragments that immunoprecipitated by anti-p53, anti-DNMT3a, or anti-DNMT3b, were amplified by PCR using primers specific for mChIP. (e) NIH3T3 cells were transfected with EV or p3XFLAG7.1-HBx. Whole-cell lysates were prepared and protein levels were evaluated by western blotting.
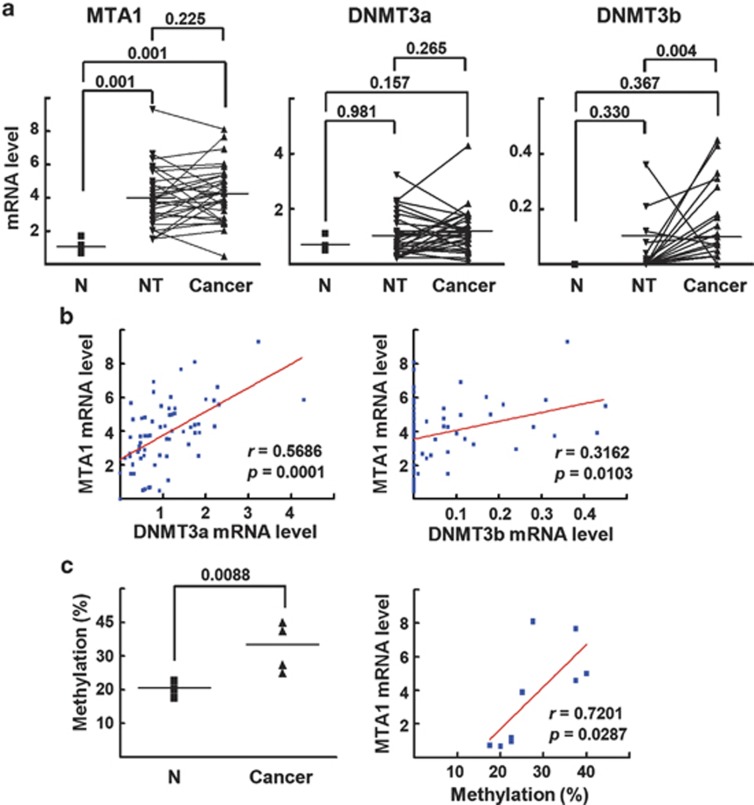
Expression level of MTA1 is correlated with the MTA1-promoter methylation in HCC specimens
Finally, we performed qRT–PCR analysis to assess the expression levels of MTA1, DNMT3a, and DNMT3b in 30 HBV-associated HCC specimens after microdissection of HCC tissues and the adjacent nontumorous tissues.26 The expression level of MTA1 did not differ significantly between the HCC tissues and the adjacent nontumorous region; however, the expression levels in both groups were elevated considerably compared with the level in the normal human livers (P=0.001) (Figure 6a). The expression level of DNMT3a in the HCC and nontumorous tissues varied considerably in the groups, and the mean values were higher than normal, but the differences were not significant. The level of DNMT3b was significantly higher in the HCC samples than in the nontumorous tissues (P=0.004), but the expression levels of DNMT3b in both the HCC and nontumorous tissues did not differ significantly compared with the level in the normal human livers. Pearson correlation analysis of MTA1 and DNMT3 expression in the human normal livers and the HCC specimens showed a strong correlation (r=0.5686, P=0.0001) for DNMT3a and a marginally higher correlation (r=0.3162, P=0.0103) for DNMT3b, which may indicate the involvement of DNMT3a and DNMT3b in the regulation of MTA1 expression (Figure 6b).
Figure 6.
Expression level of MTA1 is correlated with the MTA1 promoter methylation in HCC specimens. (a) The HBV-associated HCC tissues obtained from 30 HCC patients.26 qRT–PCR was performed with cDNA prepared from the microdissected non-tumor or cancer regions. The independent data (normal (N) vs nontumor (NT) region and normal vs cancer) and the matched data (nontumor vs cancer) of gene expression was analyzed using Wilcoxon signed rank test and Mann–Whitney test, respectively. (b) Pearson correlation analysis for comparison of mRNA level of MTA1 and DNMT3a/3b gene in six human normal liver and 30 HCC specimens. (c) Methylation status of the CpG island 1 of four normal human livers and five HCC tissues with higher MTA1 expression were analyzed by bisulfite sequencing (left). Pearson correlation analysis for comparison of MTA1 mRNA expression level and percentage of methylation of the CpG island of MTA1 promoter (right).
Bisulfite sequencing was used to analyze the CpG island 1 methylation status of four normal human livers and five HCC tissues with higher MTA1 expression. The methylation level was significantly higher (P=0.0088) in the HCC tissues. MTA1 mRNA expression and the MTA1 promoter methylation level correlated significantly (r=0.7201, P=0.0287), indicating a close association between the DNA methylation status of the MTA1 promoter and MTA1 expression level (Figure 6c). The results obtained from the HBx-transgenic mice and clinicopathological studies strongly support our hypothesis that the HBx-induced methylation of the MTA1 promoter upregulates the transcription of MTA1, which may contribute to the development of HBV-associated HCC.
Discussion
Because of the strong connection between MTA1 gene expression and progression of HBV-associated HCC, identifying the molecular mechanism responsible for the effect of HBx on the transcriptional activation of the MTA1 gene is an important task. In the present study, we demonstrated the existence of an epigenetic mechanism for MTA1 gene regulation during hepatocarcinogenesis, which was mediated by HBx protein. We observed that HBx induced the methylation of human MTA1 gene promoter CpG island1, which contains the specific DNA-binding sequences for p53 (Figure 2b). The MTA1 gene promoter also contains a CpG island2 region; however, this region did not respond to HBx-mediated methylation, probably because of the presence of DNA-binding sites for transcription factors that confer resistance to de novo methylation in cancer cells.
Epigenetic inactivation caused by hypermethylation of a promoter is well established for genes involved in the initiation and progression of HCCs.13 The levels of DNMT1, DNMT3a, and DNMT3b were increased significantly in HCC tissues compared with nonneoplastic liver tissues (Figure 6a).27, 28 In many cases, the aberrant DNA methylation is associated with gene silencing. For example, the expression levels of tumor-suppressor genes, such as p16INK4A, correlates inversely with the expression of DNMT3a.13 However, we observed a significant positive correlation between the mRNA expression levels of MTA1, DNMT3a, and DNMT3b in the present study (Figure 6b). Similar to our observation, DNA methylation-mediated derepression was reported for several genes with oncogenic potential. The methylation of the hTERT promoter at the CCCTC-binding factor-binding site inhibits the DNA binding of CCCTC-binding factor, which increases hTERT expression, especially in human tumors.29 Methylation of the survivin gene promoter inhibits the binding of p53 and causes derepression of survivin gene expression.24 Interestingly, the observation that HBx recruited DNMTs to the MTA1 promoter in our investigation (Figures 4a and 5d) raises the possibility that the function of HBx is linked to specific targeting of promoters of both tumo- suppressor genes and genes with oncogenic potential. Indeed, HBx induces hypermethylation of the IGFBP-3 promoter by recruiting DNMT1, DNMT3A1, and DNMT3A2, which suppress IGFBP-3 expression.18 By contrast, HBx suppresses the expression of p16INK4A, RAR-β2, ASPP1, and ASPP2 in HCC tissues by upregulating or recruiting DNMT1 and DNMT3A.19, 20, 21 HBx induces the transcriptional activation of DNMT1, which causes subsequent DNA hypermethylation of the promoter of E-cadherin.17 Therefore, HBx may be one of the most potent and efficient epigenetic regulators that control cellular gene expression and may have beneficial effects for viral survival and propagation through immortalization of host cells.
In this study, we found that DNA methylation-induced derepression of the MTA1 gene was closely associated with the function of p53. This observation may be related to the previous observation that the loss of p53 function increases invasion and metastasis in several in vivo models of HCC.30, 31 However, mRNA level of p53 was significantly higher in the non-tumorous and tumor tissues compared with the p53 level in the normal human livers (Supplementary Figure). However, the mRNA level of p53 may not represent the functional p53, as inactivation of p53 by mutations is frequently found in tumors associated with HBV infection.32 Further, the negative cross-talk between HBx and p53 protein has been addressed in the context of HBV-associated hepatocarcinogenesis. HBx binds to the wild-type p53 protein, inhibits sequence-specific DNA binding, and sequesters p53 in the cytoplasm, thereby preventing its nuclear entry.33, 34 Here we show a new type of cross-talk between HBx and p53, in which HBx-mediated methylation of DNA inhibits specific DNA binding of p53 (Figures 3 and 5), and p53 is then unable to interact with its cognate binding sites if a methylated cytosine is present. The survivin promoter contains a region containing a p53-binding element, and methylation of the region inhibits the binding of p53.24 In an independent study, HBx increased the expression of survivin, suggesting that the survivin promoter may be a target of HBx-mediated methylation.35 We reported recently that poly(ADP-ribose) polymerase 1 (PARP-1)-mediated poly(ADP-ribose)ylation (PARylation) of p53 is necessary for the transcriptional repression of MTA1.23 Inhibition of PARP-1 increases or alters the pattern of DNA methylation.36, 37 Our data together with those of the other groups may suggest that PARP-1-mediated PARylation on certain cellular proteins has a role in maintaining the methylation pattern of genomic DNA. Whether the PARylation of p53 is linked to HBx-mediated DNA methylation remains as an interesting question.
Several recent studies have shown that MTA1 can be a molecular target for antimetastatic therapy. Downregulation of MTA1 by RNA interference successfully suppressed the in vitro growth and experimental metastasis of mouse B16F10 melanoma cells in vivo.38 Silencing MTA1 significantly suppressed the invasion and angiogenic activity of several cancer cell lines and delayed tumor formation and development in mouse xenografts.39, 40 In this study, we found that the expression of MTA1 was significantly greater in both the cancerous regions and the adjacent noncancerous regions than in the normal liver (Figure 6a). This result suggests that MTA1 also has a role in precancerous changes in HBV-associated HCC. Epigenetic modification has emerged recently as an attractive therapeutic strategy against HBV-associated HCC. DNMT inhibitor demethylation agents, such as 5-aza-2′deoxycytidine, and histone deacetylase inhibitors, such as SAHA, tributyrin, and valproic acid, have been tested preclinically against HCC and have shown promising results.41 Interestingly, 5-aza-2′deoxycytidine can increase the sensitivity of hepatoma cell lines to 5-fluorouracil, which represses the transcriptional expression of MTA1, as demonstrated in our recent study23 and by another study.42 In the present study, we showed that MTA1 expression was upregulated by DNA methylation, which may provide a rationale for epigenetic combination therapy to HBV-associated HCC in patients with an increased MTA1 expression level.
In summary, the present study highlights the important role of DNA methylation in MTA1 expression in HBx-positive HCC. Our findings shed light on the basic molecular mechanisms involved in HBx-induced HCC progression and support a role for DNMTs as a potential target for antimetastatic therapy.
Materials and methods
Cells and cell culture
Human normal liver cell lines, Chang and WRL-68, a human colon cancer cell line, HCT 116, and a mouse embryonic fibroblast cell line, NIH3T3, were obtained from American-type culture collection. Chang X-34 cells, in which HBx gene expression is under the control of a doxycycline-inducible promoter, were described previously.8 Cells were maintained in Dubelco's modified Eagle's medium containing 10% fetal bovine serum at 37 °C in a 5% CO2/95% air incubator.
Plasmids, si-RNA, western blotting, and transient transfection
The eukaryotic expression vectors for p3XFLAG7.1-HBx was described previously.8 The human MTA1 promoter (from −860 to +216) was cloned from human BAC genomic clone RZPDB737A112145D (RZPD, Berlin, Germany) by PCR amplification and subsequent insertion into SacI and NheI site of the pGL2-Basic vector (Promega, Madison, WI, USA). The si-RNA duplexes targeting si-DNMT3a (5′-CAA CAUCGAAUCCAUGAAAUU-3′ and 5′-UUUCAUGGAUUCGAUGUUG UU-3′) and si-DNMT3b (5′-ACGCACAGCUGACGACUCAUU-3′ and 5′-UGAGUCGUCAGCUGUGCG UUU-3′), and control si-GFP (green fluorescent protein), were synthesized and purified by ST Pharm (Seoul, Korea).43 Transient transfection, reporter gene analysis, and western blotting were performed as described previously.26
Bisulfite-modified direct sequencing
Genomic DNA was extracted using the Wizard Genomic DNA purification kit (Promega) and treated with sodium bisulfite according to the protocol of EpiTect Bisulfite Kit (Qiagen, Valencia, CA, USA). The modified DNA was PCR amplified using the following primers: 5′-GGAAGGTATTTTGATAGGTGGT-3′ (sense) and 5′-CCCTATACCCCACAAATAAATC-3′ (antisense) for the region 1 (CpG island1of human MTA1 promoter); 5′-AGGGTATTAGGGGAGGATTTATTAG-3′ (sense) and 5′-AACCATTTTAAATTAAAATAAACAC-3′ (antisense) for the region 2 (CpG island2 of human MTA1 promoter). The resulting PCR products were cloned using a pGEM-T-easy vector system (Promega) and subjected to sequencing analysis.
Chromatin immunoprecipitation (ChIP)
ChIP assay was performed basically as previously described.23 Briefly, cells were fixed for 15 min in 0.75% formaldehyde, placed in lysis buffer (50 mmol/l HEPES (hydroxyethyl piperazineethanesulfonic acid)-KOH (pH 7.5), 140 mmol/l NaCl, 1 mmol/l EDTA (pH 8), 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate and fresh protease inhibitor cocktail), and then chromatin was sheared into 500–200 bp fragments by sonication. Equal amounts of sample were immunoprecipitated with, 2 μg of anti-p53 (DO-1), anti-DNMT3a, anti-DNMT3b (Santa Cruz Biotech, Santa Cruz, CA, USA), anti-p53 (PAb421) (Calbiochem, La Jolla, CA, USA), anti-FLAG (Sigma, St Louis, MO, USA), or anti-AcH3K9 (Abcam, Cambridge, MA, USA). Bound target DNA fragments were analyzed for the presence of sequences −718 to −348 (ChIP1), +89 to +366 (ChIP2) of human MTA1 promoter and −2323 to −2017 (mChIP) of mouse MTA1 promoter by PCR amplification.
DNA pull-down assay
Promoter templates biotinylated at the 5′ end of the upper strand were produced by PCR using primers, 5′-GCCCACACCAAGGCGCGCAC-3′ (sense) and biotin-5′-CACCCCACACCTCCCTTCCA-3′ (antisense). Nuclear extracts were prepared as described previously.23 Nuclear extracts (100 μg) from HCT 116 cells were incubated with the biotinylated DNA probe (10 nℳ) in a binding buffer (20 mℳ HEPES, 150 mℳ NaCl, 1 mℳ EDTA, 0.01% NP-40, and fresh protease inhibitor cocktail) containing 1 μg/ml herring sperm DNA and 20 μl streptavidin Sepharose High Performance (GE Healthcare, Piscataway, NJ, USA) at 4 °C for overnight. The protein–DNA complexes were washed three times with the binding buffer, and then subjected to SDS–PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) followed by western blotting with anti-p53 antibodies (Santa Cruz Biotech). Methylated PCR products were prepared in vitro using SssI (CpG) DNA methyltransferase (New England Biolabs, Cambridge, MA, USA).
HCC Samples and qRT–PCR
Six normal liver samples and 30 patients with HBV-associated HCC were described previously.26 All samples were collected anonymously according to the Institutional Review Board guidelines, and the histopathological features of the examined HCCs are described previously.26 For total RNA extraction from formalin-fixed paraffin-embedded tissues, each tissue section was stained with hematoxylin, and nontumor or cancer regions were microdissected with a laser microdissection system or cut directly with a needle from sections. RNA isolation and qRT–PCR was performed as previously described previously.25 The following primers and probes were used for qRT–PCR. DNMT3a: 5′-ACTACATCAGCAAGCGCAAG-3′ (forward primer), 5′-CACAGCATTCATTCCTGCAA-3′ (reverse primer), and Universal ProbeLibrary probe: No. 75. DNMT3b: 5′- CCGAGAACAAATGGCTTCAG-3′ (forward primer), 5′- TTCCTGCCACAAGACAAACA-3′ (reverse primer), and Universal ProbeLibrary probe: No. 64. MTA1: 5′-GGCAGACATCACCGACTTG-3′ (forward primer), 5′-ACACCTGGGTCTCCAACCCT-3′ (reverse primer), and Universal ProbeLibrary probe: No. 54 (Roche Diagnostics, Mannheim, Germany). Five HCC specimens showing high expression of MTA1 in tumor were selected for bisulfite sequencing.
Statistics
Experimental values are expressed as the mean±s.d. of three independent experiments. The significance of any difference was determined by Student's t-tests and expressed as a probability value. For clinicopathological studies, the independent data (normal vs non-tumor region) and the matched data (non-tumor vs cancer) of gene expression was analyzed using Wilcoxon signed rank test and Mann–Whitney test, respectively. Mean differences were considered significant at P<0.05. The relationship between the expression of DNMTs and MTA1 was analyzed by calculating Pearson correlation coefficient.
Acknowledgments
This work was supported by grants from the NRF (2009-0080757), and the SRC/ERC (R11-2007-107-01001-0).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Oncogenesis website (http://www.nature.com/oncsis)
Supplementary Material
References
- Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52:594–604. doi: 10.1016/j.jhep.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006;147:58–66. doi: 10.1016/j.lab.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Lara-Pezzi E, Serrador JM, Montoya MC, Zamora D, Yáñez-Mó M, Carretero M, et al. The hepatitis B virus X protein (HBx) induces a migratory phenotype in a CD44-dependent manner: possible role of HBx in invasion and metastasis. Hepatology. 2001;33:1270–1281. doi: 10.1053/jhep.2001.1270. [DOI] [PubMed] [Google Scholar]
- Ou DP, Tao YM, Tang FQ, Yang LY. The hepatitis B virus X protein promotes hepatocellular carcinoma metastasis by upregulation of matrix metalloproteinases. Int J Cancer. 2007;15:1208–1214. doi: 10.1002/ijc.22452. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu S, Hu T, Liu S, He Y, Sun S. Up-regulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology. 2009;50:490–499. doi: 10.1002/hep.23008. [DOI] [PubMed] [Google Scholar]
- Moon WS, Chang K, Tarnawski AS. Overexpression of metastatic tumor antigen 1 in hepatocellular carcinoma: Relationship to vascular invasion and estrogen receptor-alpha. Hum Pathol. 2004;35:424–429. doi: 10.1016/j.humpath.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Ryu SH, Chung YH, Lee H, Kim JA, Shin HD, Min HJ, et al. Metastatic tumor antigen 1 is closely associated with frequent postoperative recurrence and poor survival in patients with hepatocellular carcinoma. Hepatology. 2008;47:929–936. doi: 10.1002/hep.22124. [DOI] [PubMed] [Google Scholar]
- Yoo YG, Na TY, Seo HW, Seong JK, Park CK, Shin YK, et al. Hepatitis B virus X protein induces the expression of MTA1 and HDAC1, which enhances hypoxia signaling in hepatocellular carcinoma cells. Oncogene. 2008;27:3405–3413. doi: 10.1038/sj.onc.1211000. [DOI] [PubMed] [Google Scholar]
- Bui-Nguyen TM, Pakala SB, Sirigiri RD, Xia W, Hung MC, Sarin SK, et al. NF-kappaB signaling mediates the induction of MTA1 by hepatitis B virus transactivator protein HBx. Oncogene. 2010;29:1179–1189. doi: 10.1038/onc.2009.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui-Nguyen TM, Pakala SB, Sirigiri DR, Martin E, Murad F, Kumar R. Stimulation of inducible nitric oxide by hepatitis B virus transactivator protein HBx requires MTA1 coregulator. J Biol Chem. 2010;285:6980–6986. doi: 10.1074/jbc.M109.065987. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Taby R, Issa JP. Cancer epigenetics. CA Cancer J Clin. 2010;60:376–392. doi: 10.3322/caac.20085. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;3:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Tischoff I, Tannapfe A. DNA methylation in hepatocellular carcinoma. World J Gastroenterol. 2008;14:1741–1748. doi: 10.3748/wjg.14.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Van Nhieu JT, Prigent S, Srivatanakul P, Tiollais P, Buendia MA. Altered expression of E-cadherin in hepatocellular carcinoma: correlations with genetic alterations, beta-catenin expression, and clinical features. Hepatology. 2002;36:692–701. doi: 10.1053/jhep.2002.35342. [DOI] [PubMed] [Google Scholar]
- Yamada S, Nomoto S, Fujii T, Takeda S, Kanazumi N, Sugimoto H, et al. Frequent promoter methylation of M-cadherin in hepatocellular carcinoma is associated with poor prognosis. Anticancer Res. 2007;27:2269–2274. [PubMed] [Google Scholar]
- Wong CM, Ng YL, Lee JM, Wong CC, Cheung OF, Chan CY, et al. Tissue factor pathway inhibitor-2 as a frequently silenced tumor suppressor gene in hepatocellular carcinoma. Hepatology. 2007;45:1129–1138. doi: 10.1002/hep.21578. [DOI] [PubMed] [Google Scholar]
- Lee JO, Kwun HJ, Jung JK, Choi KH, Min DS, Jang KL. Hepatitis B virus X protein represses E-cadherin expression via activation of DNA methyltransferase 1. Oncogene. 2005;24:6617–6625. doi: 10.1038/sj.onc.1208827. [DOI] [PubMed] [Google Scholar]
- Park IY, Sohn BH, Yu E, Suh DJ, Chung YH, Lee JH, et al. Aberrant epigenetic modifications in hepatocarcinogenesis induced by hepatitis B virus X protein. Gastroenterology. 2007;132:1476–1494. doi: 10.1053/j.gastro.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Zhu YZ, Zhu R, Fan J, Pan Q, Li H, Chen Q, et al. Hepatitis B virus X protein induces hypermethylation of p16(INK4A) promoter via DNA methyltransferases in the early stage of HBV-associated hepatocarcinogenesis. J Viral Hepat. 2010;17:98–107. doi: 10.1111/j.1365-2893.2009.01156.x. [DOI] [PubMed] [Google Scholar]
- Jung JK, Park SH, Jang KL. Hepatitis B virus X protein overcomes the growth-inhibitory potential of retinoic acid by downregulating retinoic acid receptor-beta2 expression via DNA methylation. J Gen Virol. 2010;91:493–500. doi: 10.1099/vir.0.015149-0. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wu G, Bu F, Lu B, Liang A, Cao L, et al. Epigenetic silence of ankyrin-repeat-containing, SH3-domain-containing, and proline-rich-region- containing protein 1 (ASPP1) and ASPP2 genes promotes tumor growth in hepatitis B virus-positive hepatocellular carcinoma. Hepatology. 2010;51:142–153. doi: 10.1002/hep.23247. [DOI] [PubMed] [Google Scholar]
- Yoo YG, Oh SH, Park ES, Cho H, Lee N, Park H, et al. Hepatitis B virus X protein enhances transcriptional activity of hypoxia-inducible factor-1alpha through activation of mitogen-activated protein kinase pathway. J Biol Chem. 2003;278:39076–39084. doi: 10.1074/jbc.M305101200. [DOI] [PubMed] [Google Scholar]
- Lee M-H, Na H, Kim E-J, Lee H-W, Lee M-O.Poly(ADP-ribosyl)ation of p53 induces gene-specific transcriptional repression of MTA1 Oncogene(e-pub ahead of print 30 January 2012; doi: 10.1038/onc.2012.2 [DOI] [PubMed]
- Nabilsi NH, Broaddus RR, Loose DS. DNA methylation inhibits p53-mediated survivin repression. Oncogene. 2009;28:2046–2050. doi: 10.1038/onc.2009.62. [DOI] [PubMed] [Google Scholar]
- Lee SG, Rho HM. Transcriptional repression of the human p53 gene by hepatitis B viral X protein. Oncogene. 2000;19:468–471. doi: 10.1038/sj.onc.1203312. [DOI] [PubMed] [Google Scholar]
- Na TY, Shin YK, Roh KJ, Kang SA, Hong I, Oh SJ, et al. Liver X receptor mediates hepatitis B virus X protein-induced lipogenesis in hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2009;49:1122–1131. doi: 10.1002/hep.22740. [DOI] [PubMed] [Google Scholar]
- Saito Y, Kanai Y, Nakagawa T, Sakamoto M, Saito H, Ishii H, et al. Increased protein expression of DNA methyltransferase (DNMT) 1 is significantly correlated with the malignant potential and poor prognosis of human hepatocellular carcinomas. Int J Cancer. 2003;105:527–532. doi: 10.1002/ijc.11127. [DOI] [PubMed] [Google Scholar]
- Park HJ, Yu E, Shim YH. DNA methyltransferase expression and DNA hypermethylation in human hepatocellular carcinoma. Cancer Lett. 2006;233:271–278. doi: 10.1016/j.canlet.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Renaud S, Loukinov D, Abdullaev Z, Guilleret I, Bosman FT, Lobanenkov V, et al. Dual role of DNA methylation inside and outside of CTCF-binding regions in the transcriptional regulation of the telomerase hTERT gene. Nucleic Acids Res. 2007;35:1245–1256. doi: 10.1093/nar/gkl1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Lewis BC, Klimstra DS, Socci ND, Xu S, Koutcher A, Varmus HE. The absence of p53 promotes metastasis in a novel somatic mouse model for hepatocellular carcinoma. Mol Cell Biol. 2005;25:1228–1237. doi: 10.1128/MCB.25.4.1228-1237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougot D, Neuveut C, Buendia MA. HBV induced carcinogenesis. J Clin Virol. 2005;34:S75–S78. doi: 10.1016/s1386-6532(05)80014-9. [DOI] [PubMed] [Google Scholar]
- Elmore LW, Hancock AR, Chang SF, Wang XW, Chang S, Callahan CP, et al. Hepatitis B virus X protein and p53 tumor suppressor interactions in the modulation of apoptosis. Proc Natl Acad Sci USA. 1997;94:14707–14712. doi: 10.1073/pnas.94.26.14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26:2166–2176. doi: 10.1038/sj.onc.1210279. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong N, Yin L, Cai N, Ma H, You J, et al. Hepatitis B virus X protein upregulates survivin expression in hepatoma tissues. J Med Virol. 2005;77:374–381. doi: 10.1002/jmv.20466. [DOI] [PubMed] [Google Scholar]
- Zardo G, Caiafa P. The unmethylated state of CpG islands in mouse fibroblasts depends on the poly(ADP-ribosyl)ation process. J Biol Chem. 1998;273:16517–165120. doi: 10.1074/jbc.273.26.16517. [DOI] [PubMed] [Google Scholar]
- Zardo G, Marenzi S, Perilli M, Caiafa P. Inhibition of poly(ADP-ribosyl)ation introduces an anomalous methylation pattern in transfected foreign DNA. FASEB J. 1999;13:1518–1522. doi: 10.1096/fasebj.13.12.1518. [DOI] [PubMed] [Google Scholar]
- Qian H, Yu J, Li Y, Wang H, Song C, Zhang X, et al. RNA interference of metastasis-associated gene 1 inhibits metastasis of B16F10 melanoma cells in a C57BL/6 mouse model. Biol Cell. 2007;99:573–581. doi: 10.1042/bc20060130. [DOI] [PubMed] [Google Scholar]
- Rao Y, Wang H, Fan L, Chen G. Silencing MTA1 by RNAi reverses adhesion, migration and invasiveness of cervical cancer cells (SiHa) via altered expression of p53, and E-cadherin/β-catenin complex. J Huazhong Univ Sci Technolog Med Sci. 2011;31:1–9. doi: 10.1007/s11596-011-0141-9. [DOI] [PubMed] [Google Scholar]
- Kai L, Wang J, Ivanovic M, Chung YT, Laskin WB, Schulze-Hoepfner F, et al. Targeting prostate cancer angiogenesis through metastasis-associated protein 1 (MTA1) Prostate. 2011;71:268–280. doi: 10.1002/pros.21240. [DOI] [PubMed] [Google Scholar]
- Herceg Z, Paliwal A. Epigenetic mechanisms in hepatocellular carcinoma: how environmental factors influence the epigenome. Mutat Res. 2011;727:55–61. doi: 10.1016/j.mrrev.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Kanda T, Tada M, Imazeki F, Yokosuka O, Nagao K, Saisho H. 5-aza-2′-deoxycytidine sensitizes hepatoma and pancreatic cancer cell lines. Oncol Rep. 2005;14:975–979. [PubMed] [Google Scholar]
- Kim MS, Kondo T, Takada I, Youn MY, Yamamoto Y, Takahashi S, et al. DNA demethylation in hormone-induced transcriptional derepression. Nature. 2009;461:1007–1012. doi: 10.1038/nature08456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.