Abstract
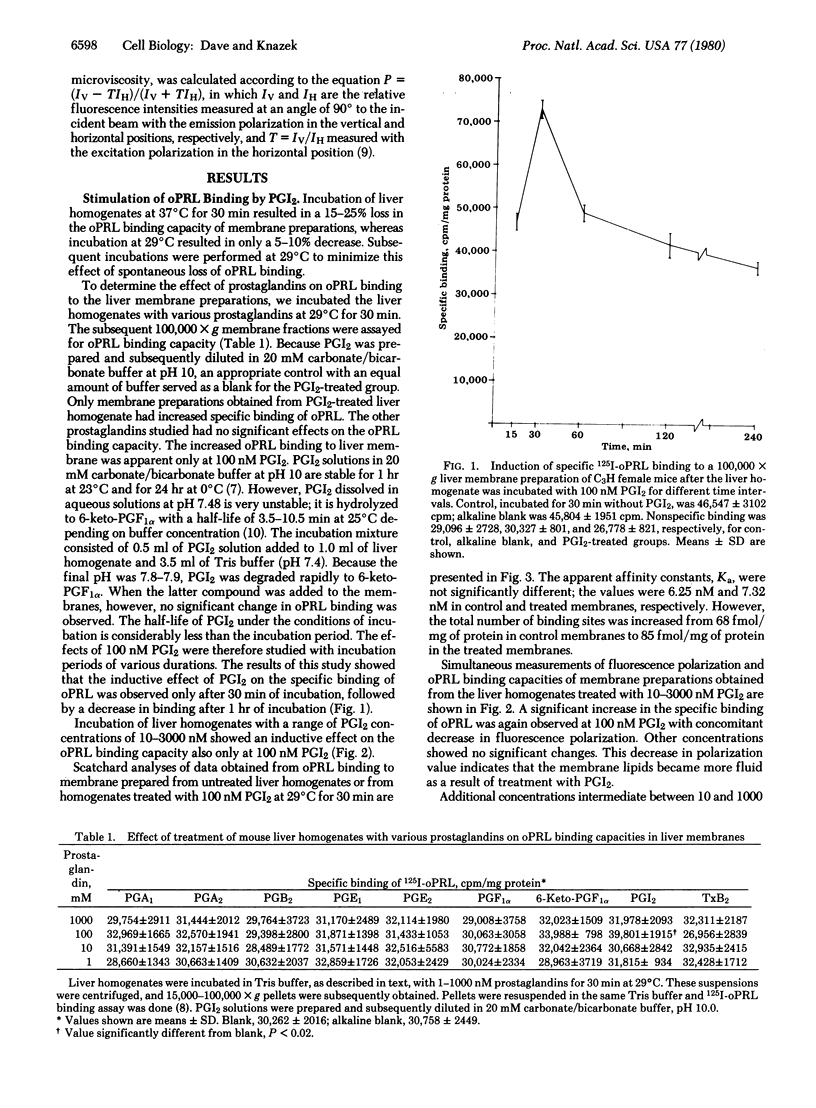
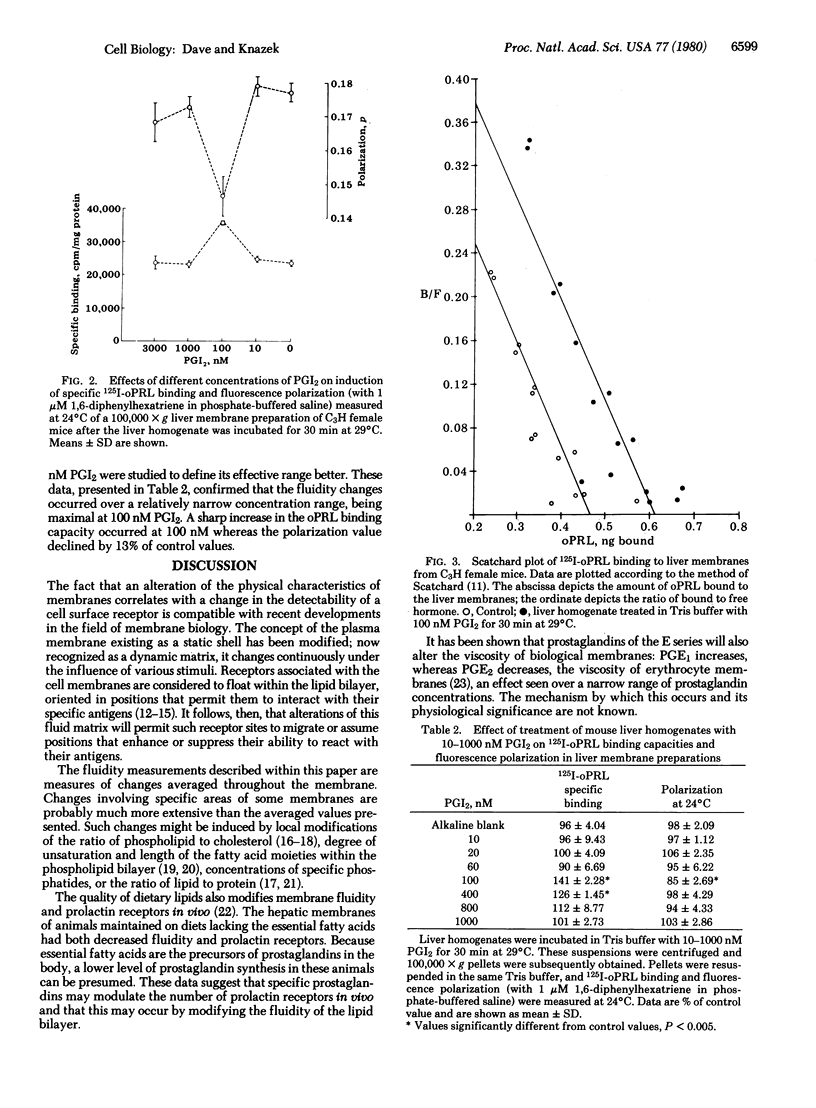
The objective of this study was to determine if prostaglandins alter the fluidity of hepatic membranes and if this, in turn, modifies their ability to bind prolactin. Liver homogenates of adult C3H female mice incubated with 1-1000 nM prostaglandin A1, A2, B2, F1 alpha, 6-keto-F1 alpha, E1, E2, I2, or thromboxane B2 provided the 100,000 X g membrane pellets for subsequent ovine prolactin binding and membrane fluidity studies. Only membrane preparations treated with prostaglandin I2 showed an increase in specific binding of ovine prolactin. The effect (40-50%) was maximal at 100 nM prostaglandin I2 after 30 min of incubation and was due to an increase in the number of receptor sites. Under the same conditions, prostaglandin I2 induced a 17% decrease in membrane microviscosity. These data suggest that specific prostaglandins may modulate the number of prolactin receptors in vivo by modifying the fluidity of the lipid bilayer and the subsequent ease with which receptors can assume active configurations within the matrix.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borochov H., Shinitzky M. Vertical displacement of membrane proteins mediated by changes in microviscosity. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4526–4530. doi: 10.1073/pnas.73.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Raff M. C. Mammalian plasma membranes. Nature. 1975 Nov 6;258(5530):43–49. doi: 10.1038/258043a0. [DOI] [PubMed] [Google Scholar]
- Cho M. J., Allen M. A. Chemical stability of prostacyclin (PGI2) in aqueous solutions. Prostaglandins. 1978 Jun;15(6):943–954. doi: 10.1016/0090-6980(78)90037-0. [DOI] [PubMed] [Google Scholar]
- Cogan U., Shinitzky M., Weber G., Nishida T. Microviscosity and order in the hydrocarbon region of phospholipid and phospholipid-cholesterol dispersions determined with fluorescent probes. Biochemistry. 1973 Jan 30;12(3):521–528. doi: 10.1021/bi00727a026. [DOI] [PubMed] [Google Scholar]
- Cooper R. A. Abnormalities of cell-membrane fluidity in the pathogenesis of disease. N Engl J Med. 1977 Aug 18;297(7):371–377. doi: 10.1056/NEJM197708182970707. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Knazek R. A., Liu S. C. Dietary essential fatty acids are required for maintenance and induction of prolactin receptors. Proc Soc Exp Biol Med. 1979 Nov;162(2):346–350. doi: 10.3181/00379727-162-40679. [DOI] [PubMed] [Google Scholar]
- Knazek R. A., Liu S. C., Gullino P. M. Induction of lactogenic binding sites in the liver of the Snell dwarf mouse. Endocrinology. 1977 Jul;101(1):50–58. doi: 10.1210/endo-101-1-50. [DOI] [PubMed] [Google Scholar]
- Kury P. G., Ramwell P. W., McConnell H. M. The effect of prostaglandins E1 and E2 on the human erythrocyte as monitored by spin labels. Biochem Biophys Res Commun. 1974 Jan 23;56(2):478–483. doi: 10.1016/0006-291x(74)90867-5. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4521–4528. doi: 10.1021/bi00665a029. [DOI] [PubMed] [Google Scholar]
- Mehdi S. Q., Nussey S. S., Shindelman J. E., Kriss J. P. The influence of lipid substitution on thyrotropin-receptor interactions in artificial vesicles. Endocrinology. 1977 Nov;101(5):1406–1412. doi: 10.1210/endo-101-5-1406. [DOI] [PubMed] [Google Scholar]
- Muller C., Shinitzky M. Modulation of transferrin receptors in bone marrow cells by changes in lipid fluidity. Br J Haematol. 1979 Jul;42(3):355–362. doi: 10.1111/j.1365-2141.1979.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Rillema J. A. Activation of casein synthesis by prostaglandins plus spermidine in mammary gland explants of mice. Biochem Biophys Res Commun. 1976 May 3;70(1):45–49. doi: 10.1016/0006-291x(76)91106-2. [DOI] [PubMed] [Google Scholar]
- Rillema J. A. Effects of prostaglandins on RNA and casein synthesis in mammary gland explants of mice. Endocrinology. 1976 Aug;99(2):490–495. doi: 10.1210/endo-99-2-490. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Henkart P. Fluidity of cell membranes--current concepts and trends. Int Rev Cytol. 1979;60:121–147. [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Microviscosity parameters and protein mobility in biological membranes. Biochim Biophys Acta. 1976 Apr 16;433(1):133–149. doi: 10.1016/0005-2736(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J., Fischkoff S., Chance B., Cooper R. A. Fluorescent probe analysis of the lipid architecture of natural and experimental cholesterol-rich membranes. Biochemistry. 1974 Apr 9;13(8):1589–1595. doi: 10.1021/bi00705a006. [DOI] [PubMed] [Google Scholar]
- Weeks J. R. A method for administration of prolonged intravenous infusion of prostacyclin (PGI2) to unanesthetized rats. Prostaglandins. 1979 Apr;17(4):495–499. doi: 10.1016/0090-6980(79)90002-9. [DOI] [PubMed] [Google Scholar]


