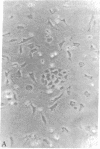
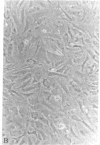
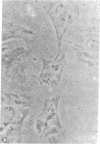
Abstract
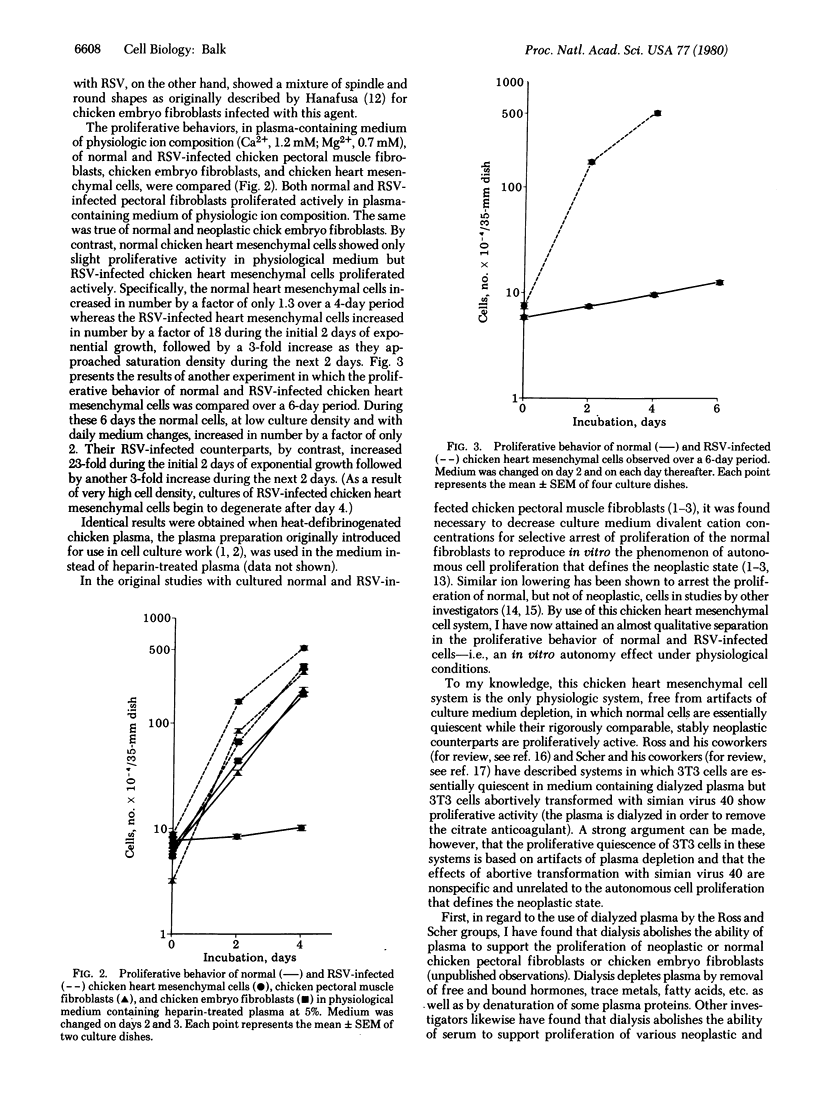
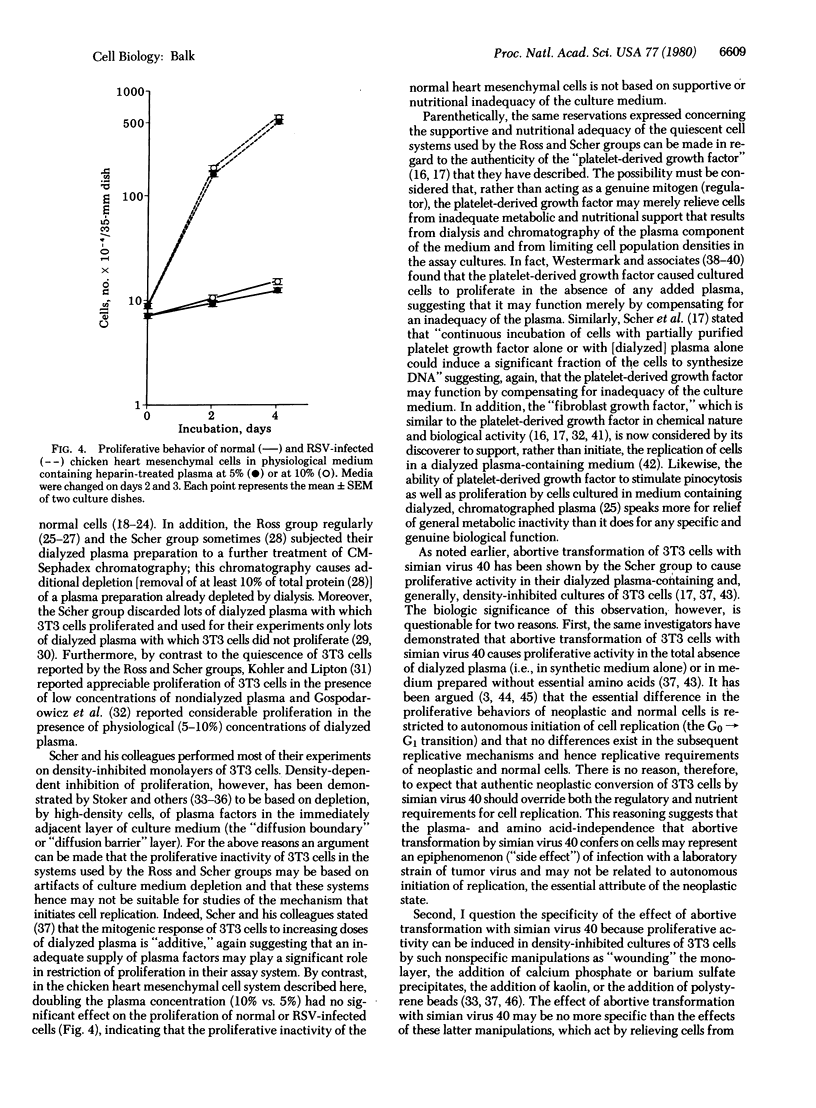
Normal as well as Rous sarcoma virus-infected chicken pectoral and chicken embryo fibroblasts proliferate actively in a plasma containing medium of physiological ion concentrations (Ca2+, 1.2 mM; Mg2+, 0.7 mM). Reduction of medium calcium and magnesium concentrations is necessary to achieve selective quiescence of normal fibroblasts in these cell systems. By contrast, normal chicken heart mesenchymal cells proliferate only sluggishly (one doubling or less during a 6-day period) in a plasma containing medium of physiologic ion concentrations, whereas Rous sarcoma virus-infected heart mesenchymal cells proliferate actively (more than four doublings during an initial 2-day phase of exponential growth). The chicken heart mesenchymal cell system therefore has great potential for studies of the mechanism that initiates cell replication and of the failure in cellular regulatory processes that is responsible for the autonomous initiation of replication of neoplastic cells. From comparison of the chicken heart mesenchymal cell system to dialyzed plasma-based systems in which 3T3 cells tend to proliferative quiescence, it is argued that this proliferative quiescence of 3T3 cells is a result of cell starvation and is not physiologically meaningful.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balk S. D. Calcium as a regulator of the proliferation of normal, but not of transformed, chicken fibroblasts in a plasma-containing medium. Proc Natl Acad Sci U S A. 1971 Feb;68(2):271–275. doi: 10.1073/pnas.68.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D., Polimeni P. I., Hoon B. S., LeStourgeon D. N., Mitchell R. S. Proliferation of Rous sarcoma virus-infected, but not of normal, chicken fibroblasts in a medium of reduced calcium and magnesium concentration. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3913–3916. doi: 10.1073/pnas.76.8.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D., Whitfield J. F., Youdale T., Braun A. C. Roles of calcium, serum, plasma, and folic acid in the control of proliferation of normal and Rous sarcoma virus-infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):675–679. doi: 10.1073/pnas.70.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. W., Colowick S. P. Stimulation of sugar uptake and thymidine incorporation in mouse 3T3 cells by calcium phosphate and other extracellular particles. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5593–5597. doi: 10.1073/pnas.74.12.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Ross R. Mediation of pinocytosis in cultured arterial smooth muscle and endothelial cells by platelet-derived growth factor. J Cell Biol. 1978 Dec;79(3):663–671. doi: 10.1083/jcb.79.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- Elmore E., Swift M. Growth of human skin fibroblasts in dialyzed fetal bovine serum. In Vitro. 1977;13(12):837–842. doi: 10.1007/BF02615132. [DOI] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks L. M., Wilson P. D. "Spontaneous" neoplastic transformation in vitro: the ultrastructure of the tissue culture cell. Eur J Cancer. 1970 Dec;6(6):517–523. doi: 10.1016/0014-2964(70)90072-1. [DOI] [PubMed] [Google Scholar]
- Frantz C. N., Stiles C. D., Scher C. D. The tumor promoter 12-O-tetradecanoyl-phorbol-13-acetate enhances the proliferative response of Balb/c-3T3 cells to hormonal growth factors. J Cell Physiol. 1979 Sep;100(3):413–424. doi: 10.1002/jcp.1041000305. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Gospodarowicz D., Greene G., Moran J. Fibroblast growth factor can substitute for platelet factor to sustain the growth of Balb/3T3 cells in the presence of plasma. Biochem Biophys Res Commun. 1975 Jul 22;65(2):779–787. doi: 10.1016/s0006-291x(75)80213-0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R. Do plasma and serum have different abilities to promote cell growth? Proc Natl Acad Sci U S A. 1980 May;77(5):2726–2730. doi: 10.1073/pnas.77.5.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Growth factors in mammalian cell culture. Annu Rev Biochem. 1976;45:531–558. doi: 10.1146/annurev.bi.45.070176.002531. [DOI] [PubMed] [Google Scholar]
- HARRIS M. Essential growth factor in serum dialysate for chick skeletal muscle fibroblasts. Proc Soc Exp Biol Med. 1959 Nov;102:468–471. doi: 10.3181/00379727-102-25287. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Partial purification and characterization of platelet factors stimulating the multiplication of normal human glial cells. Exp Cell Res. 1977 Oct 15;109(2):429–437. doi: 10.1016/0014-4827(77)90023-4. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: purification and partial characterization. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3722–3726. doi: 10.1073/pnas.76.8.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Baldwin J. H. Cell density is determined by a diffusion-limited process. Nature. 1979 Mar 15;278(5701):283–284. doi: 10.1038/278283b0. [DOI] [PubMed] [Google Scholar]
- Hunter E. Biological techniques for avian sarcoma viruses. Methods Enzymol. 1979;58:379–393. doi: 10.1016/s0076-6879(79)58153-1. [DOI] [PubMed] [Google Scholar]
- Kohler N., Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974 Aug;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- Maroudas N. G. Short-range diffusion gradients. Cell. 1974 Nov;3(3):217–219. doi: 10.1016/0092-8674(74)90134-2. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., Hamilton W. G., Ham R. G. Selenium is an essential trace nutrient for growth of WI-38 diploid human fibroblasts. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2023–2027. doi: 10.1073/pnas.73.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milo G. E., Jr, DiPaolo J. A. Neoplastic transformation of human diploid cells in vitro after chemical carcinogen treatment. Nature. 1978 Sep 14;275(5676):130–132. doi: 10.1038/275130a0. [DOI] [PubMed] [Google Scholar]
- Mitchell R. S., Balk S. D., Frank O., Baker H., Christine M. J. The failure of methotrexate to inhibit chicken fibroblast proliferation in a serum-containing culture medium. Cancer Res. 1975 Sep;35(9):2613–2615. [PubMed] [Google Scholar]
- Mitchell R. S., Elgas R. J., Balk S. D. Proliferation of Rous sarcoma virus-infected, but not of normal, chicken fibroblasts in oxygen-enriched environment: preliminary report. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1265–1268. doi: 10.1073/pnas.73.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons P. G. Selective proliferation of human tumour cells in calcium-depleted medium. Aust J Exp Biol Med Sci. 1978 Jun;56(3):297–300. doi: 10.1038/icb.1978.31. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Nist C., Kariya B., Rivest M. J., Raines E., Callis J. Physiological quiescence in plasma-derived serum: influence of platelet-derived growth factor on cell growth in culture. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):497–508. doi: 10.1002/jcp.1040970325. [DOI] [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Isberg R. R., Pledger W. J., Antoniades H. N., Scher C. D. Control of the Balb/c-3T3 cell cycle by nutrients and serum factors: analysis using platelet-derived growth factor and platelet-poor plasma. J Cell Physiol. 1979 Jun;99(3):395–405. doi: 10.1002/jcp.1040990314. [DOI] [PubMed] [Google Scholar]
- Stoker M. G. Role of diffusion boundary layer in contact inhibition of growth. Nature. 1973 Nov 23;246(5430):200–203. doi: 10.1038/246200a0. [DOI] [PubMed] [Google Scholar]
- Stoker M., Piggott D. Shaking 3T3 cells: further studies on diffusion boundary effects. Cell. 1974 Nov;3(3):207–215. doi: 10.1016/0092-8674(74)90133-0. [DOI] [PubMed] [Google Scholar]
- Swierenga S. H., Whitfield J. F., Karasaki S. Loss of proliferative calcium dependence: simple in vitro indicator of tumorigenicity. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6069–6072. doi: 10.1073/pnas.75.12.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark B., Wasteson A. A platelet factor stimulating human normal glial cells. Exp Cell Res. 1976 Mar 1;98(1):170–174. doi: 10.1016/0014-4827(76)90476-6. [DOI] [PubMed] [Google Scholar]