Abstract
In order to deal with the rising problem of antibiotic resistance, newer antibacterials are being discovered and added to existing pool. Since the year 2000, however, only four new classes of antibacterials have been discovered. These include the oxazolidinones, glycolipopeptides, glycolipodepepsipeptide and pleuromutilins. Newer drugs were added to existing classes of antibiotics, such as streptogramins, quinolones, beta-lactam antibiotics, and macrolide-, tetracycline- and trimethoprim-related drugs. Most of the antibacterials are directed against resistant S. aureus infections, with very few against resistant gram-negative infections. The following article reviews the antibacterials approved by the FDA after the year 2000 as well as some of those in clinical trials. Data was obtained through a literature search via Pubmed and google as well as a detailed search of our library database.
Keywords: Antibiotic resistance, Latest antibacterials, New antibacterials
Introduction
The need for novel antibacterials has been greater than ever in the face of increasing resistance to the older ones. Three classes of drug-resistant bacteria are a major cause of concern- Methicillin -resistant Staphylococcus aureus (MRSA), multidrug-resistant (MDR) and pan-drug-resistant (PDR) gram-negative bacteria, which include strains of Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa, and a third class comprising of MDR and extensively-drug-resistant (XDR) strains of Mycobacterium tuberculosis (MDR-TB and XDR-TB).[1]
This article reviews the antibacterials approved for clinical use by the FDA since the year 2000 and those in clinical trials, especially in the later phases. Information was obtained through a literature search via Pubmed and Google using keywords like ‘newer antibiotics’, ‘newer antibacterials’, ‘antibacterials’ and ‘antibiotics’. The internet search was accompanied by a detailed search of our library database. Since the topic is extensive, we omitted results on antitubercular drugs.
The Newer Antibacterials
Antibacterials belonging to chemical classes introduced after the year 2000
Antibacterials belonging to chemical classes introduced after 2000 are described in Table 1.
Table 1.
Antibacterials belonging to chemical classes introduced after 2000
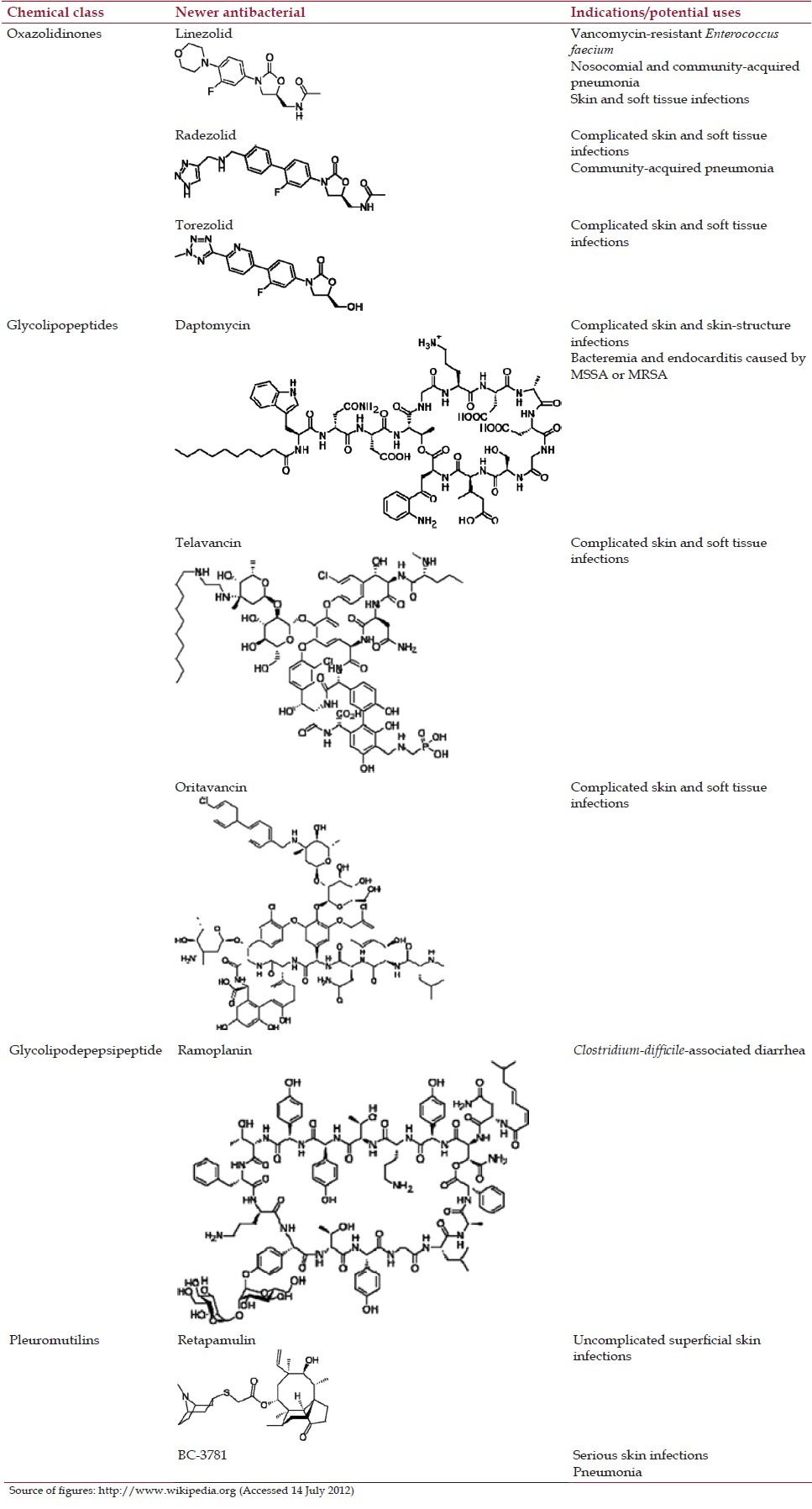
Oxazolidinones
The oxazolidinones include:
In clinical use: Linezolid
In clinical trials: Radezolid, torezolid.
The development of another oxazolidinone PF-708093 was stopped after it entered Phase I clinical trials.[2]
The oxazolidinones are a new class of drugs effective against gram-positive bacteria including MRSA, vancomycin-resistant enterococci (VRE) and Streptococcus pneumoniae. Besides, they are also effective against Mycobacterium tuberculosis and Nocardia.[3]
Linezolid
Linezolid is among the first antibacterials to be approved for clinical use this millennium. It is indicated for the treatment of vancomycin-resistant Enterococcus faecium infections, nosocomial and community-acquired pneumonia, as well as skin and soft tissue infections.
Linezolid is bacteriostatic against drug-resistant organisms like MRSA and VRE. Minimum inhibitory concentration (MIC) is less than 4 μg/ml for strains of S. aureus.[4] Linezolid inhibits bacterial protein synthesis at an early stage and inhibits the formation of a functional initiation complex. Mutations in the peptidyl transferase centre of the rRNA result in resistance to linezolid.[3]
Linezolid is associated with serious adverse effects like bone marrow suppression, peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome (when used with selective serotonin uptake inhibitors). It also shows mild monoamine oxidase (MAO) inhibitory activity.[5]
Radezolid
Radezolid has completed phase II clinical trials in complicated skin and soft tissue infections and community-acquired pneumonia with positive results. It differs from linezolid by the presence of a biaryl spacer and a heteroaryl side chain. This chemical modification increases ionization, makes the molecule hydrophilic at physiological pH and confers a dibasic character to it, thus improving the antimicrobial spectrum to include linezolid-resistant strains.[6] In vitro studies indicate that radezolid is 10-times more potent than linezolid due to higher intrinsic activity and cellular accumulation.[7] Thus, this drug could be particularly useful in persistent intracellular infections.
Torezolid
Torezolid is the active moiety of torezolid phosphate, a second-generation oral oxazolidinone. Studies claim that it shows a 4-to 16-times greater potency than linezolid against gram-positive species including MRSA. Unlike linezolid, it has been found to be bactericidal in an animal model[8] and is active against linezolid-resistant strains of S. aureus in vitro.[9] MRSA and MSSA strains also show 16 times lower frequency of spontaneous resistance as compared to strains exposed to linezolid. Torezolid has undergone phase II clinical trials for complicated skin and soft tissue infections with good efficacy and an acceptable side effect profile.[8] Its mean half-life of 8-11.1 h is approximately two-fold longer than that of linezolid, thus allowing once-daily dosing.[9]
Glycolipopeptides
Glycolipopeptides include:
In clinical use: Daptomycin, telavancin
Under clinical trials: Oritavancin.
Dalbavancin, a glycolipopeptide that requires only once-a-week dosing,[5] has been withdrawn from the market following feedback from regulatory authorities. Development of friulimicin, a lipopeptide was discontinued after a Phase I intravenous escalating-dose trial indicated that the pharmacokinetic profile of the drug was unfavourable.[2]
Daptomycin
Daptomycin is a cyclic lipopeptide with a decanoyl side-chain approved by the FDA in the year 2003 for the treatment of complicated skin and skin-structure infections caused by methicillin-susceptible or methicillin-resistant S. aureus, Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae subsp. equisimilis and Enterococcus faecalis (vancomycin-susceptible only).[10] It has also been approved for use in bacteremia and endocarditis[5] caused by MSSA or MRSA. It is inactivated by surfactant and is therefore contraindicated in pneumonia.[11]
Daptomycin exhibits rapid bactericidal activity against most gram-positive organisms including multiple-antibiotic resistant strains. Approximately 90% strains of staphylococci and streptococci are inhibited at concentrations of 0.25-0.5 μg/ml; similarly, the concentrations that inhibit 90% of E. faecalis and E. faecium are 0.5-1 and 2-4 μg/ml, respectively. The MICs for vancomycin-resistant strains tend to be higher than for vancomycin-susceptible organisms.[11]
Daptomycin acts by inserting its lipophilic tail in the bacterial cell membrane resulting in rapid membrane depolarization and potassium ion efflux. This is followed by arrest of DNA, RNA and protein synthesis, and finally cell death.
Drug interactions could occur when daptomycin is used with statins and aminoglycosides.[5] It causes reversible myopathy as one of its side effects.[11]
Telavancin
Telavancin was approved for use in 2009 for complicated skin and soft tissue infections caused by gram-positive bacteria including MRSA strains. In vitro activity against some vancomycin-resistant gram-positive organisms has been observed.[12]
Telavancin exhibits a dual mechanism of action - It inhibits peptidoglycan chain formation through blockage of both, transpeptidation and transglycosylation during cell wall formation. It also dissipates membrane potential of the bacterial cell membrane causing an increase in permeability.
Adverse effects of telavancin include vomiting, paresthesias, dyspnea, microalbuminemia, taste disturbances and thrombocytopenia.[5] Renal function should be monitored before, during and after telavancin therapy. Telavancin is not recommended in pregnancy.[13]
Oritavancin
Oritavancin is a lipoglycopeptide active against MRSA as well as VRE. It shows rapid bactericidal activity with a concentration-dependent post-antibiotic effect.[5] It inhibits transglycosylation as well as transpeptidation during peptidoglycan synthesis. It also blocks utilization of D-Ala-D-Ala or D-Ala-D-Lac containing PG precursors.[3] Currently, the FDA has accepted its New Drug Application (NDA) for standard review.
Oritavancin has a long half-life and undergoes mainly hepatic elimination, thus it may not require much dosage adjustment in renal failure patients, unlike the other lipoglycopeptides.[14]
Glycolipodepepsipeptide
In clinical trials: Ramoplanin.
Ramoplanin
Ramoplanin is a glycolipodepepsipeptide undergoing phase III clinical trials for use in Clostridium-difficile associated diarrhoea. Since it is effective only when administered orally and is not absorbed systemically, it is useful in local gastrointestinal infections.
Ramoplanin inhibits cell wall synthesis by inhibiting peptidoglycan formation. Unlike glycopeptides, it does not complex with the D-Ala-D-Ala sequence of cell wall precursors to inhibit cell wall synthesis.[15]
Pleuromutilins
The pleuromutilins include:
In clinical use: Retapamulin
In clinical trials: BC-3781.
Tiamulin and valnemulin are used in veterinary medicine. BC-3205 and BC-7013 are in early phases of clinical trials.
Retapamulin
Retapamulin is the first pleuromutilin that was approved in 2007 for topical use in the treatment of uncomplicated superficial skin infections.
Retapamulin exerts its antibacterial action by inhibiting protein synthesis. MIC90 value of retapamulin in in vitro studies was 0.12 g/ml against S. aureus, including mupirocin-resistant strains. Retapamulin appears to be approximately 1000 times as potent as mupirocin or fusidic acid against Streptococcus pyogenes.
Indications for retapamulin may increase in the future since it is effective against many common skin pathogens and has low potential for development of bacterial resistance. Side effects include pruritus and allergic contact dermatitis.[16]
BC-3781
BC-3781 is the first pleuromutilin antibacterial being developed for systemic use. It has recently completed Phase II trials and is likely to be used for the treatment of serious skin infections and pneumonia. It appears similar in efficacy to vancomycin with a good safety and tolerability profile.[17]
Newer antibacterials belonging to chemical classes in use before the year 2000
Newer antibacterials belonging to chemical classes in use before the year 2000 are described below and listed in Table 2.
Table 2.
Newer antibacterials belonging to existing chemical classes
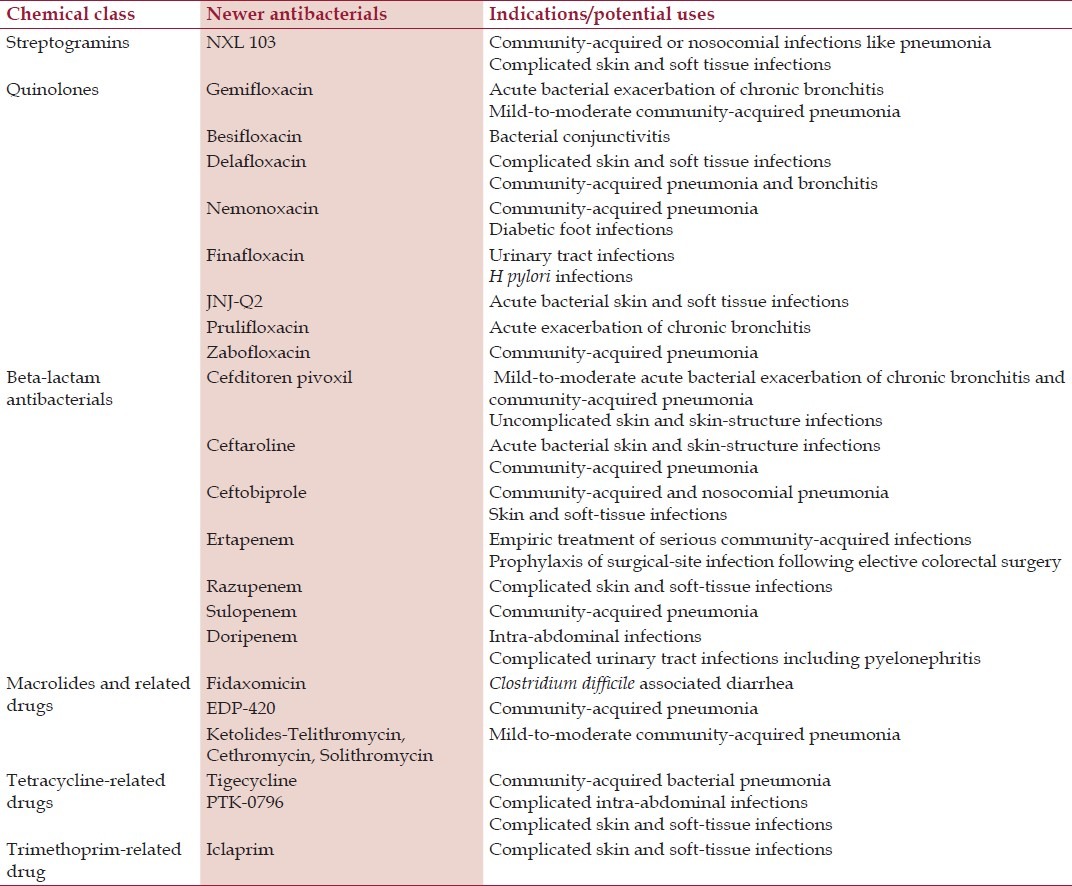
Streptogramins
Newer streptogramins are:
In clinical trials: NXL 103.
Virginamycin is a streptogramin used in fuel and ethanol industry, and in agriculture. Though not available in the United States, pristinamycin (or pristinamycine) is used in France, Belgium and some European countries
NXL-103
NXL-103 is a novel, orally administered streptogramin that has undergone Phase II trials. It contains linopristin and flopristin in a 30:70 ratio.
NXL-103 is effective against gram-positive cocci including MRSA, MRSE and VRE, gram-negative rods and anaerobes and has the potential to be used in infections like community-acquired pneumonia, community-acquired or nosocomial MRSA and VRE infections, and complicated skin and soft tissue infections. It has been shown to be up to 4 times more potent than quinupristin/dalfopristin in in vitro tests. Adverse effects in Phase I and Phase II clinical trials include vomiting, orthostatic hypotension, nausea, dizziness, headache, loose stools, and abdominal pain. Some abnormalities in liver enzymes have also been noted.[11]
Quinolones
The newer quinolones include:
In clinical use: Gemifloxacin, besifloxacin
In clinical trials: Nemonoxacin, finafloxacin, JNJ-Q2, delafloxacin, prulifloxacin, zabofloxacin.
Many of the new fluoroquinolones have anti-pseudomonal activity and additional anti-MRSA activity.[18]
Among the newer quinolones, gatifloxacin has been banned due to the risk of severe hyperglycemia. Trovafloxacin has been withdrawn from the market due to risk for hepatotoxicity. NXL-101 was discontinued after it entered Phase I clinical trials due to prolongation of QT interval. The development of DC-159a and DX-619 was discontinued after Phase I trials. WCK 771, the arginine salt of S-(–)-nadifloxacin, is under clinical trials in India, though it is not yet being tested in the United States. Sitafloxacin is a new fluoroquinolone currently marketed in Japan.
Gemifloxacin
Gemifloxacin is an oral fluoroquinolone approved in 2003 for treatment of acute bacterial exacerbation of chronic bronchitis and mild-to-moderate community-acquired pneumonia.
Gemifloxacin shows enhanced activity against gram-positive bacteria.[19] Its good potency against Strep pneumoniae appears to be due to high affinity for DNA gyrase and topoisomerase IV. Its activity against gram-positive organisms appears to be better than that of moxifloxacin, but its higher protein binding and lower serum levels result in similar activity. However, it has poor activity against methicillin-resistant strains.
Gemifloxacin also shows good activity against fluoroquinolone-resistant strains including fluoroquinolone-resistant H. influenzae.[20] It appears to be more active than ciprofloxacin against Chlamydia pneumoniae and Mycoplasma pneumoniae, but less active against Pseudomonas aeruginosa.
High concentrations are achieved in the respiratory tract after oral administration, making it an ideal drug for the treatment of respiratory tract infections.[19]
Besifloxacin
Besifloxacin was approved in 2009 as an ophthalmic suspension to treat bacterial conjunctivitis. Other formulations of the drug have not been developed to prevent indiscriminate use and thereby increase the chances of resistance to the drug.
Besifloxacin has clinical efficacy similar to moxifloxacin. It inhibits both DNA gyrase and topoisomerase IV and thus potentially reduces the chances for the development of resistance.
Besifloxacin is administered as eye drops 3 times a day. Its frequency of administration is similar to moxifloxacin and less than other fluoroquinolones, thus possibly increasing compliance. The duration between doses can vary between 4 and 12 hours, thus increasing flexibility of treatment.[21]
Quinolones undergoing clinical trials
Nemonoxacin
Nemonoxacin is a nonfluorinated quinolone that has completed phase II clinical trials in community-acquired pneumonia and diabetic foot infections.[22] It shows good activity against a variety of gram-positive and negative organisms including MRSA, VRE and other multi-drug-resistant organisms.[23]
Finafloxacin
Finafloxacin has proven activity against MRSA, VRE and other drug-resistant strains as well as anaerobes. Oral and IV formulations of finafloxacin are undergoing phase II clinical trials. Currently, it appears to be safe without prolongation of QT interval and other serious side effects associated with fluoroquinolones. High tissue levels are achieved even in acidic environments of pus, urine and secretions from infected tissues.[24] Its once-a-day administration makes it an attractive drug. Currently, it is being tested for urinary tract infections and H pylori infections.
JNJ-Q2
JNJ-Q2, a novel fluorinated 4-quinolone, is undergoing phase II clinical trials with positive results in acute bacterial skin and soft tissue infections. Most adverse effects appear to be mild in preliminary studies.[25]
Delafloxacin
Delafloxacin is a new fluoroquinolone that shows good activity against gram-positive organisms compared to earlier quinolones, including MRSA strains. It also covers gram-negative organisms and anaerobes including resistant strains. It is effective in acidic environments. It has been developed as an oral and IV formulation and is being evaluated in complicated skin and soft tissue infections, community-acquired pneumonia and bronchitis.[26]
Other quinolones
Among the other quinolones being evaluated, prulifloxacin has undergone limited studies and has the potential to be used in acute exacerbation of chronic bronchitis.[27] Zabofloxacin is undergoing phase II clinical trials for oral treatment of community-acquired pneumonia.[28]
Newer beta-lactam antibacterials
The newer beta-lactam antibacterials include:
In clinical use
Cephalosporins: Cefditoren pivoxil, ceftaroline
Carbapenems: Ertapenem and doripenem. Biapenem is approved in some European countries but not in the United States.
In clinical trials
Cephalosporin: Ceftobiprole
Carbapenems: Razupenem, sulopenem, ME 1036
Faropenem medoxomil was developed as intended treatment for community-acquired pneumonia and other respiratory tract infections. It underwent up to Phase III clinical trials. However, the development of the drug stopped after FDA issued a non-approvable letter in 2006 and requested additional clinical studies to demonstrate superiority. The development of another carbapenem, tomopenem was stopped due to unspecified reasons.[2]
Newer beta-lactam antibacterials in clinical use
Cefditoren pivoxil
Cefditoren pivoxil, a prodrug that releases cefditoren, is an oral cephalosporin approved by the FDA for clinical use in 2001. Cefditoren is a third-generation oral cephalosporin with good activity against certain respiratory tract pathogens, namely S. pneumoniae, H. influenzae and M. catarrhalis, including some β-lactamase producing strains.[29] Cefditoren pivoxil is also effective against Staphylococcus aureus (but not MRSA strains) and Streptococcus pyogenes (penicillin-susceptible strains only). MIC90 values for cefditoren against MSSA ranged from 0.5 to 1 mg/L, and were similar to those for cefuroxime and cefdinir, but lower than those for cefpodoxime, cefaclor, and cefixime.[30]
Cefditoren pivoxil is used in the treatment of mild-to-moderate acute bacterial exacerbation of chronic bronchitis and community-acquired pneumonia, pharyngitis or tonsillitis, and uncomplicated skin and skin-structure infections.[4]
Ceftaroline
Ceftaroline is the newest cephalosporin with anti-MRSA activity that obtained FDA approval in October 2010. It is used in the treatment of acute bacterial skin and skin-structure infections, and community-acquired bacterial pneumonia.
Ceftaroline is active against MSSA as well as MRSA isolates with MIC90 values of 0.25 and 1 mg/L, respectively. The low MIC values are indicative of ceftaroline's high affinity for penicillin-binding proteins. Ceftaroline is also effective against Streptococcus pyogenes, Streptococcus agalactiae, and Streptococcus pneumoniae, gram-negative bacteria like ceftazidime-susceptible E. coli and Klebsiella pneumoniae, and β-lactamase-positive and negative Haemophilus influenzae.
Ceftaroline, available as ceftaroline fosamil, is administered intravenously; dosage adjustment is required in renal failure.[31]
Ertapenem
Ertapenem is a 1-β-methyl carbapenem approved for use by the FDA in the year 2001. It is effective against gram-positive and negative aerobic as well as anaerobic bacteria excluding the nonfermenters, MRSA and drug-resistant enterococci. It appears to be effective against most resistant enterobacteriaceae producing ESBLs and/or AmpC-type β-lactamases.[32] MIC90 values in in vitro studies for most species of Enterobacteriaceae were <1 mg/L, significantly lower than those of imipenem. MIC90s for most Bacteroides fragilis group isolates ranged from 1 to 4 mg/L, and from 0.06 mg/L for Clostridium perfringens to 4 mg/L for Clostridium clostridioforme.[33]
Since ertapenem has limited in vitro activity against P. aeruginosa and Acinetobacter species, it is more suitable for the empiric treatment of serious infections acquired in the community than those acquired nosocomially. Ertapenem is also recommended for prophylaxis of surgical-site infection following elective colorectal surgery. Unlike imipenem, ertapenem does not require co-administration with cilastin.
Doripenem
Doripenem was approved for use by the FDA in 2007. Its spectrum is more similar to that of meropenem and imipenem than of ertapenem. Thus, it is effective against gram-positive and negative aerobes and anaerobes including Pseudomonas aeruginosa, Acinetobacter species, but not MRSA, VRE and other strains resistant to imipenem and meropenem. It is effective against β-lactamase producing strains of enterobacteriaceae; MICs for doripenem against extended-spectrum β-lactamase or AmpC producers are less than 1 μg/ mL, and between the ranges of 8-64 μg/mL against KPC-producing or metallo-β-lactamase-producing organisms.
Doripenem is approved for the treatment of intra-abdominal infections and complicated urinary tract infections including pyelonephritis. Dosage adjustment is required in renal failure patients.[34]
Beta-lactam antibacterials in clinical trials
Ceftobiprole
Ceftobiprole is an intravenous fourth-generation cephalosporin being developed for community-acquired pneumonia, skin and soft-tissue infections due to MRSA, and nosocomial pneumonia due to suspected or proven MRSA, including ventilator-associated pneumonia.[35]
Ceftobiprole has been submitted as a new drug application (NDA) to the FDA and has already been approved for use in some countries. It has limited activity against anaerobes and is not effective against extended spectrum β-lactamase, serine carbapenemases and metallo-β-lactamases producing species.[36] The prodrug ceftobiprole medocaril is currently formulated as an intravenous preparation.
Ceftobiprole facilitates a conformational change in penicillin-binding protein PBP2a, allowing the formation of a stable acyl-enzyme complex. It also has affinity to other staphylococcal binding proteins such as PBP1a, PBP1b, PBP2x, PBP2a, PBP2b and PBP3; this property is responsible for the high activity of ceftobiprole against staphylococci. It also binds to PBPs of other organisms such as S. pneumoniae, E coli and Pseudomonas aeruginosa with high affinity.
Adverse effects reported include a caramel-like taste disturbance, nausea, vomiting, headache and mild-to-moderate increase in liver enzymes.[36]
Carbapenems
Among the carbapenems in clinical trials, razupenem is a novel β-methyl carbapenem that has completed Phase II clinical trials in complicated skin and soft-tissue infections. It is active against methicillin-resistant staphylococci and vancomycin-resistant enterococci, including Enterococcus faecalis but not Enterococcus faecium.[1] It also shows some activity against Enterobacteriaceae.[18]
Sulopenem shows good activity against gram-positive and gram-negative organisms and anaerobes except Pseudomonas aeruginosa. It is currently undergoing Phase II clinical trials in skin and soft-tissues infections. It is also being tested for use in community-acquired pneumonia.[37]
ME 1036 is an intravenous carbapenem in early clinical trials. It shows in vitro potency against resistant gram-positive organisms, including MRSA and VRE, and ESBL-producing E. coli and K. pneumoniae but is not effective against P. aeruginosa.[38]
Newer macrolides and ketolides
Newer macrolides and ketolides include:
In clinical use: Fidaxomicin, telithromycin
In clinical trials: EDP-420, ketolides including cethromycin, solithromycin (CEM-101), PF-04287881.
Ketolides are derivatives of macrolides with replacement of L-cladinose on the macrolide ring with a 3-keto group.
Newer macrolides and ketolides approved for use
Fidaxomicin
Fidaxomicin is probably the latest antibacterial approved for use in May 2011. It is indicated in Clostridium difficile-associated diarrhoea. MIC50 and MIC90 values for C. difficile range from ≤0.016 to 0.25 μg/ml and 0.125 to 0.5 μg/ml, respectively. Rates of recurrence for some strains of Clostridium difficile have also been found to be lower with fidaxomicin as compared to vancomycin in clinical studies. Thus, it may be a preferred drug in cases of recurrence.[39]
Fidaxomicin exerts its bactericidal effect by inhibiting bacterial RNA polymerase. It is minimally or not absorbed following oral administration, thus systemic side effects are reduced. It does not affect the normal flora of the lower gastrointestinal tract since it does not show any activity against gram-negative organisms.[18]
Telithromycin
Telithromycin is the first ketolide in the market approved for use in 2004 for the treatment of mild-to-moderate community-acquired pneumonia caused by Streptococcus pneumoniae (including MDR isolates), Haemophilus influenzae, Moraxella catarrhalis, Chlamydophila pneumoniae or Mycoplasma pneumoniae.[40]
Telithromycin is effective against erythromycin-resistant pneumococci. This is because of its enhanced binding to domain II of rRNA. MIC values for pneumococci vary from of 0.008 μg/mL to 0.015 μg/mL.[41]
Telithromycin was initially also indicated for the treatment of acute bacterial sinusitis and acute exacerbation of chronic bronchitis. However, it was withdrawn for these indications in 2007 due to its ability to cause serious adverse effects. Post-marketing surveillance revealed that telithromycin was associated with hepatotoxicity, exacerbation of myasthenia gravis, visual disturbances, and loss of consciousness. Its hepatotoxic potential resulted in narrowing of its indications to the treatment of mild-to-moderate community-acquired pneumonia only.[40]
Newer macrolides and ketolides undergoing clinical trials
EDP-420
EDP-420 is the first bridged bicyclic macrolide currently undergoing Phase II studies for the treatment of community-acquired pneumonia. It has shown good activity against respiratory tract pathogens including some drug-resistant strains. It also appears to show some activity against Mycobacterium avium. It has a long half-life that may permit less frequent dosage and for shorter periods.[42]
Cethromycin
Cethromycin is a new ketolide that has been submitted as a New Drug Application for the treatment of mild-to-moderate pneumonia. It has completed phase III studies with positive results.
Cethromycin is highly active against S. pneumoniae, including drug-resistant strains. It also shows some activity against gram-negative organisms including Moraxella cattarhalis, Haemophilus influenzae and atypical pathogens.
Unlike telithromycin, cethromycin does not appear to be hepatotoxic in clinical trials.[40]
Solithromycin
Solithromycin is a new fluoroketolide entering Phase II clinical trials for community-acquired pneumonia. It inhibits synthesis of bacterial ribosomal protein in a number of gram-positive as well as negative bacteria, including resistant strains.[43]
Newer tetracycline-related antibacterials
Newer antibacterials related to tetracyclines include:
In clinical use: Tigecycline
In clinical trials: PTK-0796.
Tigecycline
Tigecycline is the glycylcycline and is a structural derivative of minocycline.[5] It was approved for use in the year 2005 for complicated skin and soft-tissue infections, community-acquired bacterial pneumonia and complicated intra-abdominal infections. Its efficacy against gram-negative as well as positive organisms makes it a useful drug in mixed infections.[11]
Tigecycline also shows potent activity against a number of resistant organisms. It binds avidly to the ribosome and does not undergo active efflux easily in gram-positive organisms.[5] However, it is susceptible to efflux from organisms like Pseudomonas aeruginosa, Proteus spp., Providencia spp., and Morganella spp., which makes these organisms inherently drug resistant to tigecycline.
MIC90 values for A. baumannii of 1-2 mg/L have been reported to be the lowest among all antimicrobials including carbapenems.[44]
Dosage reduction may be required in severe hepatic impairment. It has excellent tissue penetration, thus supporting its use in deep tissue infections.
Gastrointestinal adverse effects like nausea, vomiting, diarrhoea and heartburn are commonly observed with tigecycline. It is contraindicated in pregnancy and children below 8 years of age.[5]
PTK-0796
PTK-0796, a first-in-class aminomethylcycline, is being developed as a once-daily treatment for MRSA, VRE, and some resistant gram-negative pathogens, including A. baumannii. It has entered Phase III trials for the use in complicated skin and soft-tissue infections.
PTK-0796 shows activity against gram-positive and gram-negative bacteria as well as atypical and anaerobic bacteria, including resistant strains such as MRSA, VRE and gram-negatives producing ESBL.[18] The oral form may enable people to continue the same treatment at home following discharge from the hospital.
Newer trimethoprim-related drug
In clinical trials: Iclaprim.
Iclaprim
Iclaprim has undergone Phase III trials and has recently been accepted as New Drug Application by the FDA for the treatment of complicated skin and soft-tissue infections. Iclaprim, a dihydrofolate reductase inhibitor, is being developed as a single agent, though it does show synergistic effect when administered with some sulfonamides.
Iclaprim shows good activity against S. aureus and S. pneumoniae, including several resistant strains. Its effectiveness against H. influenzae, Moraxella catarrhalis and Legionella pneumophila may also make it useful in respiratory tract infections. It is being developed as an intravenous as well as oral formulation.[45]
Conclusions
Misuse of the older antibacterials has resulted in the problem of resistance that we face today. Unfortunately, the process of discovering a new antibacterial is a lengthy one. Only four new classes of antibacterials have been discovered in the last 11 years. The drugs in the pipeline are also not an extensive list. In addition, a number of drugs are discontinued during development. Though newer antibacterials are the need of the hour, it is also necessary to implement policies restricting their use. They should be available for use only for the specific conditions they are developed for. Their use in trivial infections and topical application should be avoided. Regulatory bodies can make it mandatory that these drugs should be available only in tertiary care hospital, thus preventing their rampant use and over-the-counter utility.
Most of the newly approved antibacterials are aimed to treat resistant gram-positive infections like those caused by MRSA and VRE. Research also needs to be directed towards other serious infections like those caused by resistant gram-negative bacteria and anaerobes.
Attempts have also been made during the discovery process to improve the pharmacokinetic properties of the newer antibacterial drugs. This should help to improve patient acceptability of the drugs and ensure that the patients complete the entire course, again to reduce the possibility of developing resistance.
The list of antibacterials likely to come out in the market in the next few years is short. An advice to use these drugs judiciously only in situations that they are specifically indicated for should accompany the medications. They should be administered under careful supervision to ensure that they do not suffer the same fate of their predecessors.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009;325:1089–93. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryder NS. Discontinued drugs in 2008: Anti-infectives. Expert Opin Investig Drugs. 2010;19:1–21. doi: 10.1517/13543780903473150. [DOI] [PubMed] [Google Scholar]
- 3.Devasahayam G, Scheld WM, Hoffman PS. Newer antibacterial drugs for a new century. Expert Opin Investig Drugs. 2010;19:215–34. doi: 10.1517/13543780903505092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunton LB, Chabner BA, Knollmann BC, editors. 12th ed. New York, NY: McGraw Hill Medical; 2011. Goodman and Gilman's: The Pharmacological Basis of Therapeutics. [Google Scholar]
- 5.Manfredi R, Sabbatani S. Novel pharmaceutical molecules against emerging resistant gram-positive cocci. Braz J Infect Dis. 2010;14:96–108. doi: 10.1590/s1413-86702010000100020. [DOI] [PubMed] [Google Scholar]
- 6.Lemaire S, Tulkens PM, Van Bambeke F. Cellular pharmacokinetics of the novel biaryloxazolidinone radezolid in phagocytic cells: Studies with macrophages and polymorphonuclear neutrophils. Antimicrob Agents Chemother. 2010;54:2540–8. doi: 10.1128/AAC.01723-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemaire S, Kosowska-Shick K, Appelbaum PC, Verween G, Tulkens PM, Van Bambeke F. Cellular pharmacodynamics of the novel biaryloxazolidinone radezolid: Studies with infected phagocytic and nonphagocytic cells, using Staphylococcus aureus, Staphylococcus epidermidis, Listeria monocytogenes, and Legionella pneumophila. Antimicrob Agents Chemother. 2010;54:2549–59. doi: 10.1128/AAC.01724-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prokocimer P, Bien P, Surber J, Mehra P, DeAnda C, Bulitta JB, et al. Phase 2, randomized, double-blind, dose-ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral torezolid phosphate in patients with complicated skin and skin structure infections. Antimicrob Agents Chemother. 2011;55:583–92. doi: 10.1128/AAC.00076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betriu C, Morales G, Rodríguez-Avial I, Culebras E, Gómez M, López-Fabal F, et al. Comparative activities of TR-700 (torezolid) against staphylococcal blood isolates collected in Spain. Antimicrob Agents Chemother. 2010;54:2212–5. doi: 10.1128/AAC.01653-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steenbergen JN, Alder J, Thorne GM, Tally FP. Daptomycin: A lipopeptide antibiotic for the treatment of serious Gram-positive infections. J Antimicrob Chemother. 2005;55:283–8. doi: 10.1093/jac/dkh546. [DOI] [PubMed] [Google Scholar]
- 11.Politano AD, Sawyer RG. NXL-103, a combination of flopristin and linopristin, for the potential treatment of bacterial infections including community-acquired pneumonia and MRSA. Curr Opin Investig Drugs. 2010;11:225–36. [PMC free article] [PubMed] [Google Scholar]
- 12.Dunbar LM, Tang DM, Manausa RM. A review of telavancin in the treatment of complicated skin and skin structure infections (cSSSI) Ther Clin Risk Manag. 2008;4:235–44. doi: 10.2147/tcrm.s1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonkowski J, Daniels AR, Peppard WJ. Role of telavancin in treatment of skin and skin structure infections. Clin Cosmet Investig Dermatol. 2010;3:127–33. doi: 10.2147/CCID.S9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guskey MT, Tsuji BT. A comparative review of the lipoglycopeptides: Oritavancin, dalbavancin, and telavancin. Pharmacotherapy. 2010;30:80–94. doi: 10.1592/phco.30.1.80. [DOI] [PubMed] [Google Scholar]
- 15.Montecalvo MA. Ramoplanin: A novel antimicrobial agent with the potential to prevent vancomycin-resistant enterococcal infection in high-risk patients. J Antimicrob Chemother. 2003;51(Suppl 3):iii31–5. doi: 10.1093/jac/dkg274. [DOI] [PubMed] [Google Scholar]
- 16.Moody MN, Morrison LK, Tyring SK. Retapamulin: What is the role of this topical antimicrobial in the treatment of bacterial infections in atopic dermatitis? Skin Therapy Lett. 2010;15:1–4. [PubMed] [Google Scholar]
- 17.Nabriva Therapeutics presents extended Phase II results for pleuromutilin antibiotic BC-3781. [Accessed December 1, 2011]. at http://www.drugs.com/clinical_trials/nabriva-therapeutics-presents-extended-phase-iiresults-pleuromutilin-antibiotic-bc-3781-12362.html .
- 18.Abbanat D, Morrow B, Bush K. New agents in development for the treatment of bacterial infections. Curr Opin Pharmacol. 2008;8:582–92. doi: 10.1016/j.coph.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Saravolatz LD, Leggett J. Gatifloxacin, gemifloxacin, and moxifloxacin: The role of 3 newer fluoroquinolones. Clin Infect Dis. 2003;37:1210–5. doi: 10.1086/378809. [DOI] [PubMed] [Google Scholar]
- 20.Morrissey I, Tillotson G. Activity of gemifloxacin against Streptococcus pneumoniae and Haemophilus influenzae. J Antimicrob Chemother. 2004;53:144–8. doi: 10.1093/jac/dkh092. [DOI] [PubMed] [Google Scholar]
- 21.Comstock TL, Karpecki PM, Morris TW, Zhang JZ. Besifloxacin: A novel anti-infective for the treatment of bacterial conjunctivitis. Clin Ophthalmol. 2010;4:215–25. doi: 10.2147/opth.s9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.TaiGen announces presentation of nemonoxacin at the joint meeting of ICAAC/IDSA. [Accessed December 1, 2001]. at http://www.drugs.com/clinical_trials/taigen-announces-presentation-nemonoxacin-joint-meetingicaac-idsa-5844.html .
- 23.van Rensburg DJ, Perng RP, Mitha IH, Bester AJ, Kasumba J, Wu RG, et al. Efficacy and safety of nemonoxacin versus levofloxacin for community-acquired pneumonia. Antimicrob Agents Chemother. 2010;54:4098–106. doi: 10.1128/AAC.00295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins PG, Stubbings W, Wisplinghoff H, Seifert H. Activity of the investigational fluoroquinolone finafloxacin against ciprofloxacin-sensitive and -resistant Acinetobacter baumannii isolates. Antimicrob Agents Chemother. 2010;54:1613–5. doi: 10.1128/AAC.01637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow BJ, He W, Amsler KM, Foleno BD, Macielag MJ, Lynch AS, et al. In vitro antibacterial activities of JNJ-Q2, a new broad-spectrum fluoroquinolone. Antimicrob Agents Chemother. 2010;54:1955–64. doi: 10.1128/AAC.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemaire S, Tulkens PM, Van Bambeke F. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:649–58. doi: 10.1128/AAC.01201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blasi F, Aliberti S, Tarsia P, Santus P, Centanni S, Allegra L. Prulifloxacin: A brief review of its potential in the treatment of acute exacerbation of chronic bronchitis. Int J Chron Obstruct Pulmon Dis. 2007;2:27–31. doi: 10.2147/copd.2007.2.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safety and efficacy study of oral zabofloxacin in community acquired pneumonia. [Accessed December 1, 2011]. at http://clinicaltrials.gov/ct2/show/NCT01081964 .
- 29.Barberán J, Mensa J. Cefditoren and community-acquired lower respiratory tract infections. Rev Esp Quimioter. 2009;22:144–50. [PubMed] [Google Scholar]
- 30.Manaktala C, Singh AK, Verma M, Sachdeva A, Sharma H, Roy A, et al. Efficacy and tolerability of cefditoren pivoxil in uncomplicated skin and skin structure infections in Indian patients. Indian J Dermatol. 2009;54:350–6. doi: 10.4103/0019-5154.57612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girish C, Balakrishnan S. Ceftaroline fosamil: A novel anti-Methicillin-resistant Staphylococcus aureus cephalosporin. J Pharmacol Pharmacother. 2011;2:209–11. doi: 10.4103/0976-500X.83298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah PM, Isaacs RD. Ertapenem, the first of a new group of carbapenems. J Antimicrob Chemother. 2003;52:538–42. doi: 10.1093/jac/dkg404. [DOI] [PubMed] [Google Scholar]
- 33.Wexler HM. In vitro activity of ertapenem: Review of recent studies. J Antimicrob Chemother. 2004;53(Suppl 2):ii11–21. doi: 10.1093/jac/dkh204. [DOI] [PubMed] [Google Scholar]
- 34.Paterson DL, Depestel DD. Doripenem. Clin Infect Dis. 2009;49:291–8. doi: 10.1086/600036. [DOI] [PubMed] [Google Scholar]
- 35.El Solh A. Ceftobiprole: A new broad spectrum cephalosporin. Expert Opin Pharmacother. 2009;10:1675–86. doi: 10.1517/14656560903048967. [DOI] [PubMed] [Google Scholar]
- 36.Ohlsen K. Novel antibiotics for the treatment of Staphylococcus aureus. Expert Rev Clin Pharmacol. 2009;2:661–72. doi: 10.1586/ecp.09.26. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton-Miller JM. Chemical and microbiologic aspects of penems, a distinct class of beta-lactams: Focus on faropenem. Pharmacotherapy. 2003;23:1497–507. doi: 10.1592/phco.23.14.1497.31937. [DOI] [PubMed] [Google Scholar]
- 38.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 39.Shah D, Dang MD, Hasbun R, Koo HL, Jiang ZD, DuPont HL, et al. Clostridium difficile infection: Update on emerging antibiotic treatment options and antibiotic resistance. Expert Rev Anti Infect Ther. 2010;8:555–64. doi: 10.1586/eri.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rafie S, MacDougall C, James CL. Cethromycin: A promising new ketolide antibiotic for respiratory infections. Pharmacotherapy. 2010;30:290–303. doi: 10.1592/phco.30.3.290. [DOI] [PubMed] [Google Scholar]
- 41.Lonks JR, Goldmann DA. Telithromycin: A ketolide antibiotic for treatment of respiratory tract infections. Clin Infect Dis. 2005;40:1657–64. doi: 10.1086/430067. [DOI] [PubMed] [Google Scholar]
- 42.Jiang LJ, Or YS. Pharmacokinetics of EDP-420 after multiple oral doses in healthy adult volunteers and in a bioequivalence study. Antimicrob Agents Chemother. 2009;53:3218–25. doi: 10.1128/AAC.00022-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llano-Sotelo B, Dunkle J, Klepacki D, Zhang W, Fernandes P, Cate JH, et al. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob Agents Chemother. 2010;54:4961–70. doi: 10.1128/AAC.00860-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seputiene V, Povilonis J, Armalyte J, Suziedelis K, Pavilonis A, Suziedeliene E. Tigecycline - How powerful is it in the fight against antibiotic-resistant bacteria? Medicina (Kaunas) 2010;46:240–8. [PubMed] [Google Scholar]
- 45.Neuner EA, Ritchie DJ, Micek ST. New antibiotics for healthcare-associated pneumonia. Semin Respir Crit Care Med. 2009;30:92–101. doi: 10.1055/s-0028-1119813. [DOI] [PubMed] [Google Scholar]


