Pregabalin and Methylcobalamine are both capable reliving the symptoms of peripheral neuropathy but their effectiveness when used in a fixed dose combination still remains a subject of evaluation, especially in the orthopaedic setting. This post marketing surveillance study evaluates efficacy and tolerability of a fixed dose combination of Methylcobalamin 750 μg and Pregabalin 75 mg in the management of the same. The fixed dose combination of was prescribed for 4 weeks to the selected patients at 300 orthopaedic clinics all over India involving 1327 patients. Pain was assessed on a 10 point Numerical Pain Intensity Scale while physicians and patients overall assessment of efficacy and tolerability was done on a four-point scale. Results demonstrated a reduction in the pain which was evidenced by a visual analogue score of 38.87 and 73.99% at second and fourth week respectively. Eighty seven per-cent doctors and 88.11% patients expressed response to therapy as good to excellent. Tolerability was expressed as good to excellent by 81.48% of patients. Adverse events were of mild severity. Thus the fixed dose combination was efficacious and well tolerated in neuropathic pain with minimal adverse effects.
This multi-centre open label, non-interventional observational, Phase IV (Post marketing surveillance) trial was planned to determine the efficacy and tolerability of a FDC of Methylcobalamine and Pregabalin in cases of painful peripheral neuropathy. The study was conducted in accordance with the Declaration of Helsinki and its subsequent revisions, with strict adherence to protocol which was approved by the independent ethic committee.
Patients of either sex between the ages of 18-65 years with painful peripheral neuropathy were recruited in the study from the outpatient department of 300 orthopaedic clinics across India. The nature of the study was explained to them and informed consent was taken. The primary requirement for inclusion was neuropathic patients with a score of > 4 on neurogenic pain diagnostic questionnaire (NPDQ).[1] The main exclusion criteria included any contraindication to Methylcobalamin and Pregabalin, complex regional pain syndrome (type 1 and 2), pregnancy, lactating mothers and also women with no reliable history of contraception in childbearing age.
Eligible patients consumed the FDC of Methylcobalamin and Pregabalin, orally, as prescribed by the treating physician for duration of 4 weeks. Medication for control of diabetes and those considered necessary for the patients well-being and which did not interfere with the study medication were prescribed at the discretion of treating investigator.
Patients were screened during Visit 1. The efficacy and safety was assessed on two visits (on completion of second weeks) and third visit (on completion of fourth weeks). Pain was assessed on a 10 point Numerical Pain Intensity Scale or the visual analogue scale (VAS);[2] the patients were asked to rate their pain and the corresponding visual analogue score was recorded. The pain assessed during the first visit served as the baseline score. Physicians and Patients performed the overall assessment of efficacy and tolerability at the end of the study on a four-point scale of; Excellent, Good, Moderate and Poor. The nature and severity of the adverse events reported assessed was assessed at the end of the study.
The primary outcome measure was the change in the pain level from the baseline on the VAS score. Secondary outcome measures included; (i) Adverse Events that were either spontaneously reported by the patient or noticed by the physician. (ii) Doctors Global Assessment of Response to Therapy (DGART), (iii) Patients Global Assessment of Response to Therapy (PGART) and (iii) Patients Global Assessment of Tolerability to Therapy (PGATT).
Statistical analysis was done using IBM SPSS 19.0 (Statistical Package for the Social Sciences). Demographic data was presented as descriptive statistics and baseline and end of surveillance (EOS) characteristics were compared by using the t-test for quantitative variables. Paired t-test was performed to assess the statistical significance of the differences between baseline and surveillance visits, under each group separately. All analyses were prospectively planned and conducted on an intention-to-treat (ITT) basis. Patient data loss was kept to a minimum by defining ITT eligibility as any patient with at least one baseline and one follow-up visit.
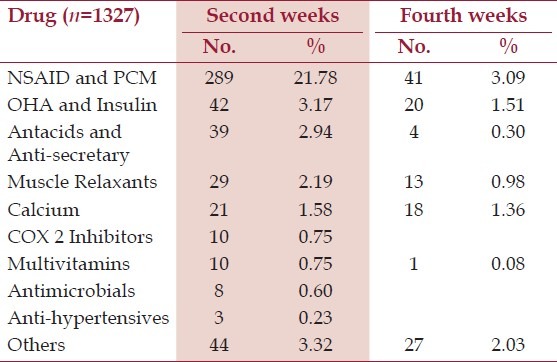
A total of 1525 patients were screened, of which 63.90% were males and 36.10% were females. The mean (±SD) age of patient recruited was 47.98 (±10.27) years. All patients data was included for safety analysis but since 198 scored <4 on the NPDQ,[Table 1] there were 1327 eligible patients for efficacy analysis. One patient dropped out due to early relief, 26 withdrew due to no relief while for five patients follow up data was not available. The baseline signs and symptom in patients with NPDQ score > 4 is summarized in Table 2. A total of 30.37% (n=403) reported use of concomitant medication at second weeks, whereas on completion of study only 8.29% (n=110) were taking additional drugs, this reduction was seen predominantly for pills that decreased pain or relaxed the muscles [Table 3] Participants that completed the trial remained compliant to study medication by adhering to the treatment as per study protocol.
Table 1.
Patients baseline Neuropathic pain diagnostic questionnaire (NPDQ) scores (n=1525)
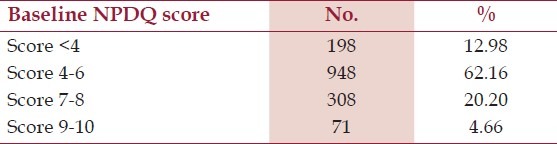
Table 2.
Baseline clinical signs and symptom distribution in study population
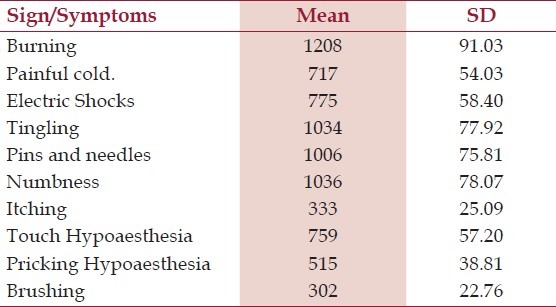
Table 3.
Concomitant medication received by the patients
The mean (±SD) VAS scores at baseline was 7.74 (±1.54). At second visit and end of study, a higher proportion of patients reported relief of pain from baseline which was shown by mean scores of 4.73 (±1.41) and 2.01 (±1.40) respectively . The reduction in the pain as evidenced by change form baseline in the mean VAS score for pain was 38.87% and 73.99% at second and fourth week respectively.
Eighty eight per cent doctors and 88.11% patients expressed response to therapy as good to excellent. Tolerability to therapy was expressed as good to excellent by 81.48% of patients. Adverse events reported were of mild severity.
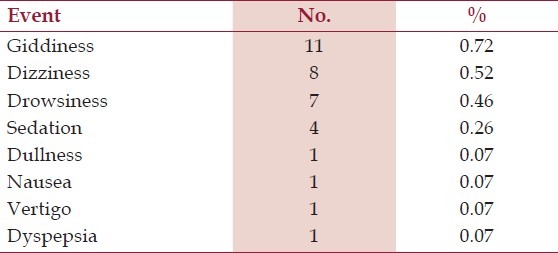
Thirty one patients experienced a total of 33 adverse events. Only one participant reported severe vertigo and another dizziness which lead to study dropouts, the former was not related to study medication as confirmed by the investigator. There was also a report of severe giddiness after start of therapy which was adequately managed tablet Betahistine 8 mg . Dyspepsia related to the concomitant Non-Steroidal Anti-inflammatory Drugs use, was treated using a proton pump inhibitor. Giddiness and drowsiness in three patients resolved without any intervention [Table 4].
Table 4.
Adverse events reported by the patients (n=1525)
Pain, one of the cardinal symptoms of neuropathy, was reduced by the FDC of Methylcobalamin and Pregabalin and significant effect was seen from second week onwards to end of study. Majority of the physicians expressed that FDC of Methylcobalamin and Pregabalin clearly demonstrated good to excellent response to therapy. Patients were of similar opinion regarding the efficacy and tolerability to the study drug. Also, most of the adverse events that occurred did not warrant discontinuation of the treatment and the overall tolerability profile of the study medications was not affected.
Pregabalin's anticonvulsant and analgesic action is by binding to the α2-δ protein subunit of voltage-gated calcium channels, thereby reducing excitatory neurotransmitter release. Pregabalin exhibits linear pharmacokinetics after oral administration, with low intersubject variability.[3] Pregabalin was effective in relieving central neuropathic pain, improving sleep, anxiety, and overall patient status in patients with spinal cord injury.[4]
A Cochrane Systematic Review of various studies on Pregabalin has proven its efficacy in neuropathic pain conditions and fibromyalgia.[5] The neurologically active form of vitamin B12 is Methylcobalamin is.[6] In a four-month, double-blind, placebo-controlled trial of type 1 and 2 diabetics with neuropathy, it was found that Methylcobalamin (1500 mcg per day) significantly improved somatic and autonomic symptoms in the treatment group compared to placebo.[7] Systematic review of several controlled clinical trials on vitamin B12 concluded Methylcobalamin therapy benefits on autonomic symptoms were as consistent as its effects on pain and somatosensory symptoms and which were relatively reliable.[8] Clinical studies have also demonstrated that Vitamin B 12 is more effective than nortriptyline in improving painful diabetic neuropathy.[9,10]
In the view of the finding of this study we conclude that a FDC of Methylcobalamin 750 μg and Pregabalin 75 mg is well tolerated and efficacious in treating the pain of neuropathic origin.
References
- 1.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Vranken JH, Dijkgraaf MG, Kruis MR, van der Vegt MH, Hollmann MW, Heesen M. Pregabalin in patients with central neuropathic pain: A randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain. 2008;136:150–7. doi: 10.1016/j.pain.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Blommel ML, Blommel AL. Pregabalin: An antiepileptic agent useful for neuropathc pain. Am J Health.Syst Pharm. 2007;64:1475–82. doi: 10.2146/ajhp060371. [DOI] [PubMed] [Google Scholar]
- 4.Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury A placebo-controlled trial. Neurol. 2006;67:1792–800. doi: 10.1212/01.wnl.0000244422.45278.ff. [DOI] [PubMed] [Google Scholar]
- 5.Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;(3):CD007076. doi: 10.1002/14651858.CD007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ide H, Fujiya S, Asanuma Y, Tsuji M, Sakai H, Agishi Y. Clinical usefulness of intrathecal injection of methylcobalamin in patients with diabetic neuropathy. Clin Ther. 1987;9:183–92. [PubMed] [Google Scholar]
- 7.Yaqub BA, Siddique A, Sulimani R. Effects of methylcobalamin on diabetic neuropathy. Clin Neurol Neurosurg. 1992;94:105–11. doi: 10.1016/0303-8467(92)90066-c. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Lai MS, Lu CJ. Effectiveness of vitamin B12 on diabetic neuropathy: Systematic review of clinical controlled trials. Acta Neurol Taiwan. 2005;14:48–54. [PubMed] [Google Scholar]
- 9.Dangour AD, Allen E, Clarke R, Elbourne D, Fasey N, Fletcher AE, et al. A randomised controlled trial investigating the effect of vitamin B12 supplementation on neurological function in healthy older people: The Older People and Enhanced Neurological function (OPEN) study protocol [ISRCTN54195799] Nutr J. 2011;10:22. doi: 10.1186/1475-2891-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talaei A, Siavash M, Majidi H, Chehrei A. Vitamin B12 may be more effective than nortriptyline in improving painful diabetic neuropathy. Int J Food Sci Nutr. 2009;60(Suppl 5):71–6. doi: 10.1080/09637480802406153. [DOI] [PubMed] [Google Scholar]


