Abstract
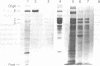
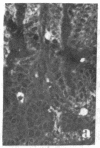
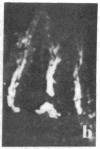
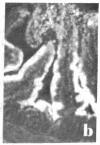
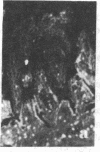
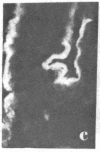
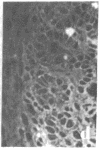
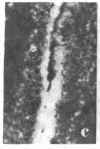
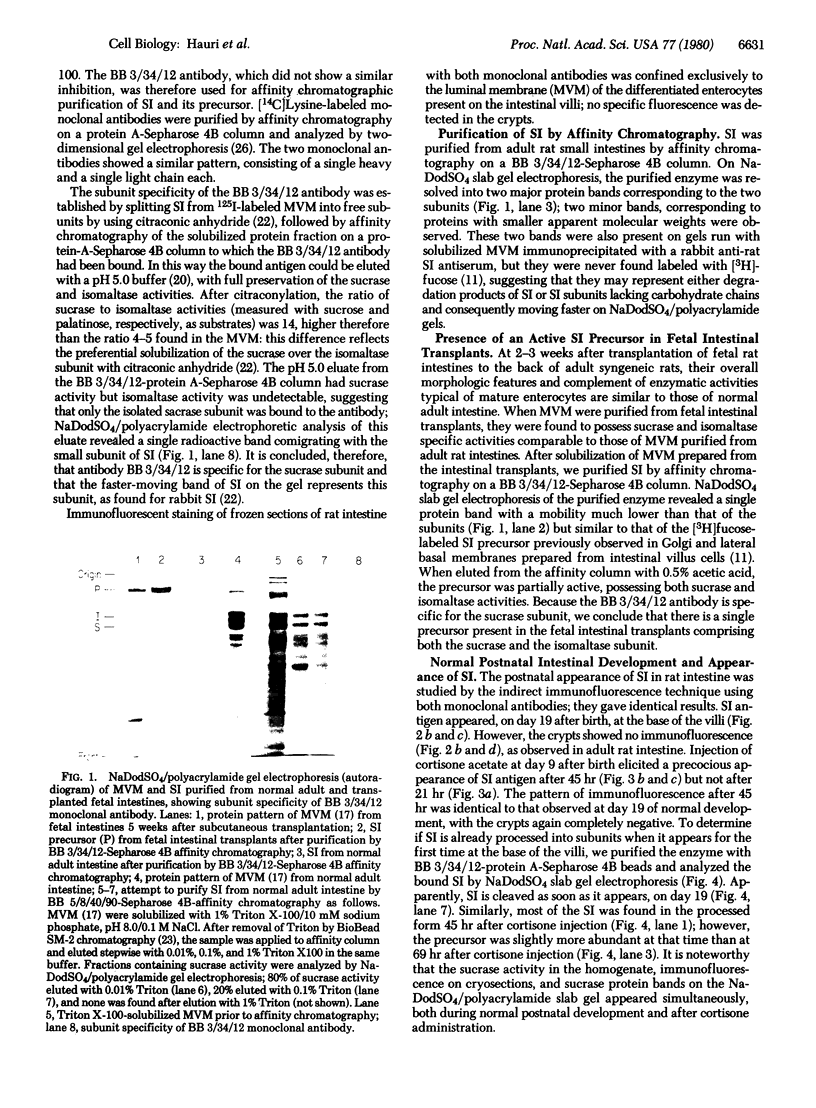
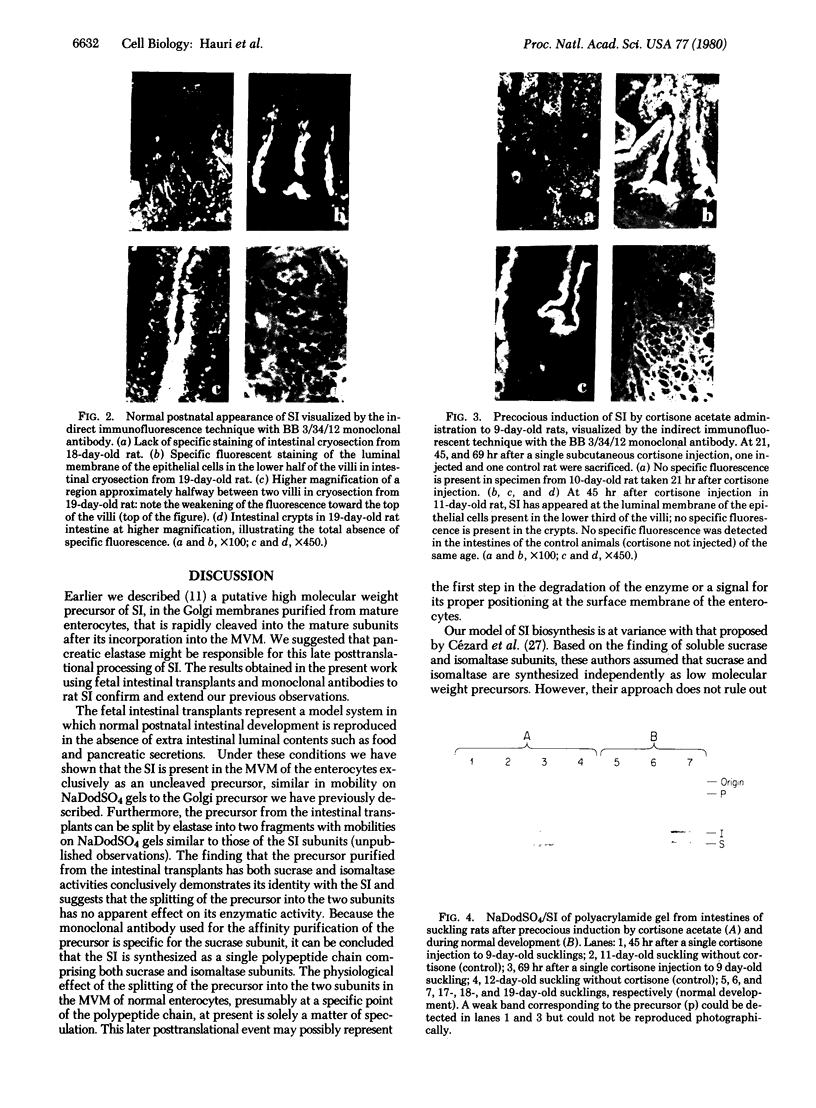
Two monoclonal antibodies, designated BB 3/34/12 and BB 5/8/40/90, have been produced to rat intestinal sucrase/isomaltase (SI) by the hybridoma technique using microvillus membranes as antigen. The BB 3/34/12 antibody was shown to be specific for the sucrase subunit. These antibodies provided new information regarding the biosynthesis and postnatal development of SI. In rat intestinal fetal transplants, SI was found exclusively in the form of an enzymatically active high molecular weight precursor, confirming our previous observations concerning the role of luminal proteases for the processing of SI in the microvillus membrane. The SI precursor, purified by affinity chromatography using the BB 3/34/12 antibody, had both sucrase and isomaltase activities, suggesting that a single precursor protein generates both sucrase and isomaltase subunits by proteolytic cleavage. The initial appearance of SI during normal postnatal development in the rat intestine was found to be confined to the cells present at the base of the villi. The same localization was observed after precocious induction of SI by cortisone acetate. In both cases, no immunofluorescence was observed in the crypts, suggesting that only the differentiated enterocyte is capable of synthesizing this enzyme. Even at the earliest times of appearance, newly synthesized SI was found almost completely split into its subunits, suggesting that the protease(s) responsible for the processing of the precursor in the microvillus membrane develop(s) in parallel with SI or earlier.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asp N. G., Dahlqvist A. Human small intestine -galactosidases: specific assay of three different enzymes. Anal Biochem. 1972 Jun;47(2):527–538. doi: 10.1016/0003-2697(72)90147-9. [DOI] [PubMed] [Google Scholar]
- Brunner J., Hauser H., Braun H., Wilson K. J., Wacker H., O'Neill B., Semenza G. The mode of association of the enzyme complex sucrase.isomaltase with the intestinal brush border membrane. J Biol Chem. 1979 Mar 25;254(6):1821–1828. [PubMed] [Google Scholar]
- Cezard J. P., Conklin K. A., Das B. C., Gray G. M. Incomplete intracellular forms of intestinal surface membrane sucrase-isomaltase. J Biol Chem. 1979 Sep 25;254(18):8969–8975. [PubMed] [Google Scholar]
- Doell R. G., Rosen G., Kretchmer N. Immunochemical studies of intestinal disaccharidases during normal and precocious development. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1268–1273. doi: 10.1073/pnas.54.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubs R., Gitzelmann R., Steinmann B., Lindenmann J. Catalytically inactive sucrase antigen of rabbit small intestine: the enzyme precursor. Helv Paediatr Acta. 1975 May;30(1):89–102. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Frank G., Brunner J., Hauser H., Wacker H., Semenza G., Zuber H. The hydrophobic anchor of small-intestinal sucrase--isomaltase: N-terminal sequence of isomaltase subunit. FEBS Lett. 1978 Dec 1;96(1):183–188. doi: 10.1016/0014-5793(78)81090-4. [DOI] [PubMed] [Google Scholar]
- Freiburghaus A. U., Dubs R., Hadorn B., Gaze H., Hauri H. P., Gitzelmann R. The brush border membrane in hereditary sucrase-isomaltase deficiency: abnormal protein pattern and presence of immunoreactive enzyme. Eur J Clin Invest. 1977 Oct;7(5):455–459. doi: 10.1111/j.1365-2362.1977.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Green J. R. The development of sucrase in the intestinal brush-border membrane of the suckling rat [proceedings]. Biochem Soc Trans. 1978;6(6):1202–1204. doi: 10.1042/bst0061202. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Leapman S. B., Deutsch A. A., Grand R. J., Folkman J. Transplantation of fetal intestine: survival and function in a subcutaneous location in adult animals. Ann Surg. 1974 Jan;179(1):109–114. doi: 10.1097/00000658-197401000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson I., Bryden A. Mitomycin C treated cells as feeders. Exp Cell Res. 1971 Nov;69(1):240–241. doi: 10.1016/0014-4827(71)90335-1. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard D., Malo C. Insulin-evoked precocious appearance of intestinal sucrase activity in suckling mice. Dev Biol. 1979 Apr;69(2):661–665. doi: 10.1016/0012-1606(79)90319-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Wands J., Trelstad R. L., Isselbacher K. J. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979 Feb;80(2):248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]