Abstract
Her2 overexpression in ER-positive breast cancer cells such as BT474 (BT) cells has been found to confer resistance to tamoxifen, and suppression of Her2 improves the antiproliferative effects of tamoxifen. In this study, the responsiveness to tamoxifen in BT/HerR, Herceptin-resistant BT cell lines established through constant Herceptin exposure, was evaluated. Compared with BT cells, improvement of sensitivity to tamoxifen in BT/HerR was demonstrated by ER functional analysis and cell proliferation assay. Tamoxifen in the resistant cell line was found to inhibit E2-stimulating estrogen-responsive gene pS2 expression more effectively than in BT cells in real time PCR assay. Western analysis showed cross-phosphorylation between ER and downstream components of Her2 was attenuated in BT/HerR cells. ER redistribution from cytoplasm to nucleus could be found in these cells through immunofluorescence and confocal studies and importantly, chromatin immunoprecipitation (ChIP) studies demonstrated that tamoxifen induced occupancy of the pS2 promoter by ER and nuclear receptor corepressor NCoR instead of coactivator AIB1 in these cells. Finally, combination of tamoxifen and Herceptin was found to improve the sensitivity of BT/HerR cells to Herceptin. Our results suggest that the ER genomic pathway in the ER-positive and Herceptin-resistant breast cancer cells may be reactivated, allowing tamoxifen therapy to be effective again, and a combination of tamoxifen and Herceptin can be a potential therapeutic strategy for ER-positive and Herceptin-resistant human breast cancer.
Keywords: BT474 cells, Her2, ER, cross-talk, Herceptin, tamoxifen, endocrine resistance
Introduction
The Her2/neu/c-ErbB2 oncogene encodes a 185-kDa transmembrane tyrosine kinase receptor, belonging to the epidermal growth factor receptor family that is amplified or overexpressed in 30% of breast cancers and is a poor prognostic factor (Slamon, et al. 1989). Trastuzumab/Herceptin is a humanized monoclonal antibody directed against Her2, which inhibits growth and proliferation of cancer cells overexpressing Her2 (Carter, et al. 1992). Herceptin has also shown efficacy in combination with conventional chemotherapeutic agents (Dieras, et al. 2001). However, while Her2 overexpressing cell lines are shown to be sensitive to Herceptin, greater than 70% of patients whose tumors overexpress Her2 do not respond to Herceptin monotherapy (Vogel, et al. 2002). In addition, a significant percentage of women who initially respond to Herceptin may develop acquired resistance to therapy after prolonged treatment. The precise mechanism of Herceptin resistance has not been fully characterized. Overexpression of constitutively active Akt (Clark, et al. 2002), insulin-like growth factor receptor (Lu, et al. 2001), loss of PTEN function (Nahta, et al. 2006) or Muc4/sialomucin complex (Price-Schiavi, et al. 2002) may potentially be involved in the resistance phenotype in breast cancer. Many strategies of combination therapy for Herceptin-resistant breast cancer cells have been considered, i.e. the combination of Herceptin with EGFR kinase inhibitors such as AG1478 or Iressa (Lenferink, et al. 2000; Moasser, et al. 2001) and a COX-2-inactive PDK-1 inhibitor, OSU-03012 (Tseng, et al. 2006). New potential therapeutic strategies, such as the combination of Herceptin with IGF-1R kinase inhibitors or anti-IGFR antibodies, are being evaluated.
More than 60% of human breast cancers are estrogen receptor (ER)-positive. Until now, the selective estrogen receptor modulator (SERM), tamoxifen, is the most common endocrine therapy used for these cancers. While approximately 50–60% of ER-positive breast cancer patients benefit from tamoxifen, some of the ER-positive breast cancer patients who do not respond to tamoxifen have overexpression of Her2 (Slamon et al. 1989). There has been much experimental evidence to suggest that tamoxifen resistance in breast cancer cells involves overexpression of Her2 and its crosstalk with ER, which is associated with ER nongenomic activity (Pietras, et al. 1995; Shou, et al. 2004). Tamoxifen or estrogen bound membrane/cytoplasmic ER, through multiple interactions with signaling intermediate molecules such as Shc (Song, et al. 2002)and modulator of nongenomic action of estrogen receptor (MNAR) (Wong, et al. 2002), can activate the EGFR/Her2 signaling pathway. Activated downstream kinases, e.g. phosphatidylinositol 3′-kinase (PI3K)/Akt and the Erk1/2 MAPK, can phosphorylate nuclear ER and its coactivators (Kato, et al. 1995; Schiff, et al. 2004; Shou et al. 2004; Sun, et al. 2001), thus up-regulating genomic ER activity and enhancing gene expression including genes in the EGFR/Her2 pathways, which in turn further augment EGFR/Her2 signaling, thus completing the cooperative cycle between the two activities of ER and their crosstalk with the EGFR/Her2 signaling pathway. In Her2-overexpressing cells, the resulting activation of downstream kinases can lead to tamoxifen resistance by modifying the activity of various transcription factors and blocking the inhibitory effects of tamoxifen on nuclear ER. Corresponding to this observation, a variety of in vitro and in vivo models have shown that suppression of Her2 improves the antiproliferative effects of tamoxifen (Kurokawa, et al. 2000; Pietras et al. 1995; Tang, et al. 1996), thus again demonstrating a causal association between Her2 overexpression and acquisition of resistance to endocrine therapy such as tamoxifen.
To explore the mechanism of Herceptin resistance and find the potential therapeutic strategy for Herceptin-resistant human breast carcinomas, several clones of BT474 (BT) human breast carcinoma cells that are resistant to Herceptin (BT/HerR) were isolated and identified over a 5-month selection process (Chan, et al. 2005). BT is an ER- positive invasive human breast ductal carcinoma cell line with very high Her2 expression, which is very sensitive to Herceptin but resistant to tamoxifen, and has been used extensively for investigating the mechanisms of Herceptin and Her2 biology (Yakes, et al. 2002; Zhou, et al. 2007). BT/HerR subclones appear to have acquired a Herceptin-resistant PI-3K signaling mechanism, because the downstream PI-3K/Akt signaling pathway is sustained in BT/HerR cells in the presence of Herceptin, but significantly down-regulated in BT cells exposed to Herceptin (Chan et al. 2005). Our previous results showed, while total and phosphorylated cellular Her2 protein remained high, phosphorylated Akt levels in the presence of Herceptin in BT/HerR subclones were decreased compared to wild type BT cells in the absence of Herceptin (Chan et al. 2005). Based on these findings and the known crosstalk between ER and Her2 in tamoxifen resistance, we hypothesized that BT/HerR and BT cells might have different responses to estrogen and endocrine treatment, such as tamoxifen. This study was undertaken to explore the sensitivity to tamoxifen in BT/HerR cells, and our results suggest that combined therapy with Herceptin and tamoxifen might be a viable therapeutic strategy for ER-positive and Herceptin-resistant human breast carcinomas.
Materials and methods
Cell culture and reagents
BT cells were routinely cultured in DMEM with 4.0 mM L-Glutamine, 4,500 mg/L Glucose (Hyclone, Logan, UT, USA) supplemented with 10% heat inactivated fetal bovine serum, 1% penicillin/streptomycin and 1 mM sodium pyruvate (Gibco BRL, Gaithersburg, MD, USA). BT/HerR 0.2 clone D and BT/HerR 1.0 clone E were maintained in the same medium with the continuous presence of 1 and 0.2μmol/L Herceptin, respectively [20]. Estrogen-driven cell assays were performed with steroid-deprived medium, where DMEM and FBS were replaced with DMEM without phenol red and charcoal-stripped FBS (Hyclone, Logan, UT, USA). Herceptin (Genentech, South San Francisco, CA, USA) was purchased from the City of Hope National Medical Center Pharmacy (Duarte, CA, USA) and tamoxifen from Sigma Chemicals (Louis, MO, USA). Source of tamoxifen, E2 and ICI
CellTiter 96 AQueous proliferation assay
The response of BT or BT/HerR cells to tamoxifen or Herceptin alone, or a combination of both agents, was examined using CellTiter 96® AQueous cell proliferation assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Cells were steroid-deprived for 72h and seeded in 96-well plates. After overnight incubation, steroid-deprived media containing 1 μM 17β-estradiol (E2), 1 μM tamoxifen or indicated concentrations of Heceptin or combination of both agents were added to the cells and left for 6 days on the cells. Each data point was performed in triplicate. At the indicated times, cells were incubated in the presence of 20 μL CellTiter 96 AQueous One Reagent per 100 μL medium for 1 h, and the reaction was measured colorimetrically at 490 nm in a SpectraMax M5 Reader (Molecular Devices, Sunnyvale, CA, USA). Background levels were determined in medium supplemented with DMSO at the appropriate dose and findings were graphed as mean ± SD.
Real time RT-PCR analysis
Cells were deprived of steroid for 72 h and incubated in the presence or absence of 1 nM 17β-estradiol (E2) with or without 1 μM tamoxifen (Tam) for 24h. Whole cell RNA was prepared using Trizole® Reagent (Invitrogen, Carlsbad, CA, USA) and first strand cDNA was synthesized using SuperScript™ III Reverse Transcriptase (Invitrogen), according to manufacturer’s instructions. Real-time PCR was performed using the iQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The reactions were carried out in a 96-well plate using SYBR Green Supermix (Bio-Rad) according to manufacturer’s instructions. Primers used were as follows: pS2, 5′-ATACCATCGACGTCCCTCCA-3′ (Forward), primer 5′-CACCTCAGACACGCTT-3′ (Reverse); β-actin, 5′-AGAAGGAGATCACTGCCCTGGCACC-3′ (Forward), 5′-CCTGCTTGCTGATCCACATCTGCTG-3′ (Reverse). PCRs were performed with HotStarTaq DNA polymerase (Qiagen, Valencia, CA, USA) under the following conditions: a preheating at 94 °C for 15 min, followed by 30 cycles of 94°C for 30 s, 60°C for 30 s, 72 °C for 1 min, followed by a 72°C extension for 10 min. The PCR product specificity was tested at the end of each run by melting temperature analysis. Single melting curve peaks at about 84.5°C were obtained. Each sample was analyzed in triplicate. The value for pS2 mRNA was divided by β-actin mRNA control value to give the relative expression level.
Transient transfection and luciferase ER reporter assays
BT or BT/HerR cells were transfected with pGL3(ERE)3-luciferase vector using Lipofectamine 2000 (Invitrogen) according to the protocol of the manufacturer. A total of 1.5 × 105 cells per well were seeded onto 24-well plates and incubated overnight. A mixture of 1 μg of the reporter plasmid, 1.5 μL of Lipofectamine 2000 and OptiMEM (Invitrogen) was added into each well and after a 4-hour incubation, cells were cultured with fresh medium containing DMSO or 1 μM E2 with or without 1 μM tamoxifen or 10 nM ICI in phenol red–free medium with 10% charcoal-dextran–treated FBS. After a 24-hour incubation following the transfection, the cells were lysed in 1x Reporter Lysis Buffer (Promega). Luciferase activity was measured using the Luciferase Assay System (Promega). Protein concentration was measured by the method of Bradford (Bio-Rad) (Bradford 1976). The relative luciferase activity was calculated by dividing the light unit of luciferase activity by protein concentration of each sample.
Immunofluorescence and confocal studies
Cells were cultured in regular media (10% serum) or steroid-deprived media in the absence or presence of 1 nM E2. Subcellular localization of ERα in BT or BT/HerR was determined by indirect immunofluorescence. Cells grown on glass coverslips were fixed in ice-cold methanol:acetone (1:1) at 4°C for 10 min. Cells were incubated with the primary antibodies, mouse ERα antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C for overnight. After being washed three times in PBS, cells were then incubated with FITC-labeled (green) secondary antibody (Chemicon, Temecula, CA, USA). The Vectashield® Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA) was used to costain the DNA and was color-coded in blue. Confocal analysis was performed with a Zeiss laser-scanning confocal microscope (Carl Zeiss, Oberkochen, Germany). Each image represents Z-sections at the same cellular level and magnification.
Cell Extracts and Western analysis
Cells were deprived of steroid for 72h and serum starved for 24h, and then treated with DMSO, 1nM E2 or 1μM tamoxifen (Tam) for 24h. Isolation of cell extracts and western analysis was previously described (Lou, et al. 2007). The following antibodies were used: total ERα and Her2, phospho-Ser118- ERα, phospho-Ser167- ERα, total and phospho-forms of Akt (Ser437) and MAPK (Thr202/Tyr204) (Cell Signaling, Boston, MA, USA). β-actin (Chemicon) was used as a control for equal loading and transfer. Quantitative densitometry of the immuno-images was performed using the Model GS-700 Imaging Densitometer with Molecular Analyst Software (Bio-Rad) and expressed as the ratios to the density of β-actin bands. Phosphorylation levels of the experiments were estimated by densitometry, and were calculated as the ratio of pMAPK/tMAPK, pAkt/tAkt, or pER/tER.
ChIP assays
Cells were deprived of steroid for 72h and serum starved for 24h, and then treated with DMSO, 1nM E2, or 1μM tamoxifen (Tam) for 45 minutes. ChIP assays were carried out with the ChIP assay kit (Upstate, Charlottesville, VA, USA) as previously described (Lou et al. 2007). One-tenth of the immunoprecipitated DNA and 1% of the input DNA were analyzed by PCR. Antibodies used for ChIP assays included: AIB1 and NCoR (Affinity Bioreagents, Golden, CO, USA), ERα and histone deacetylase 3 (HDAC3) (Santa Cruz). PCRs were performed with HotStarTaq DNA polymerase (Qiagen) under the following conditions: a preheating at 94 °C for 15 min, followed by 35 cycles of 94°C for 30 s, 60°C for 30 s, 72 °C for 1 min, followed by a 72°C extension for 10 min. PCR products were analyzed by electrophoresis in a 1.5% agarose gel, which was then stained with ethidium bromide and photographed under UV illumination. Primers for the promoter region of the pS2 gene: 5′-TTCATGAGCTCCTTCCCTTC-3′ (forward) and 5′-ATGGGAGTCTCCTCCAACCT-3′ (reverse). Representative experiments from at least three independent experiments are shown.
Statistics
Overall differences between control and treatment groups, or differences between different treatment groups, were determined by two-way ANOVA. Direct comparisons between control and treatment effects were assessed using a Student’s t test. Differences were considered significant at the p < 0.05 level.
Results
Different sensitivity of tamoxifen in BT and BT/HerR cells
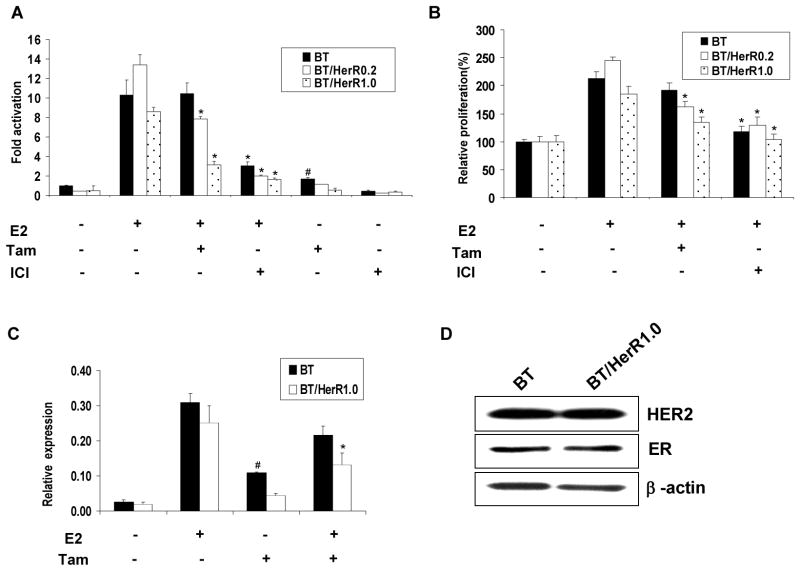
To determine whether BT/HerR and BT cells might have different sensitivities to estrogen and endocrine treatment, we first investigated the response of BT and BT/HerR cells to endocrine therapy. Current endocrine therapy of breast cancer includes selective estrogen receptor modulators (SERM) such as tamoxifen, the pure ER antagonist ICI 182,780 (fulvestrant) and aromatase inhibitors such as letrozole. Because very low levels of aromatase activity were found in BT and BT/HerR cells (data not shown), only the response to tamoxifen or ICI 182,780 (ICI) was examined. Two kinds of BT/HerR cells (BT/HerR0.2 and BT/HerR1.0, respectively) were selected to estimate the different sensitivity of BT cells to tamoxifen or ICI. In the ER functional analysis, while both BT and BT/HerR cells were found to be responsive to estrogen, BT/HerR cells were more sensitive to tamoxifen than BT cells (Fig. 1A). In BT/HerR0.2 or BT/HerR1.0 cells, tamoxifen could significantly decrease E2-stimulated luciferase activity, confirming their antagonistic functions. However, in BT cells, tamoxifen had no effect on E2-stimulated luciferase activity and, inversely, tamoxifen alone increased ERE-luciferase transcription to 1.7-fold above basal level in BT cells, indicating a partial agonist function. In addition, BT and BT/HerR cells responded similarly to pure ER antagonist ICI, which could abrogate the estrogen signal in both types of cells by accelerating ERα degradation. To elucidate the biological significance of this difference in tamoxifen function in BT and BT/HerR cells, we performed a cell proliferation assay and found that BT and BT/HerR cells had the same response to ICI, but not to tamoxifen. BT cells were resistant to tamoxifen treatment, whereas estrogen-driven proliferation of BT/HerR cells was inhibited by tamoxifen (57% inhibition in BT/HerR0.2 cells and 60% inhibition in BT/HerR1.0 cells, respectively) (Fig. 1B). To further demonstrate the different sensitivities of tamoxifen in BT cells and BT/HerR cells, levels of the estrogen– responsive gene pS2 mRNA in these two kinds of cell lines were analyzed by real time PCR. Tamoxifen was found to inhibit E2-stimulated pS2 expression in BT/HerR1.0 cells more effectively than in BT cells (Fig. 1C). In addition, tamoxifen alone in the absence of estrogen in BT cells could stimulate pS2 expression, suggesting again that tamoxifen in BT cells functions as an agonist. By Western analysis, ER and Her2 levels in BT and BT/HerR1.0 cells were found to be similar (Fig. 1D). These results suggest that both BT and BT/HerR cells exhibit wild-type ER function in response to E2, but there is a clear difference in sensitivity to tamoxifen in both growth and ER functional assays.
Figure 1.
Different sensitivity to tamoxifen in BT and BT/HerR cells. (A) Effect of tamoxifen and ICI on ER-mediated transcription in BT, BT/HerR0.2 and BT/HerR1.0 cells. Cells were transiently transfected with an ERE-containing luciferase reporter construct (ERE) in steroid-deprived medium, followed by a 16h incubation in the presence or absence of 1 nM 17β-estradiol (E2) with or without 1 μM tamoxifen (Tam) or 10nM ICI. Normalized luciferase activity from triplicate wells was expressed relative to the luciferase activity of each cell line in the absence of 17β-estradiol, tamoxifen and ICI. (B) Effects of tamoxifen and ICI on the proliferation in BT, BT/HerR0.2 and BT/HerR1.0 cells. Cells were deprived of steroid for 72 h and then incubated in the presence or absence of 1 nM 17β-estradiol with or without 1 μM tamoxifen or 10 nM ICI for 6 days. Cell proliferation from triplicate wells was expressed relative to optical density at 490 nm of each cell line in the absence of 17β-estradiol, tamoxifen and ICI. Results in A and B are expressed as mean ± SD of at least three separate experiments. *, P<0.05, compared to control in presence of 17β-estradiol. #, P<0.05, compared to control in absence of 17β-estradiol, tamoxifen and ICI. (C) Real time-PCR analysis of the estrogen responsive gene pS2 in BT and BT/HerR cells. BT and BT/HerR1.0 cells were deprived of steroid for 72 h and incubated in the presence or absence of 1 nM 17β-estradiol (E2) with or without 1 μM tamoxifen (Tam) for 24h. The expression of pS2 from triplicate wells was quantified and normalized to that of β-actin. Results are expressed as mean ± SD of at least three separate experiments. *, P<0.05, compared to BT cells. #, P<0.05, compared to control in absence of 17β-estradiol and tamoxifen. (D). Levels of ER and Her2 receptors in BT and BT/HerR cells. BT and BT/HerR1.0 cells were cultured in regular media to 80% confluence. Lysates of cells were prepared and tested for ER and HER2 content by immunoblot analysis. β-actin was used as a control for equal loading and transfer. Blots are representative of three experiments.
Attenuated cross-phosphorylation between ER and HER2 downstream components in BT and BT/HerR cells
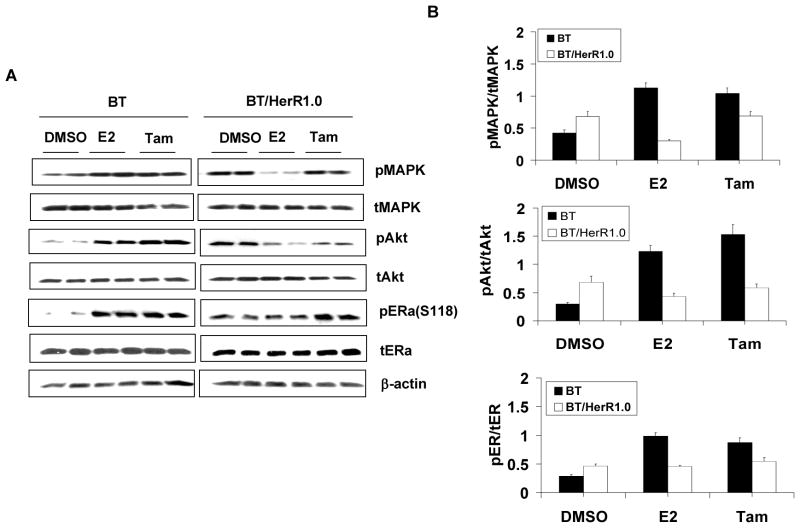
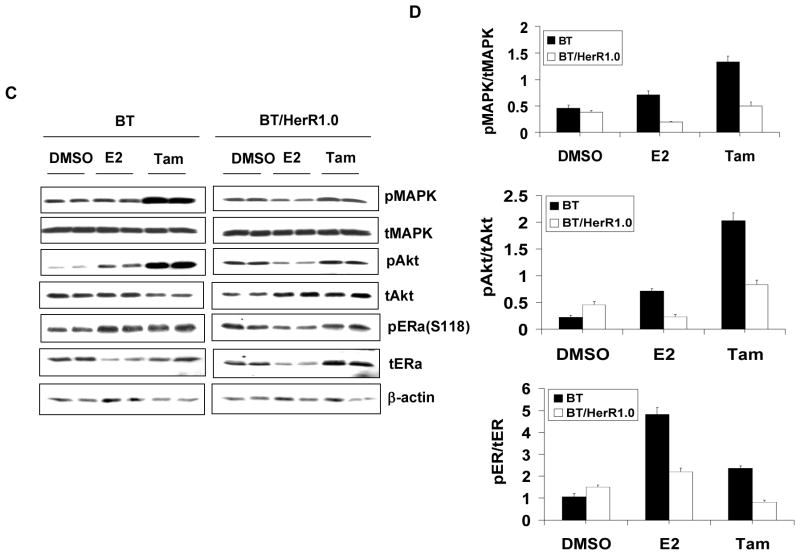
The crosstalk between ER and Her2 involves ER nongenomic activity and is thought to be very important in the development of tamoxifen resistance (Pietras et al. 1995; Shou et al. 2004). To explore the mechanism of differential sensitivities to tamoxifen in BT cells and BT/HerR cells, the nongenomic activity of ER in BT cells and BT/HerR1.0 cells was examined. Our previous studies have found that the total and phosphorylated Her2 levels in BT/HerR cells are not significantly changed compared to BT cells, and signaling through the PI3K/Akt pathway is sustained in the presence of Herceptin in BT/HerR (Chan et al. 2005). To further explore our previous findings, we examined the changes of downstream components of the Her2 pathway, such as MAPK and Akt, in the presence of E2 and tamoxifen. As shown by Western analysis (Fig. 2A and 2B), after 20-minute treatment with estrogen and tamoxifen, both estrogen and tamoxifen could stimulate phosphorylation of MAPK and Akt in BT cells compared to the DMSO control. However, while the basal phosphorylated Akt was slightly elevated in BT/HerR1.0 cells compared to BT cells (Fig. 2B), both estrogen and tamoxifen in these cells could not stimulate phosphorylation of MAPK and Akt. These results indicate that ER nongenomic activity in BT/HerR cells is attenuated, and tamoxifen could function as an agonist in BT cells, but not in BT/HerR cells. To determine the effect of attenuated ER nongenomic activity on ER function in BT/HerR cells, ER phosphorylation in BT and BT/HerR1.0 cells treated with or without estrogen or tamoxifen was examined using a specific anti-phospho-Ser118 or Ser167 ERα antibody. In BT cells, activated MAPK by estrogen and tamoxifen could lead to potentiation of phospho-ERα at Ser118, which was previously reported to be phosphorylated by MAPK [17]. However, estrogen and tamoxifen-stimulated phospho-ERα at Ser118 was found to be attenuated in BT/HerR1.0 cells compared to that in BT cells. Under our experimental conditions, phosphorylation of ERα at Ser167, which could be phosphorylated by Akt (Campbell, et al. 2001), could not be detected in either cell line (data not shown). Our results are similar to those in a previous study on BT cells (Wang et al. 2005), implying that Ser-167 phosphorylation is not abundant in BT and BT/HerR cells. The effects of estrogen and tamoxifen treatments on phosphorylated levels of MAPK, AKt and ER were still significant after 24 hours (Fig. 3A and 3B), indicating that they are not transient. In addition, estrogen treatment for 24 hours could down-regulate the total ER level and tamoxifen treatment for 24 hours could up-regulate the total ER level in these two cell lines (Fig. 3A and 3B), which is consistent with other’s findings (Wang, et al. 2005).
Figure 2.
Cross-phosphorylation between ER and HER2 downstream components in BT and BT/HerR cells. BT and BT/HerR1.0 cells were deprived of steroid for 72h and serum starved for 24h, and then treated with DMSO, 1nM estrogen (E2), 1μM tamoxifen (Tam) for 20 min (A and B) or 24h (C and D). Levels of total (t) and phosphorylated (p) MAPK, Akt and ERα at Ser118 were measured in cellular extracts by immunoblot analysis. β-actin was used as a control for equal loading and transfer. Figures are representative of three experiments. Phosphorylation levels of the experiments were estimated by densitometry, and were calculated as the ratio of pMAPK/tMAPK, pAkt/tAkt, or pER/tER.
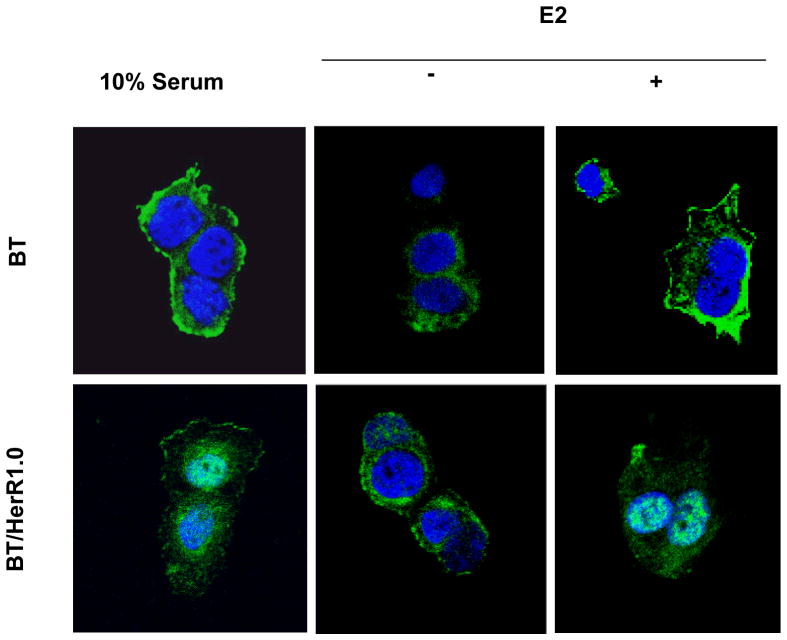
Figure 3.
Subcellular localization of ER in BT and BT/HerR cells. BT and BT/HerR1.0 cells were cultured in regular media (10% serum) or steroid-deprived media in the absence (−) or presence (+) of 1 nM 17β-estradiol (E2). Cellular localization of ER was determined by indirect immunofluorescence as indicted in “Materials and Methods”. Confocal microscopy was used to show the presence of ER (green) in the cytoplasmic compartment and nucleus (blue, DNA counterstain). Cells were deprived of steroid for 72 h and then stimulated with E2 for 1h to examine the effects on ER localization.
Localization of ER in BT and BT/HerR cells
Since subcellular localization of ER has been linked to the responsiveness of breast cancer cells to tamoxifen (Yang, et al. 2004), we analyzed subcellular localization of ER in BT cells and BT/HerR1.0 cells by immunofluorescence and confocal studies. In regular media, more ER in BT cells was found to localize in the membrane/cytoplasm than in the nucleus, but in BT/HerR1.0 cells, more ER localized in the nucleus (Fig. 4A), indicating that the genomic pathway of ER in BT/HerR cells is more important than the nongenomic pathway. To further confirm these results, cells were deprived of endogenous estrogen for 3 days and then treated with 1 μM E2 for 1h, and the effects of estrogen on ER localization were examined. Estrogen stimulation could promote ER translocation from the nucleus to the membrane/cytoplasm in BT cells, but from the membrane/cytoplasm to the nucleus in BT/HerR1.0 cells (Fig. 4B). These results show ER subcellular localization in BT/HerR cells is different from BT cells, which suggests that enhancement of the ER genomic pathway is one of the potential mechanisms related with increased sensitivity to tamoxifen in the BT/HerR cells.
Figure 4.
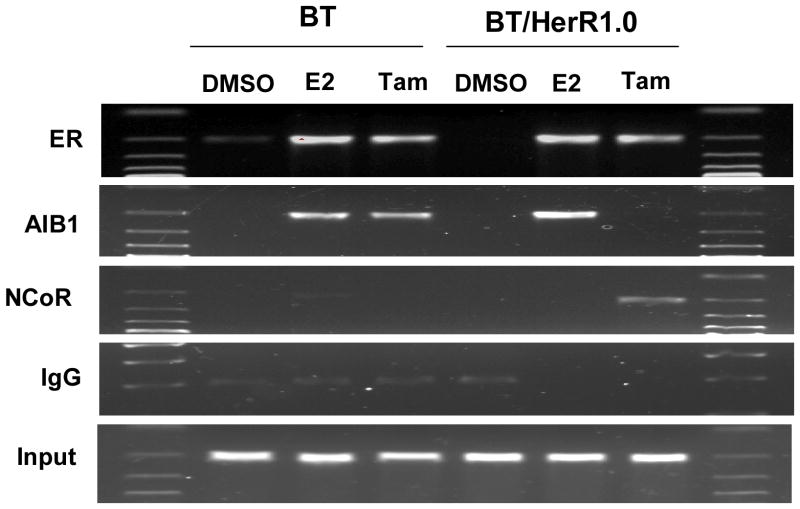
Different recruitment of nuclear receptor coactivator or corepressor on estrogen-responsive pS2 promoter by tamoxifen bound ER in BT and BT/HerR cells. BT and BT/HerR1.0 cells were deprived of steroid for 72h and serum starved for 24h, and then treated with DMSO, 1nM 17β-estradiol (E2), or 1μM tamoxifen (Tam) for 45 minutes. Occupancy of the estrogen-responsive pS2 promoter by ER; the coactivator AIB1; the corepressor NCoR was examined by ChIP assay as indicted in “Materials and Methods”. Relevant pS2 promoter sequences were PCR amplified from complexes immunoprecipitated with each antibody. Input lane shows DNA that was PCR amplified from extracts before immunoprecipitation. Gels are representative of three experiments.
Different recruitment of nuclear receptor coactivator or corepressor on pS2 promoter by tamoxifen bound ER in BT and BT/HerR cells
One mechanism for tamoxifen resistance may be related to a change in the balance of association of ER with nuclear receptor coactivators or corepressors (Smith, et al. 1997). Tamoxifen had been shown to function as an agonist in BT cells but an antagonist in BT/HerR cells (Fig. 1A, 1C and Fig. 3). Next, we employed ChIP assays to address if there was a different recruitment of nuclear receptor coactivator or corepressor on the pS2 promoter by tamoxifen-bound ER in these two kinds of cell lines. In BT cells, E2 induced occupancy of the pS2 promoter by ER and coactivator AIB1. Tamoxifen, like E2, also induced occupancy by ER and coactivator AIB1, but not corepressor NCoR, which is consistent with its agonist function in these cells. In BT/HerR1.0 cells, estrogen induced occupancy of the pS2 promoter by ER and coactivator AIB1, but tamoxifen induced occupancy by ER and corepressor NCoR instead of coactivator AIB1, which is consistent with the antagonist function of tamoxifen in these cells (Fig. 4). The different recruitment of nuclear receptor coactivator or corepressor on the pS2 promoter by tamoxifen-bound ER in BT and BT/HerR cells may explain the improvement of tamoxifen sensitivity in BT/HerR cells.
Combination of tamoxifen and Herceptin improves the sensitivity of BT/HerR cells to Herceptin
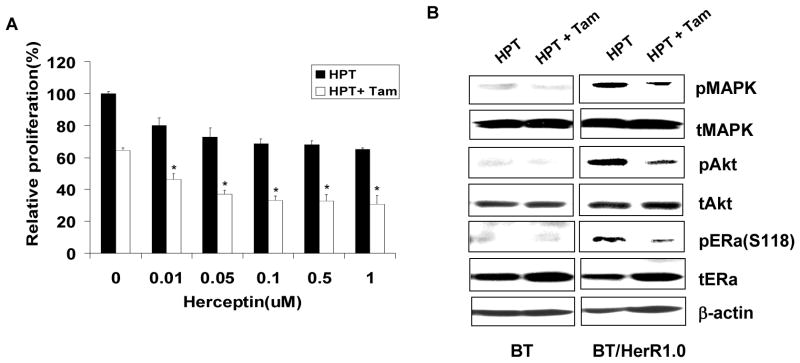
The cross talk between ER and Her2 signaling pathways suggests that targeting both ER and growth factor receptors simultaneously could be a fruitful approach to the treatment of breast cancer. The combination of tamoxifen and Herceptin has been reported to synergistically inhibit the proliferation of BT cells (Argiris, et al. 2004; Wang et al. 2005). Although BT/HerR cells are resistant to Herceptin, the sensitivity of these cells to tamoxifen was improved. Hence, we next investigated if the combination of tamoxifen and Herceptin could improve the sensitivity of BT/HerR cells to Herceptin. Compared to Herceptin or tamoxifen treatment alone, the combination of Herceptin with tamoxifen significantly inhibited the proliferation of BT/HerR1.0 cells (Fig. 5A). Western analysis of signaling proteins showed that, consistent with other’s findings (Wang et al. 2005), BT cells were very sensitive to Herceptin alone and the combination of tamoxifen and Herceptin did not have any additional effect on phosphorylation of MAPK, Akt or ERα at Ser118 (Fig. 5B). Though phosphorylation of MAPK, Akt and ERα at Ser118 was sustained in BT/HerR1.0 cells in the presence of Herceptin compared to BT cells, combination of Herceptin with tamoxifen significantly down-regulated phosphorylation of downstream signaling components of HER2 pathway and phospho-ERα at Ser118. These results indicate combination of Herceptin with tamoxifen can sensitize BT/HerR cells to Herceptin, suggesting that combined therapy with Herceptin and tamoxifen might be a viable therapeutic strategy for ER-positive and Herceptin-resistant human breast cancer.
Figure 5.
Combination of tamoxifen and Herceptin improves the sensitivity of BT/HerR cells to Herceptin. (A) BT and BT/HerR1.0 cells were deprived of steroid for 72h and then incubated in the presence of 1 nM 17β-estradiol (E2) with increasing concentrations of Herceptin (HPT) or its combination with 1 μM tamoxifen (Tam) for 6 days. Cell proliferation was expressed relative to optical density at 490 nm of each cell line in the presence of 17β-estradiol. *, P<0.05, combined agents versus Herceptin alone or tamoxifen alone.(B) BT and BT/HerR1.0 cells were deprived of steroid for 72h and serum starved for 24h, and then treated with 1 μM Herceptin alone or its combination with 1μM tamoxifen for 24h. Levels of total (t) and phosphorylated (p) MAPK, AKT and ERα at Ser118 were measured in cellular extracts by Western analysis. β-actin was used as a control for equal loading and transfer. Figures are representative of three experiments.
Discussion
Currently Herceptin is the only Her2-targeted therapy approved by the FDA for the treatment of metastatic breast cancer (MBC). While Herceptin is a powerful drug, many patients who achieve an initial response to Herceptin-based regimens develop resistance within one year. Adding the expensive prices of these drugs, it is very important to explore new potential therapeutic strategies to improve the sensitivity of Herceptin. Besides the reported strategies of combination therapy for Herceptin resistance in breast cancer, Herceptin-resistant cells have recently been shown to retain sensitivity to green tea polyphenol epigallocatechin-3 gallate (EGCG), providing a novel strategy for treatment of Herceptin-resistant breast cancers (Eddy, et al. 2007). In the present study, we demonstrated the improvement of tamoxifen responses in BT/HerR cells and tried to explore new therapeutic strategies to improve sensitivity to Herceptin. Our results showed that there was a significant difference in response to tamoxifen between BT and BT/HerR cells (Fig 1A, 1B). BT/HerR cells were much more sensitive to tamoxifen than BT cells, and tamoxifen in these cells could function as an antagonist. In contrast, BT cells are resistant to tamoxifen, as observed by another laboratory (Zhou et al. 2007), and tamoxifen has a partial agonist function in these cells as well as in MCF-7/HER2 cells, which have been reported to be resistant to tamoxifen (Kurokawa et al. 2000; Shou et al. 2004). In addition, we observed that the two kinds of cell lines had similar sensitivity to the pure ER antagonist, ICI. The different responses to tamoxifen and ICI in these cells are associated with the different mechanisms of the two agents affecting ER function. ICI may block both the transcriptional activating function 2(AF-2) and transcriptional activating function 1(AF-1) of ER by attenuating dimerization of the receptor and accelerating receptor degradation, but tamoxifen only blocks AF2 of ER by competing with the receptor and allows activation of AF1 (Dowsett, et al. 2005). Therefore, tamoxifen in breast cancer cells only partially inactivates ER-regulated transcription or even has a partial agonist function in HER2 overexpressing cells such as MCF-7/Her2 (Kurokawa et al. 2000; Shou et al. 2004) and BT cells (Fig. 1A, 1C and Fig. 2). The different sensitivities to tamoxifen in BT and BT/HerR cells are not attributable to differences in ER and Her2 level because total ER and Her2 levels in these two kinds of cells were similar (Fig. 1D). Considering the important role of interaction of the Her2 and ER pathways in the development of tamoxifen-resistance (Pietras et al. 1995; Shou et al. 2004), a change in cross-talk between ER and Her2 signaling pathways might be involved in this different response to tamoxifen in these two kinds of cell lines.
In Her2-overexpressing tumors, the cross-talk between ER and Her2 pathways is associated with ER nongenomic activity, and the nongenomic rapid ER action in these cells may become more prominent. For example, tamoxifen has been shown to rapidly activate EGFR, Her2, AKt, and ERK/MAPK in MCF-7/Her2 cells, establishing a cycle of cell survival and proliferation via bi-directional cross-talk between growth factor and ER signaling pathways (Shou et al. 2004). In BT cells, which express ER and overexpress Her2 and AIB1 (Anzick, et al. 1997; Yakes et al. 2002; Zhou et al. 2007), effects of estrogen and tamoxifen on phosphorylation of downstream effectors of EGFR/Her2 are similar to those in MCF-7/Her2 cells (Shou et al. 2004) (Fig. 2). In MCF-7/Her2 cells, the EGF receptor tyrosine kinase inhibitor gefitinib could eliminate this cross-talk and restore tamoxifen’s antitumor effects, and Herceptin was also demonstrated to reverse tamoxifen resistance by reducing downstream MAPK/ERR1/2 signaling (Kurokawa et al. 2000). These findings suggest that Herceptin-mediated down-regulation of the Her2 pathway may be associated with increased sensitivity to tamoxifen. Our results showed the attenuated activation of Akt and MAPK by E2 or tamoxifen in BT/HerR1.0 cells (Fig. 2). Consistent with the attenuated change in phosphorylated MAPK, E2 and tamoxifen-stimulated phospho-ERα at Ser118 was also attenuated. These results suggest that the nongenomic activities of ER in BT/HerR cells is decreased, which leads to attenuation of the cross-talk between growth factor and ER signaling pathways, and may be involved in the increased sensitivity to tamoxifen in BT/HerR cells. The reason for the attenuated activation of AKT and MAPK by E2 or tamoxifen in BT/HerR cells is currently not clear and may be associated with chronic Herceptin exposure. For example, phosphorylated Akt levels in the presence of Herceptin in BT/HerR cells has been found to be decreased than in BT cells in the absence of Herceptin (Chan et al. 2005). However, in the absence of Herceptin, the basal phosphorylated Akt level in BT/HerR1.0 cells was not decreased and inversely was somehow increased, compared to BT cells (Fig. 2). Hence, the precise mechanism of attenuated activation of Akt and MAPK by E2 or tamoxifen in BT/HerR cells will require further research.
Besides the cross-talk between ER and Her2, recent research has shown that subcellular localization of ER may play a mechanistic role in determining the responsiveness of breast cancer cells to tamoxifen (Yang et al. 2004). Her2 overexpression in MCF-7/Her2 cells promotes the relocalization of ER from the nucleus to the cytoplasm, enhances interactions of ER with Her2, inhibits ER transactivation function, and induces resistance to tamoxifen-mediated growth inhibition of breast cancer cells. Conversely, down-regulation of Her2 by Herceptin leads to suppression of MAPK stimulation, restoration of ER to the nucleus, and potentiation of the growth-inhibitory action of tamoxifen. Different subcellular localization of ER in specific cells represents the different activating status of genomic pathway or nonegenomic pathway of ER. Our data showed different subcellular localization of ER in BT cells and BT/HerR1.0 cells, which suggest exclusion of ER from the nucleus in BT cells might act to deprive the anti-estrogenic agents of their target in the proper cellular compartment. On the other hand, entering into the nucleus of ER from the cytoplasm in BT/HerR1.0 cells might indicate a restoration of the ER genomic pathway, leading to subsequent increased sensitivity to tamoxifen (Fig. 1, 3). The restoration of the ER genomic pathway in BT/HerR cells is not due to the changes in Her2 and ER levels because the steady-state ER, total and phosphorylated Her2 levels in BT cells and BT/HerR1.0 cells are the same (Fig 1D). On the other hand, our results suggest that attenuated activation of Akt and MAPK by estrogen or tamoxifen could confer the suppressed cross-phosphorylation between ER and HER2 downstream components, which may be associated with the restoration of the ER genomic pathway and subsequent improvement of tamoxifen sensitivity in BT/HerR cells.
It is well known that ER directly binds to estrogen response element residing in the promoter region of the target gene and, by recruiting coregulatory proteins, regulates gene transcription. In addition to the activation of ER phosphorylation, activated MAPK in tamoxifen-resistant cells can phosphorylate ER coactivator AIB1 (Shou et al. 2004) and the resistant status may be related to a change in the balance of the association of ER with coactivators or corepressors (Smith et al. 1997). A recent report describes how the forced overexpression of Her2 in estrogen-dependent, tamoxifen-sensitive MCF-7 cells results in tamoxifen resistance (Shou et al. 2004). In MCF-7 cells, tamoxifen induces occupancy of the pS2 promoter by ER, corepressor NCoR and histone deacetylase (HDAC3). However, in MCF-7/Her2 cells, tamoxifen induces occupancy by ER and coactivator complexes including AIB1, P300, and CBP, resulting in the formation of acetylated histones, thereby explaining agonist effects of tamoxifen on steroid-regulated gene expression. Given the improvement of the ER genomic pathway and increased tamoxifen sensitivity in BT/HerR cells, recruitment of nuclear receptor coactivator or corepressor on pS2 promoter by tamoxifen-bound ER in these two kinds of cell lines should be different. Consistent with the observation in MCF-7 and MCF-7/HER2 cells, our results showed that tamoxifen in BT cells induced occupancy by ER and coactivator AIB1, which corresponds to its agonist function in these cells, whereas tamoxifen in BT/HerR1.0 cells induced occupancy by corepressor NcoR. The replacement of coactivator complexes with corepressor complexes in the presence of tamoxifen-bound ER is consistent with an antagonist function of tamoxifen in BT/HerR1.0 cells. The different recruitment of nuclear receptor coactivator or corepressor on pS2 promoter in BT and BT/HerR cells might explain the improvement of tamoxifen sensitivity in BT/HerR cells.
In summary, the results presented here show for the first time the enhancement of tamoxifen sensitivity in BT/HerR cells, relative to BT cells, and the following mechanisms are implicated: (a) attenuated activation of MAPK and Akt pathway by estrogen and tamoxifen, indicating the decreased ER nongenomic activity; (b) different subcelluar localization from BT cells, indicating improvement of genomic pathway of ER; and (c) the replacement of coactivator complexes with corepressor complexes on ERE-containing promoters in the presence of tamoxifen-bound ER. These results serve as direct evidences for antagonist activity of tamoxifen in BT/HerR cells. Although there is an inverse relationship between the degree of ER positivity and Her2 expression, a significant proportion of human breast cancers are both ER-positive and overexpressing Her2 (Berry, et al. 2000). Compared to ER-positive, Her2 overexpressing and tamoxifen-resistant BT cells, the increased sensitivity to classical endocrine therapy such as tamoxifen in Herceptin-resistant BT/HerR cells suggests ER genomic pathway in these cells can be improved, thus allowing tamoxifen therapy to be effective again. In spite of the increased sensitivity to tamoxifen in BT/HerR cells, our data imply mono-therapy with tamoxifen might have only modest benefits on BT/HerR cells, i.e., 60% inhibition of proliferation in BT/HerR1.0 cells compared to the control (Fig. 1B and Fig. 5A). However, combination of tamoxifen and Herceptin was demonstrated to increase the sensitivity of BT/HerR1.0 cells to Herceptin (Fig. 5), suggesting combined therapy with Herceptin and tamoxifen might be a viable therapeutic strategy for Herceptin-resistant human breast cancer cells.
Supplementary Material
Acknowledgments
The research project described in this paper has been supported by NIH grants ES08258 (SC), CA44735 (SC) and CA33572 (the COH Cancer Center grant).
References
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- Argiris A, Wang CX, Whalen SG, DiGiovanna MP. Synergistic interactions between tamoxifen and trastuzumab (Herceptin) Clin Cancer Res. 2004;10:1409–1420. doi: 10.1158/1078-0432.ccr-1060-02. [DOI] [PubMed] [Google Scholar]
- Berry DA, Muss HB, Thor AD, Dressler L, Liu ET, Broadwater G, Budman DR, Henderson IC, Barcos M, Hayes D, et al. HER-2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer. J Clin Oncol. 2000;18:3471–3479. doi: 10.1200/JCO.2000.18.20.3471. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Metz MZ, Kane SE. Differential sensitivities of trastuzumab (Herceptin)-resistant human breast cancer cells to phosphoinositide-3 kinase (PI-3K) and epidermal growth factor receptor (EGFR) kinase inhibitors. Breast Cancer Res Treat. 2005;91:187–201. doi: 10.1007/s10549-004-7715-1. [DOI] [PubMed] [Google Scholar]
- Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- Dieras V, Beuzeboc P, Laurence V, Pierga JY, Pouillart P. Interaction between Herceptin and taxanes. Oncology. 2001;61(Suppl 2):43–49. doi: 10.1159/000055401. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Nicholson RI, Pietras RJ. Biological characteristics of the pure antiestrogen fulvestrant: overcoming endocrine resistance. Breast Cancer Res Treat. 2005;93(Suppl 1):S11–18. doi: 10.1007/s10549-005-9037-3. [DOI] [PubMed] [Google Scholar]
- Eddy SF, Kane SE, Sonenshein GE. Trastuzumab-resistant HER2-driven breast cancer cells are sensitive to epigallocatechin-3 gallate. Cancer Res. 2007;67:9018–9023. doi: 10.1158/0008-5472.CAN-07-1691. [DOI] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- Lenferink AE, Simpson JF, Shawver LK, Coffey RJ, Forbes JT, Arteaga CL. Blockade of the epidermal growth factor receptor tyrosine kinase suppresses tumorigenesis in MMTV/Neu + MMTV/TGF-alpha bigenic mice. Proc Natl Acad Sci U S A. 2000;97:9609–9614. doi: 10.1073/pnas.160564197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou G, Li Y, Chen B, Chen M, Chen J, Liao R, Zhang Y, Wang Y, Zhou D. Functional analysis on the 5′-flanking region of human FXR gene in HepG2 cells. Gene. 2007;396:358–368. doi: 10.1016/j.gene.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- Moasser MM, Basso A, Averbuch SD, Rosen N. The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2- overexpressing tumor cells. Cancer Res. 2001;61:7184–7188. [PubMed] [Google Scholar]
- Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–2446. [PubMed] [Google Scholar]
- Price-Schiavi SA, Jepson S, Li P, Arango M, Rudland PS, Yee L, Carraway KL. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99:783–791. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10:331S–336S. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Smith CL, Nawaz Z, O’Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ. Linkage of rapid estrogen action to MAPK activation by ERalpha-Shc association and Shc pathway activation. Mol Endocrinol. 2002;16:116–127. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- Sun M, Paciga JE, Feldman RI, Yuan Z, Coppola D, Lu YY, Shelley SA, Nicosia SV, Cheng JQ. Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res. 2001;61:5985–5991. [PubMed] [Google Scholar]
- Tang CK, Perez C, Grunt T, Waibel C, Cho C, Lupu R. Involvement of heregulin-beta2 in the acquisition of the hormone-independent phenotype of breast cancer cells. Cancer Res. 1996;56:3350–3358. [PubMed] [Google Scholar]
- Tseng PH, Wang YC, Weng SC, Weng JR, Chen CS, Brueggemeier RW, Shapiro CL, Chen CY, Dunn SE, Pollak M, et al. Overcoming trastuzumab resistance in HER2- overexpressing breast cancer cells by using a novel celecoxib-derived phosphoinositide-dependent kinase-1 inhibitor. Mol Pharmacol. 2006;70:1534–1541. doi: 10.1124/mol.106.023911. [DOI] [PubMed] [Google Scholar]
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- Wang CX, Koay DC, Edwards A, Lu Z, Mor G, Ocal IT, Digiovanna MP. In vitro and in vivo effects of combination of Trastuzumab (Herceptin) and Tamoxifen in breast cancer. Breast Cancer Res Treat. 2005;92:251–263. doi: 10.1007/s10549-005-3375-z. [DOI] [PubMed] [Google Scholar]
- Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci U S A. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- Yang Z, Barnes CJ, Kumar R. Human epidermal growth factor receptor 2 status modulates subcellular localization of and interaction with estrogen receptor alpha in breast cancer cells. Clin Cancer Res. 2004;10:3621–3628. doi: 10.1158/1078-0432.CCR-0740-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yau C, Gray JW, Chew K, Dairkee SH, Moore DH, Eppenberger U, Eppenberger-Castori S, Benz CC. Enhanced NF kappa B and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007;7:59. doi: 10.1186/1471-2407-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.