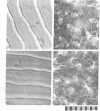
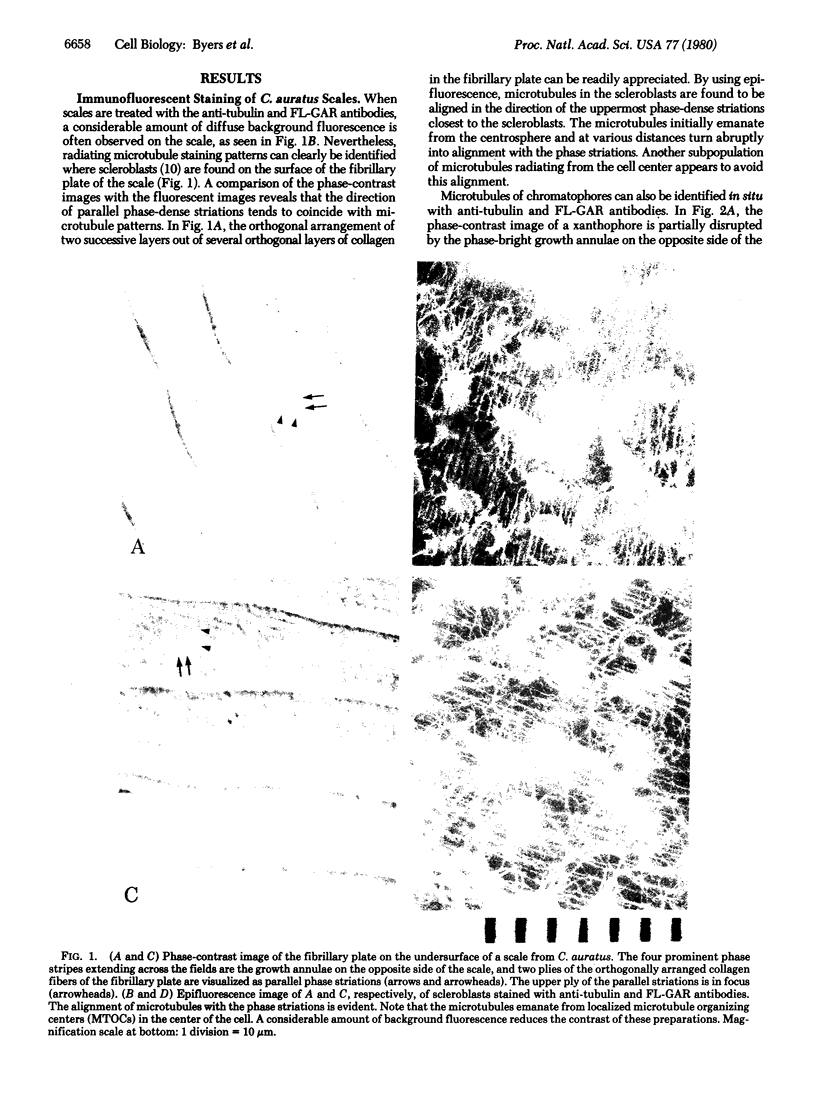
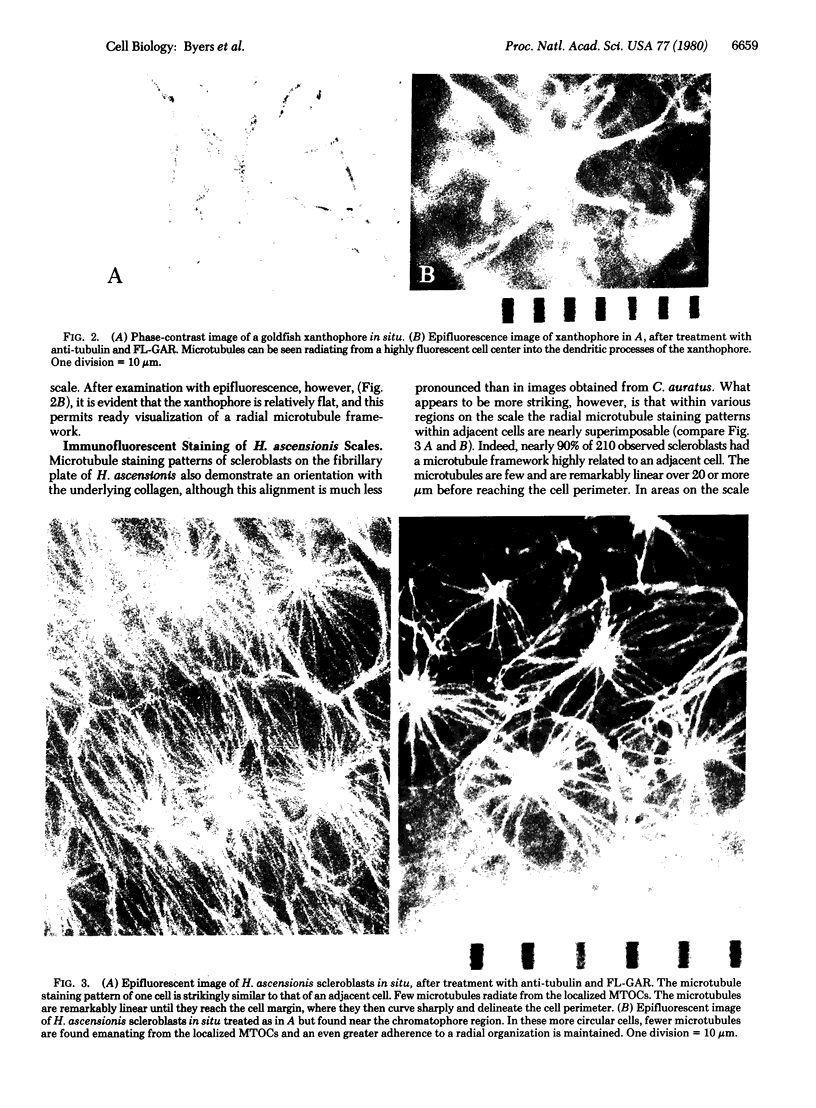
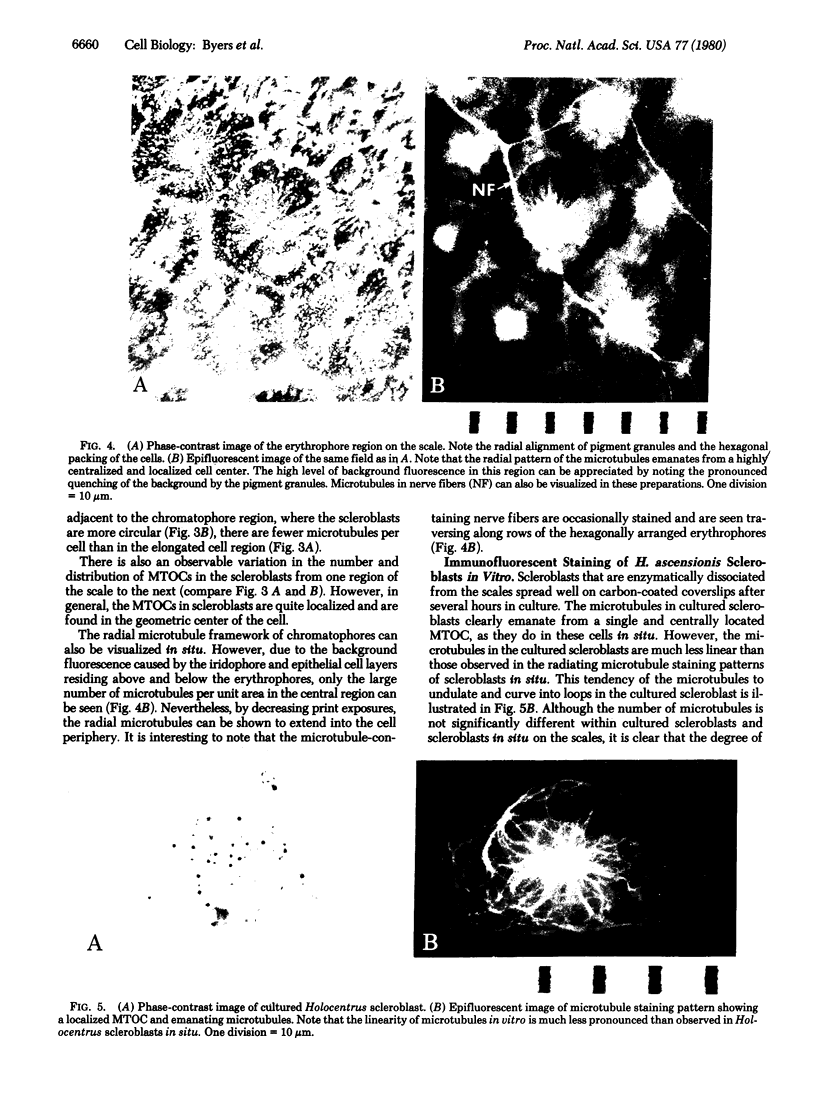
Abstract
Microtubule staining patterns can be visualized within cells in situ on the surface of fish scales from the squirrel fish, Holocentrus ascensionis, and the common goldfish, Carassius auratus, after incubation with antibodies to sea urchin tubulin and fluorescein-labeled goat antibodies to rabbit immunoglobulin G. Chromatophores in situ from both species reveal a radial microtubule framework that orients the alignment of pigment granules. Innervating fibers of erythrophores on the H. ascensionis scale can also be observed. In situ, pseudo-epithelial cells called scleroblasts show microtubule patterns with a remarkable degree of similarity within a selected region. Over 90% of the cells have a microtubule framework that is nearly superimposable from cell to adjacent cell. The microtubules in scleroblasts are few and form a simple radial framework with a localized microtubule organizing center (MTOC). Microtubules in scleroblasts in vitro emanate from localized MTOCs but are much less radially organized than in situ. Scleroblasts in situ on the scale of C. auratus show microtubules that curve abruptly into coalignment with phase striations on the fibrillary plate. The phase striations arise from the orthogonal plies of collagen in intimate association with the scleroblasts. The role of microtubules in scleroblasts may thus be to provide orientation for collagen fibrillogenesis, analogous to their role in orientation of cellulose fibers in plants. That cells in situ exhibit highly related and coordinated microtubule staining patterns reaffirms that the cytoskeleton plays an important role in the organization of differentiated tissues.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Buehler G. Daughter 3T3 cells. Are they mirror images of each other? J Cell Biol. 1977 Mar;72(3):595–603. doi: 10.1083/jcb.72.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht-Buehler G. Phagokinetic tracks of 3T3 cells: parallels between the orientation of track segments and of cellular structures which contain actin or tubulin. Cell. 1977 Oct;12(2):333–339. doi: 10.1016/0092-8674(77)90109-x. [DOI] [PubMed] [Google Scholar]
- BYERS B., PORTER K. R. ORIENTED MICROTUBULES IN ELONGATING CELLS OF THE DEVELOPING LENS RUDIMENT AFTER INDUCTION. Proc Natl Acad Sci U S A. 1964 Oct;52:1091–1099. doi: 10.1073/pnas.52.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers H. R., Porter K. R. Transformations in the structure of the cytoplasmic ground substance in erythrophores during pigment aggregation and dispersion. I. A study using whole-cell preparations in stereo high voltage electron microscopy. J Cell Biol. 1977 Nov;75(2 Pt 1):541–558. doi: 10.1083/jcb.75.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J Cell Biol. 1976 Dec;71(3):848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Simultaneous localization of myosin and tubulin in human tissue culture cells by double antibody staining. J Cell Biol. 1978 Apr;77(1):182–195. doi: 10.1083/jcb.77.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira L. C., Toledo A. M., Porter K. R. Observations on the structure of the skin of the teleost Fundulus heteroclitus (L). Arch Histol Jpn. 1970 Jun;32(1):1–15. doi: 10.1679/aohc1950.32.1. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Tropomyosin antibody: the specific localization of tropomyosin in nonmuscle cells. J Cell Biol. 1975 Jun;65(3):549–561. doi: 10.1083/jcb.65.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol J. B., Jr, Gibbins J. R., Porter K. R. A reinterpretation of the structure and development of the basement lamella: an ordered array of collagen in fish skin. Dev Biol. 1969 Oct;20(4):304–331. doi: 10.1016/0012-1606(69)90017-7. [DOI] [PubMed] [Google Scholar]
- Schliwa M., Osborn M., Weber K. Microtubule system of isolated fish melanophores as revealed by immunofluorescence microscopy. J Cell Biol. 1978 Jan;76(1):229–236. doi: 10.1083/jcb.76.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon F. Detailed neurite morphologies of sister neurolbastoma cells are related. Cell. 1979 Jan;16(1):165–169. doi: 10.1016/0092-8674(79)90197-1. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Lopata M. A., Kirschner M. W. Aggregation of microtubule initiation sites preceding neurite outgrowth in mouse neuroblastoma cells. Cell. 1979 Feb;16(2):253–263. doi: 10.1016/0092-8674(79)90003-5. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Lopata M. A., Kirschner M. W. Multiple sites for the initiation of microtubule assembly in mammalian cells. Cell. 1979 Feb;16(2):239–252. doi: 10.1016/0092-8674(79)90002-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Groeschel-Stewart U. Antibody to myosin: the specific visualization of myosin-containing filaments in nonmuscle cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4561–4564. doi: 10.1073/pnas.71.11.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]