Abstract
Introduction
Sleep deficits associated with sleep apnea and insomnia increase the risk of vascular inflammation and insulin resistance. This study examined the hypothesis that inflammation markers are higher in those diabetic patients who experience sleep deficits compared with those without any history of a sleep disorder.
Methods
Fasting blood was obtained after written informed consent, and sleep disorder histories were obtained from type 2 diabetic patients (n=81) attending clinics of the Louisiana State University Health Sciences Center.
Results
There was a significant correlation between body weight and leptin, and leptin in turn was significantly correlated with 10-kDa interferon-γ–induced protein (IP-10) levels and insulin resistance in type 2 diabetic patients. Fasting blood levels of leptin, IP-10, and insulin resistance were significantly elevated in patients with sleep deficits compared with diabetics with normal sleep patterns. There were no differences in glycosylated hemoglobin (HbA1c) or fasting glucose in patients with sleep deficits compared with those with normal sleep patterns. Sleep deficits increase circulating levels of leptin, IP-10, and insulin resistance compared to levels seen in patients with diabetes who reported no difficulty with sleep. Patients with sleep apnea had significantly lower hydrogen sulfide (H2S) levels compared with patients with normal sleep patterns or patients with insomnia. Low levels of circulating H2S could contribute to higher vascular inflammation in patients with sleep apnea.
Conclusions
These results suggest that sleep apnea is associated with a decrease in circulating H2S and sleep disorders increase the risk of inflammation and insulin resistance, which can contribute to the increased risk of vascular disease in subjects with type 2 diabetes.
Introduction
There is growing evidence that sleep disorders are associated with an increased risk of cardiovascular disease (CVD), high blood pressure, diabetes, and sleep deprivation–caused driving accidents.1–4 Insomnia is an inability to fall asleep or stay asleep. Sleep apnea is defined by pauses in breathing while asleep and is caused by obstruction of the airway or by the inability of the brain to normally regulate respiration. Both sleep apnea and insomnia are associated with a higher risk of inflammation and metabolic syndrome and frequently occur in overweight subjects.5,6 Diabetes causes increased vascular inflammation and is also associated with obesity.7 This study was originally designed to examine the relationship between the levels of 10-kDa interferon-γ–induced protein (IP-10), hydrogen sulfide (H2S), insulin resistance, and leptin and body weight in type 2 diabetic patients. An additional retrospective review of charts was conducted to find subjects with a history of insomnia or sleep apnea. The objective was to examine whether levels of inflammatory markers, insulin resistance, and glycemia are higher in those diabetic patients with histories of sleep disorders compared with diabetic patients without any sleep disorder.
Methods
Patient enrollment
Informed written consent was obtained from all patients according to the protocol approved by the Louisiana State University Health Sciences Center (LSUHSC) Institutional Review Board (IRB). All patients included in this study were adults with type 2 diabetes. A history to detect sleep disorders was obtained during the visit to obtain informed consent to participate in the study. One hundred diabetic subjects were enrolled in this study. All patients who gave written informed consent were invited to return to have blood drawn after fasting overnight. Of the 100 subjects who gave informed consent, 81 subjects came to the clinic for the blood draw.
Inclusion/exclusion criteria
Adult type 2 diabetic patients attending the diabetic clinic of the LSUHSC hospital were included in the study. Patients were excluded if they had any history of CVD, sickle cell disease, treatment with insulin, or metabolic disorders, including uncontrolled hypertension, hypothyroidism, or hyperthyroidism. Patients were excluded if they showed signs of significant hepatic dysfunction, defined as any underlying chronic liver disease or liver function tests greater than 1.5 times the upper limit of normal or renal dysfunction, defined as a serum creatinine greater than 1.5 mg/dL. Women with a positive pregnancy test or those nursing infants were also excluded. Subjects who were taking any supplemental vitamins or herbal products were not included in this study.
Blood collection
Blood was drawn from patients after an overnight fast (8 h). Following blood collection, serum tubes for chemistry profile, EDTA tubes for glycosylated hemoglobin (HbA1c), and complete blood counts were promptly delivered to the LSUHSC clinical laboratories. Additional tubes of EDTA-blood were brought to the research laboratory. Clear plasma was separated via centrifugation at 3000 rpm (1500×g) for 15 min. All plasma samples were stored at −80°C for analyses of the biochemical parameters.
Leptin, IP-10, insulin, and insulin resistance and H2S assays
Leptin, IP-10, and insulin levels in the plasma were determined by the sandwich enzyme-linked immunosorbent assay (ELISA) method using commercially available kits from Fisher Thermo Scientific Co. (Rockford, IL). All appropriate controls and standards as specified by the manufacturer's kit were used. In the cytokine assay, control samples were analyzed each time to check the variation from plate to plate on different days of analysis. Insulin resistance was calculated from blood glucose and insulin levels using the homeostasis model assessment of insulin resistance (HOMA-IR) formula.8 HOMA-IR was calculated with the formula: [plasma insulin (μU/mL)×glucose (mg%)] divided by 405. Plasma H2S was determined as described previously.9
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise mentioned. Data were analyzed using analysis of variance (ANOVA) statistically with Sigma Stat. A P value of less than 0.05 for a statistical test was considered significant.
Results
Table 1 gives the ages, body weights, glucose, HbA1c, and other blood chemistry profiles of diabetic patients without and with a history of insomnia or sleep apnea. There were no differences in the ages, body weights, body mass index (BMI), glucose, HbA1c, calcium, potassium, creatinine, or blood urea nitrogen (BUN)/creatinine levels of patients among the various groups. However, there was an increase in the insulin levels in diabetic patients with insomnia, sleep apnea, or both compared to diabetics reporting normal sleep patterns.
Table 1.
Ages, Body Weight, Glycemic Control, and Chemistry Profile of Diabetic Patients with Normal Sleep (D), with Insomnia and Sleep Apnea (IN+SA), Insomnia (IN), and Sleep Apnea (SA)
| Diabetes (n=67) | Insomnia+sleep apnea (n=14) | Insomnia (n=9) | Sleep apnea (n=5) | ||
|---|---|---|---|---|---|
| 1 | Age | 48.9±1.1 | 52.5±3.1 | 51.6±4.6 | 54.2±2.4 |
| 2 | M/F | 17/50 | 3/11 | 2/7 | 1/4 |
| 3 | Body weight (kg) | 102.5±3.7 | 106.4±9.9 | 96.8±12.4 | 121.6±15.2 |
| 4 | BMI | 36.5±1.1 | 37.7±3.1 | 36.3±4.2 | 40.6±4.1 |
| 5 | Glucose (mg%) | 138.7±7.1 | 136.2±12.9 | 141.4±19.4 | 128.0±14.4 |
| 6 | HbA1c (%) | 7.8±0.2 | 7.5±0.5 | 7.4±0.7 | 7.6±0.5 |
| 7 | Insulin (μU/mL) | 15.0±1.2# | 23±3.5* | 24±5.1* | 21±5.0* |
| 8 | Calcium (mg/dL) | 9.3±0.1 | 9.2±0.1 | 9.2±0.2 | 9.1±0.2 |
| 9 | Potassium (mM) | 4.2±0.04 | 4.15±0.08 | 4.05±0.1 | 4.32±0.12 |
| 10 | BUN/creatinine (ratio) | 17.16±0.76 | 18.46±1.76 | 17.94±1.82 | 19.30±3.84 |
| 11 | Creatinine (mg/dL) | 0.86±0.02 | 0.85±0.06 | 0.90±0.08 | 0.78±0.08 |
| 12 | HOMA | 4.99±0.54# | 7.87±1.65* | 9.09±2.6 | 5.94±1.01 |
HOMA insulin resistance was calculated with the formula: [plasma insulin (μU/mL)×glucose (mg%)] divided by 405. Values are mean±standard error. Differences in # vs.* are significant (P<0.05).
M/F, male/female; BMI, body mass index; HbA1c, glycosylated hemoglobin; BUN, blood urea nitrogen; HOMA, homeostasis model assessment.
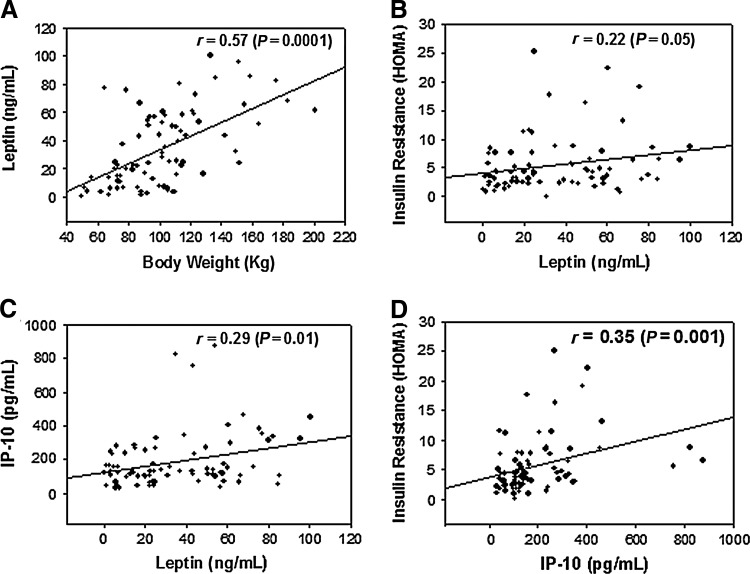
Figure 1 illustrates a significant relationship between body weight and blood levels of leptin (Fig. 1A). Leptin showed a significant relationship with levels of IP-10 (Fig. 1C) and insulin resistance (Fig. 1B) in type 2 diabetic patients. Blood levels of IP-10 and insulin resistance were also positively correlated in type 2 diabetic patients (Fig. 1D).
FIG. 1.
The relationship between body weight and leptin (A), leptin and insulin resistance (B), leptin and 10-kDa interferon-γ–induced protein (IP-10) (C), and IP-10 and insulin resistance (D).
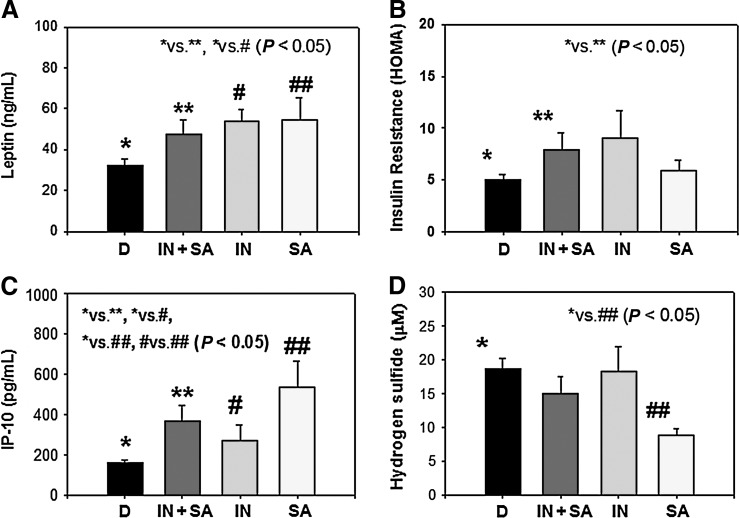
Figure 2 shows blood levels of leptin, insulin resistance, IP-10, and H2S type 2 diabetic patients separated into the following groups: Diabetics without history of sleep disorder (D), diabetics with history of both insomnia and sleep apnea (IN+SA), diabetics with a history of insomnia (IN), and diabetics with a history of sleep apnea (SA). Leptin and IP-10 levels were significantly elevated in diabetic patients with a sleep disorder, whether IN, SA, or both compared with diabetics with a history of normal sleep. HOMA-IR was significantly greater in those with both IN and SA compared to D, but not in the subgroups with IN or SA. The small number of subjects in these groups may have contributed to the lack of significance. There were no differences in H2S levels in diabetics without sleep disorders or those with IN or IN+SA. However, there were significantly lower levels of H2S in diabetics with SA compared with diabetic patients with normal sleep history or those only complaining of IN.
FIG. 2.
Blood levels of leptin (A), insulin resistance (B), 10-kDa interferon-γ–induced protein (IP-10) (C), and hydrogen sulfide in diabetic patients with normal sleep (D), with insomnia and sleep apnea (IN+SA), insomnia (IN), or sleep apnea (SA). Values are mean±standard error (SE). Differences were significant (P<0.05) between D versus IN+SA and D versus IN for leptin, between D versus IN+SA for homeostasis model assessment (HOMA), between D versus IN+SA, D versus IN, D versus IN, IN versus SA for IP-10, and between D versus SA for H2S levels.
Discussion
Epidemiological studies suggest that short sleep duration is correlated with an increased risk of developing obesity, metabolic syndrome, and diabetes.10–14 Glucose tolerance has been shown to be impaired after 6 days of sleep restricted from 12 h to 4 h per night.11 Sleep apnea is the most common category of sleep-disordered breathing. The muscle tone of the body ordinarily relaxes during sleep. At the level of the throat, the human airway is composed of collapsible walls of soft tissue that can obstruct breathing during sleep. Chronic sleep apnea requires treatment to prevent low blood oxygen, sleep deprivation, and other complications. Individuals with low muscle tone and soft tissue around the airway (e.g., those with obesity) or those with structural features that give rise to a narrowed airway are at high risk for obstructive sleep apnea (OSA). The elderly are more likely to have OSA than young people, and men are more likely to suffer sleep apnea than women. The risk of OSA rises with increasing body weight and age. Markers of vascular inflammation, leptin, and insulin resistance levels are elevated in the blood of many diabetic patients. Our study suggests a significant increase in leptin with increasing body weight. Leptin, in turn, has a significant relationship with blood levels of IP-10 and insulin resistance in type 2 diabetic patients.
Leptin is produced by adipocytes and is believed to contribute to the central regulation of food intake.15,16 Its reduction may contribute to the development of obesity. Except for very rare genetic syndromes, obesity is associated with highly elevated serum concentrations of leptin due to leptin resistance. Human studies with normal subjects observed an increase in serum leptin in subjects with sleep restriction but not in controls.17 The studies in the literature suggest that leptin is a proinflammatory hormone.18–21 Evidence from our study suggests that sleep deprivation increases circulating leptin and is an additional risk factor that increases inflammation in type 2 diabetic patients.
The IP-10 chemokine is an interferon-inducible protein-10.22 The presence of IP-10 in atherosclerotic plaques and its upregulation after balloon injury in the rat carotid artery suggest a direct inflammatory role for IP-10.23 The proinflammatory effect of IP-10 is mediated predominantly through recruitment of effector T cells at sites of inflammation. IP-10 gene disruption is associated with enhanced expression of antiinflammatory cytokine IL-10 and a decrease in the development of atherosclerotic lesion formation in hypercholesterolemic apolipoprotein E (ApoE) knockout mice.24 A growing body of evidence suggests that IP-10 is involved in the pathogenesis of atherosclerosis and chronic inflammation of the lungs.24 There are limited reports regarding the role of IP-10 in sleep disorders. In our study, the significantly higher levels of IP-10 in diabetic patients who also have sleep disorders suggest that lack of sleep is an additional risk factor for the elevated CVD seen in type 2 diabetes. Insulin resistance plays a key role in the regulatory pathway that progresses from hyperglycemia to monocyte and endothelial cell activation in the enhanced vascular inflammation of diabetes.25 This study indicates that sleep deficits increase the leptin and insulin resistance and IP-10 levels in type 2 diabetic patients. Elevated leptin or leptin resistance, IP-10, and insulin resistance levels may play crucial roles in accelerating the development of CVD in type 2 diabetic patients with a history of sleep deficits.
Our data demonstrate that sleep apnea may cause lower circulating H2S levels. H2S is gaining acceptance as a signaling molecule and has been shown to elicit a variety of biological effects that may mediate protection from CVD.26,27 H2S is produced in vivo from L-cysteine by the action of two enzymes, cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE).28 CSE is mainly expressed in the thoracic aorta, portal vein, ileum, heart, liver, kidney, and vascular smooth muscle, whereas CBS is highly expressed in the central and peripheral nervous systems.27 Recent studies have shown evidence for two other H2S-producing enzymes, 3-mercaptopyruvate sulfurtransferase and cysteine aminotransferase, which produce H2S in the brain as well as in vascular endothelium.29–31 H2S inhibits oxidative stress, promotes stimulation of adenosine triphosphate–senstive potassium (KATP) channels, and relaxation and vasodilation in vascular smooth muscle cells and of the human corpus cavernous smooth muscle.27,29–34 Genetic deletion of the CSE enzyme in mice markedly reduces H2S levels in the serum, heart, aorta, and other tissues, and mice lacking CSE display pronounced hypertension and diminished endothelium dependent vasorelaxation.27 Furthermore, ApoE knockout mice treated with a H2S donor showed reduced atherosclerotic plaque size compared to controls.35 These studies suggest that H2S is a physiological neuromodulator, vasodilator, and regulator of blood pressure.27 H2S can also increase glucose metabolism via activation of the phosphoinositide 3-kinase (PI3K)/phosphatidylinositol 3,4,5-trisphosphate (PIP3)/Akt/protein kinase Cζ(PKCζ(/λ insulin signaling pathways.36 A significant decrease in circulating H2S in diabetic patients with sleep apnea compared with diabetic patients with normal sleep histories is an interesting observation. The subjects with a history of sleep apnea are known to have a higher incidence of hypertension.1 On the other hand, an increase in arterial blood pressure was observed in rats supplemented with an inhibitor of CSE that catalyzes formation of H2S and the development of hypertension in CSE kockout mice model.28,37 Ex vivo studies also report relaxation of mesentery artery beds after H2S supplementation.38 The effect of H2S on smooth muscle relaxation is likely to be mediated by the opening of KATP channels located on vascular smooth muscle cells and conductance by calcium-activated postassium channels (KCa channels) located on vascular endothelial cells. H2S causes opening of these two channels, leading to membrane hyperpolarization and smooth muscle relaxation. Diabetes is associated with lower blood levels of H2S and vascular inflammation.39,40 Whether the deoxygenation associated with sleep apnea is another risk factor in lowering H2S levels is not known and needs investigation. Further studies are also needed to investigate whether lower blood levels of H2S are associated with a higher incidence of hypertension in subjects with sleep apnea. It needs to be determined whether L-cysteine, an endogenous precursor of H2S, has a beneficial effect on improved sleep and vascular inflammation.
In conclusion, sleep deficits increase levels of leptin, IP-10, and insulin resistance and thus can accelerate vascular inflammation in type 2 diabetic patients. Sleep apnea, but not insomnia, is associated with modest but significantly lower blood levels of H2S in diabetic patients. Further studies in a large patient population are needed to establish the role of altered H2S in the pathophysiology of sleep apnea in diabetic patients.
Acknowledgments
The authors are grateful to nurse coordinator John Rowell, R.N., for the outstanding help in conducting this study. S.K.J. is supported by grants from The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the Office of Dietary Supplements of the National Institutes of Health RO1 DK072433 and the Malcolm Feist Chair in Diabetes. The authors thank Ms. Georgia Morgan for excellent editing of this manuscript.
Author Disclosure Statement
None of the authors have any conflict of interest. No competing financial interests exist.
References
- 1.Pedrosa RP. Drager LF. Gonzaga CC, et al. Obstructive sleep apnea: The most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 2.Sharma SK. Agrawal S. Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365:2277–2286. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 3.Koren D. Katz LEL. Brar PC, et al. Sleep architecture and glucose and insulin homeostasis in obese adolescents. Diabetes Care. 2011;34:2442–2447. doi: 10.2337/dc11-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavie L. Polotsky V. Cardiovascular aspects in obstructive sleep apnea syndrome—molecular issues, hypoxia and cytokine profiles. Respiration. 2009;78:361–370. doi: 10.1159/000243552. [DOI] [PubMed] [Google Scholar]
- 5.Fredheim JM. Rollheim J. Omland T. Type 2 diabetes and pre-diabetes are associated with obstructive sleep apnea in extremely obese subjects: A cross-sectional study. Cardiovasc Diabetol. 2011;10:84. doi: 10.1186/1475-2840-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ursavas A. Ilcol YO. Nalci N, et al. Ghrelin, leptin, adiponectin, and resistin levels in sleep apnea syndrome: Role of obesity. Ann Thorac Med. 2010;5:161–165. doi: 10.4103/1817-1737.65050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyholm B. Nielsen MF. Kristensen K, et al. Evidence of increased visceral obesity and reduced physical fitness in healthy insulin-resistant first-degree relatives of type 2 diabetic patients. Eur J Endocrinol. 2004;150:207–214. doi: 10.1530/eje.0.1500207. [DOI] [PubMed] [Google Scholar]
- 8.Jain SK. Velusamy T. Croad JL. Rains JL. Bull R. L-cysteine supplementation lowers blood glucose, glycated hemoglobin, CRP, MCP-1, oxidative stress and inhibits NF-κB activation in the livers of Zucker diabetic rats. Free Radic Biol Med. 2009;46:1633–1638. doi: 10.1016/j.freeradbiomed.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu YZ. Wang ZJ. HO P, et al. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol. 2007;102:261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 10.Zizi F. Jean-Louis G. Brown CD, et al. Sleep duration and the risk of diabetes mellitus: Epidiomological evidence and pathophysiologyic insights. Curr Diab Rep. 2010;10:43–47. doi: 10.1007/s11892-009-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donga E. van Dijk M. van Dijk JG, et al. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care. 2010;33:1573–1577. doi: 10.2337/dc09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beihl DA. Liese AD. Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethinic cohort. Ann Epidemiol. 2009;19:351–357. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Bhusan B. Misra A. Guleria R. Obstructive sleep apnea is independently associated with the metabolic syndrome in obese Asian Indians in Northern India. Metabol Syndr Relat Disord. 2010;8:431–435. doi: 10.1089/met.2009.0125. [DOI] [PubMed] [Google Scholar]
- 14.Calvin AD. Albuquerque FN. Lopez-Jimenez F. Somers VK. Obstructive sleep apnea, inflammation, and metabolic syndrome. Metabol Syndr Relat Disord. 2009;7:271–278. doi: 10.1089/met.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantzoros CS. Magkos F. Brinkoetter M. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567–E584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malli F. Papaioannou AI. Gourgoulianis KI. Danill Z. The role of leptin in the respiratory system: An overview. Respir Res. 2010;11:152. doi: 10.1186/1465-9921-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Leeuwen WMA. Hublin C. Sallinen M, et al. Prolonged sleep restriction affects glucose metabolism in healthy young men. Int J Endocrinol. 2010;2010:1–7. doi: 10.1155/2010/108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sari R. Balci MK. Apaydin C. The relationship between plasma leptin levels and chronic complications in patients with type 2 diabetes mellitus. Metabol Syndr Relat Disord. 2010;8:499–503. doi: 10.1089/met.2009.0127. [DOI] [PubMed] [Google Scholar]
- 19.Soodini GR. Hamdy O. Adiponectin and leptin in relation to insulin sensitivity. Metabol Syndr Relat Disord. 2004;2:114–123. doi: 10.1089/met.2004.2.114. [DOI] [PubMed] [Google Scholar]
- 20.Lord GM. Leptin as a proinflammatory cytokine. Contrib Nephrol. 2006;151:151–164. doi: 10.1159/000095326. [DOI] [PubMed] [Google Scholar]
- 21.Matarese G. Moschos S. Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 22.Shimada A. Morimoto J. Kodama K, et al. Elevated Serum IP-10 levels observed in type 1 diabetes. Diabetes Care. 2001;24:510–515. doi: 10.2337/diacare.24.3.510. [DOI] [PubMed] [Google Scholar]
- 23.Wang X. Yue TL. Ohlstein EH, et al. Interferon-inducible protein-10 involves vascular smooth muscle cell migration, proliferation, and inflammatory response. J Biol Chem. 1996;271:24286–24293. doi: 10.1074/jbc.271.39.24286. [DOI] [PubMed] [Google Scholar]
- 24.Reape TJ. Groot PHE. Chemokines and atherosclerosis. Atherosclerosis. 1999;147:213–225. doi: 10.1016/s0021-9150(99)00346-9. [DOI] [PubMed] [Google Scholar]
- 25.Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxidant Redox Signal. 2011;15:1911–1926. doi: 10.1089/ars.2010.3739. [DOI] [PubMed] [Google Scholar]
- 26.Kabil O. Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L. Rose P. Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 28.Yang G. Wu L. Jiang B, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishigami M. Hiraki K. Umemura K. Ogasawara Y. Ishii K. Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;21:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 30.Shibuya N. Tanaka M. Yoshida M, et al. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 31.Shibuya N. Mikami Y. Kimura Y. Nagahara N. Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 32.d'Emmanuele di Villa Bianca R. Sorrentino R. Maffia P, et al. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc Natl Acad Sci USA. 2009;106:4513–4518. doi: 10.1073/pnas.0807974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura Y. Goto Y. Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 34.Dong XB. Yang CT. Zheng DD, et al. Inhibition of ROS-activated ERK1/2 pathway contributes to the protection of H2S against chemical hypoxia-induced injury in H9c2 cells. Mol Cell Biochem. 2012;362:149–157. doi: 10.1007/s11010-011-1137-2. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y. Zhao X. Jin H, et al. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoperotein E knockout mice. Arteriosclerosis Thromb Vasc Biol. 2009;29:173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 36.Manna P. Jain SK. Hydrogen sulfide and L-Cysteine increase PIP3 and glucose utilization by inhibiting PTEN and activating PI3K/AKT/ PKCζ/γ in 3T3L1 adipocytes. J Biol Chem. 2011;286:39848–39859. doi: 10.1074/jbc.M111.270884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao W. Ndisang JF. Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol. 2003;81:848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]
- 38.Cheng Y. Ndisang JF. Tang G, et al. Hydrogen sulfide induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Cir Physiol. 2004;287:H2316–H2383. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 39.Jain SK. Bull R. Rains JL. Bass PF. Levine SN. Reddy S. McVie R. Bocchinni JA. Low levels of hydrogen sulfide in the blood of diabetic patients and streptozotocin-treated rats cause vascular inflammation. Antioxid Redox Signal. 2010;12:1333–1638. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiteman M. Gooding KM. Whatmore JL, et al. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulfide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]