Abstract
Background
The normal human intravenous endotoxin model has been used for more than 50 years. It was once considered a possible model of sepsis, but, because no infection is present, it is better described as a model of systemic inflammation. We demonstrate herein that at least three of four systemic inflammatory response syndrome (SIRS) criteria are achieved with the model.
Methods
Otherwise healthy human volunteers were given Escherichia coli endotoxin 2 ng/kg intravenously. Vital signs were monitored, and blood samples were collected over time for assessment of white blood cells (WBCs), cytokines, counter-regulatory hormones, and monocyte receptors.
Results
The means of three variables (core temperature, heart rate, WBC) met the SIRS criteria. Compared with baseline, cytokines were elevated acutely, with tumor necrosis factor-alpha (TNFα) exhibiting temporal primacy over the other cytokines. Counter-regulatory hormones (cortisol, epinephrine) also were elevated acutely. Finally, the monocyte cell-surface receptors cluster of differentiation molecule (CD) 11b and TNF receptor-II were elevated and decreased, respectively.
Conclusions
The experimental human endotoxin model satisfies SIRS criteria and probably is best described as a model of Toll-like receptor 4 agonist-induced systemic inflammation.
The normal volunteer human endotoxin model was developed in the late 1960s at the U.S. National Institutes of Health (NIH) by Doctor Sheldon Wolff and at the University of Maryland by Doctor Sheldon Greisman [1–4]. Subsequently, work with this model was continued at the NIH by Doctors Anthony Suffredini and Joseph Parrillo [5,6] and at Harvard University by Doctor Douglas Wilmore [7,8]. Doctor Lowry brought the model to Cornell University Medical College (CUMC, now Weill Cornell Medical College) in the mid-1980s. This time period preceded the 1992 definition of the systemic inflammatory response syndrome (SIRS) as a clinical entity distinct from sepsis [9]. Earlier investigators using the human endotoxin model argued that it was a potential model of “sepsis,” an error that has dogged the model even to the present. As we believe most investigators now would acknowledge, the human endotoxin model is not of sepsis, but rather of moderate systemic inflammation. Perhaps it is not appreciated as widely that the relevant variables in the model approach or exceed SIRS criteria, as will be demonstrated herein.
Steve Lowry's research endeavors began in earnest in the 1970s at the NIH, where he was a Fellow with Doctors Steven Rosenberg and Murray Brennan at the Surgery Branch of the National Cancer Institute. It was during this time that Steve's curiosity and passion for science and research developed, so much so that Doctor Brennan subsequently invited Steve to join him at the Memorial Sloan-Kettering Cancer Center (MSKCC) in New York City. There, Steve completed a fellowship in surgical oncology and was appointed an assistant attending surgeon. While at MSKCC, Doctor Lowry's work and potential came to the attention of Doctor G. Tom Shires, then the Lewis Atterburg Stimson Professor and chair of surgery at CUMC. Doctor Shires recruited Steve as an assistant professor of Surgery and, while at CUMC, Steve blossomed fully as a scientist and researcher. After only three years, he was awarded an R01 grant that continued without interruption for 26 years, including 10 years with Method to Extend Research in Time (MERIT) status. At first, Doctor Lowry's research focused on nutrition and metabolism issues in normal human subjects and in burn patients, but broadened over the years into a multi-pronged approach that included in vitro cellular and whole-animal investigations as well as studies in intensive care unit (ICU) patients. The prong of research that Steve adopted and that became, perhaps, the mainstay of his laboratory was the human endotoxin challenge model, which is the focus of this paper.
Subjects and Methods
All studies were approved by and performed under the guidelines of the Robert Wood Johnson Medical School Institutional Review Board. After giving informed consent and being screened for normality by history and physical examination, healthy human volunteers, 18–35 years and of both genders, were given Escherichia coli O:113 endotoxin (kindly supplied through Doctor Suffredini at the NIH Clinical Center) intravenously (IV) at a dose of 2 ng/kg. The number of subjects evaluated is given in the legends to each of the figures. Symptoms were documented by questionnaires in which the following commonly experienced symptoms were listed: Headache, chills, muscle aches, ocular photosensitivity, and nausea or vomiting. Vital signs and blood samples were obtained at the time points indicated in the figures. White blood cells (WBCs) were counted by the clinical laboratory, and cytokine concentrations were quantified by sandwich enzyme-linked immunosorbent assays using recombinant standards. The plasma cortisol concentration (hydrocortisone) was measured by direct radioimmunoassay using a polyclonal rabbit antiserum. Expression of cluster of differentiation molecule (CD)11b and tumor necrosis factor receptor (TNFR)-II by blood monocytes was determined by two-color fluorescence flow cytometry. Data were analyzed by one-way repeated-measures analysis of variance (ANOVA) to determine statistical significance (p≤0.05).
Results
Symptoms
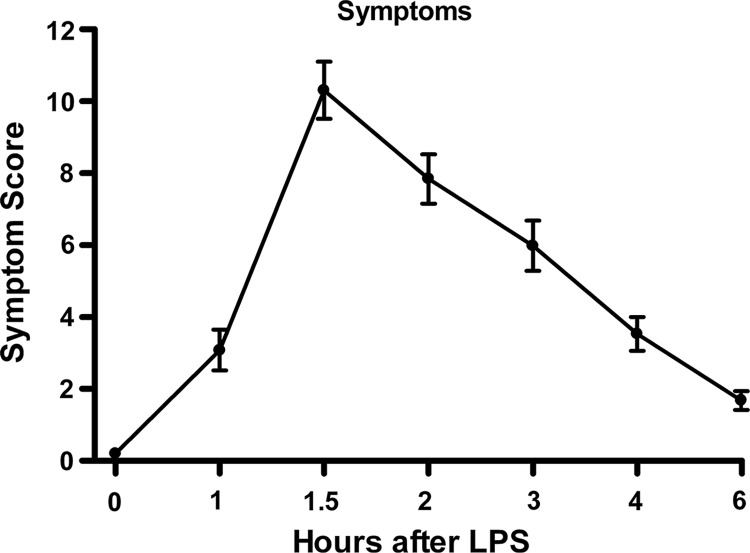
Symptoms (chills, myalgia, photosensitivity, nausea) were recorded and combined into an omnibus “symptom score” (Fig. 1). The mean symptom score at baseline (0 h) was zero. Substantial symptoms were manifested by 1 h after IV endotoxin administration, peaked at 2 h, and then declined until at least 6 h after endotoxin was given.
FIG. 1.
Omnibus symptom scores (mean±standard error) in normal human volunteers (n=66) receiving intravenous endotoxin. One-way repeated-measure analysis of variance was performed; endotoxin effect was significant at p<0.0001.
SIRS variables
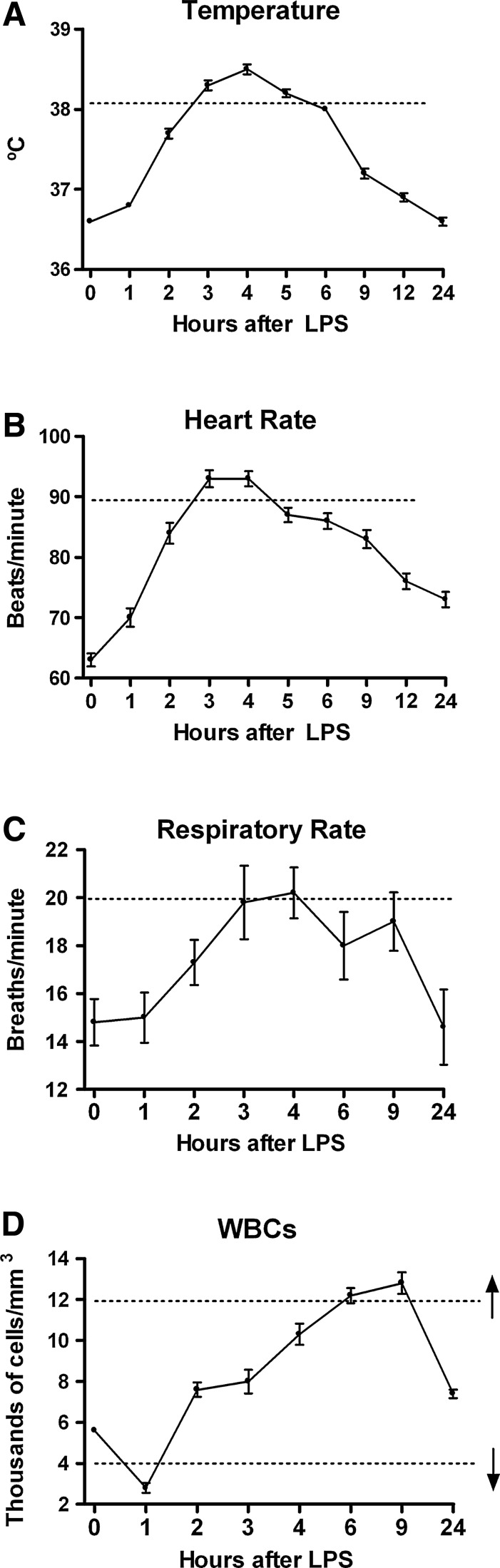
The variables that comprise the SIRS definition according to the 1992 Consensus Conference “Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis” [9] and reiterated and refined in 2001 [10] are temperature (hyperthermia or hypothermia), tachycardia, tachypnea, and abnormal WBC count (leukocytosis or leukopenia).
As shown in Figure 2A, the core temperature increased beginning as early as 1 h after endotoxin administration, with maximum temperatures attained at 3–5 h and resolution by 9 h. The maximum mean core temperature approached 38.5°C, above the SIRS criterion for hyperthermia (38.3°C). To meet the SIRS criterion for tachycardia, the heart rate must exceed 90 beats/min. As shown in Figure 2B, by 3–4 h after endotoxin injection, the mean heart rate met or exceeded this value and then diminished, although it had not normalized completely by the end of the 24-h study period. The respiratory rate (Fig. 2C) approached the SIRS criterion of 20 breaths/min by 4 h after endotoxin administration. However, the small number of subjects and relatively large variability among subjects make a definitive statement difficult as to whether the SIRS criterion for respiratory rate was satisfied. Unfortunately, PaCO2 data were not collected. The mean WBC count was ∼5,800/mm3 at baseline, decreased below the SIRS leukopenia criterion of 4,000/mm3, and then increased to more than 12,000 mm3 at 9 h post-endotoxin (Fig. 2D), thus also fulfilling the SIRS leukocytosis criterion for this parameter (12,000 mm3). Table 1 summarizes the fulfilment of the SIRS criteria, or lack thereof, after endotoxin administration for core temperature, heart rate, respiratory rate, and WBC number. Only the variable of respiratory rate may not meet the consensus criteria for SIRS in this model, although a Type II error cannot be ruled out in view of the small number of subjects. Fulfilling only two of the consensus criteria is sufficient to diagnose SIRS clinically.
FIG. 2.
Response of systemic inflammatory response syndrome (SIRS) variables in normal human volunteers receiving intravenous endotoxin (LPS, 2 ng/kg) at time 0. (A) Core (rectal) temperature (n=72). (B) Heart rate (n=72). (C) Respiratory rate (n=5). (D) White blood cell count (n=60). Graphs depict mean±standard error. Dashed lines indicate SIRS criterion value for each variable. Hypothermia is not so indicated for core temperature (panel A) because that criterion is off-scale. Both leukopenia and leukocytosis are indicated in panel D. One-way repeated-measures analysis of variance was performed. For all variables, endotoxin effect was significant at p<0.0001.
Table 1.
Variables That Do (Yes) or May Not (No) Reach Corresponding Systemic Inflammatory Response Syndrome Criterion in Normal Human Being Endotoxin Model
| Variable | Model |
|---|---|
| Core temperature elevation | Yes |
| Increase in heart rate | Yes |
| Increase in respiratory rate | No |
| Increase in white blood cell count | Yes |
Inflammatory mediators
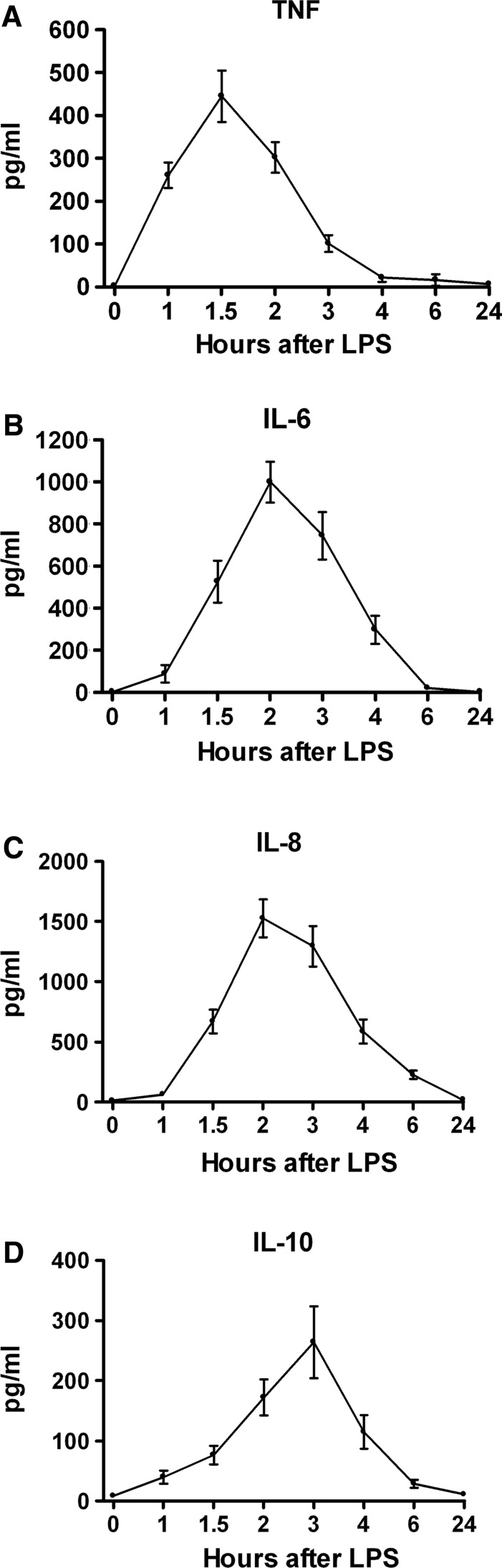
Doctor Lowry and his colleagues and collaborators played a pivotal role in the discovery and characterization of several inflammatory mediators (e.g., cytokines, chemokines, interleukins [ILs]) [11–15]. Of special importance was the discovery of cachectin/tumor necrosis factor (TNF)-alpha, one of the most potent and proximal mediators, which is believed to be an important driver of the inflammatory response, as espoused in the cytokine theory of inflammatory disease [11,12].
Figure 3A depicts the plasma TNF-α response to endotoxin administration in normal human volunteers. The mean TNF-α concentration, which was zero at baseline, increased beginning 1 h after endotoxin administration, peaked at 1.5 h, and again became undetectable by 4 h. By contrast, the mean concentrations of other pro-inflammatory mediators such as IL-6 (which also has anti-inflammatory effects) and IL-8 peaked at 2 h after endotoxin administration, later than TNF-α. The potent anti-inflammatory mediator IL-10 peaked at 3 h (Fig. 3B–D). These relations point to the temporal primacy of the TNF-α response to endotoxin in this model.
FIG. 3.
Plasma cytokine responses in normal human volunteers receiving intravenous endotoxin (LPS, 2 ng/kg) at time 0. (A) Tumor necrosis factor-alpha (n=24). (B) Interleukin (IL)-6 (n=32). (C) IL-8 (n=30). (D) IL-10 (n=31). Graphs depict mean±standard error. One-way repeated-measures analysis of variance was performed. For all cytokines, endotoxin effect was significant at p<0.0001.
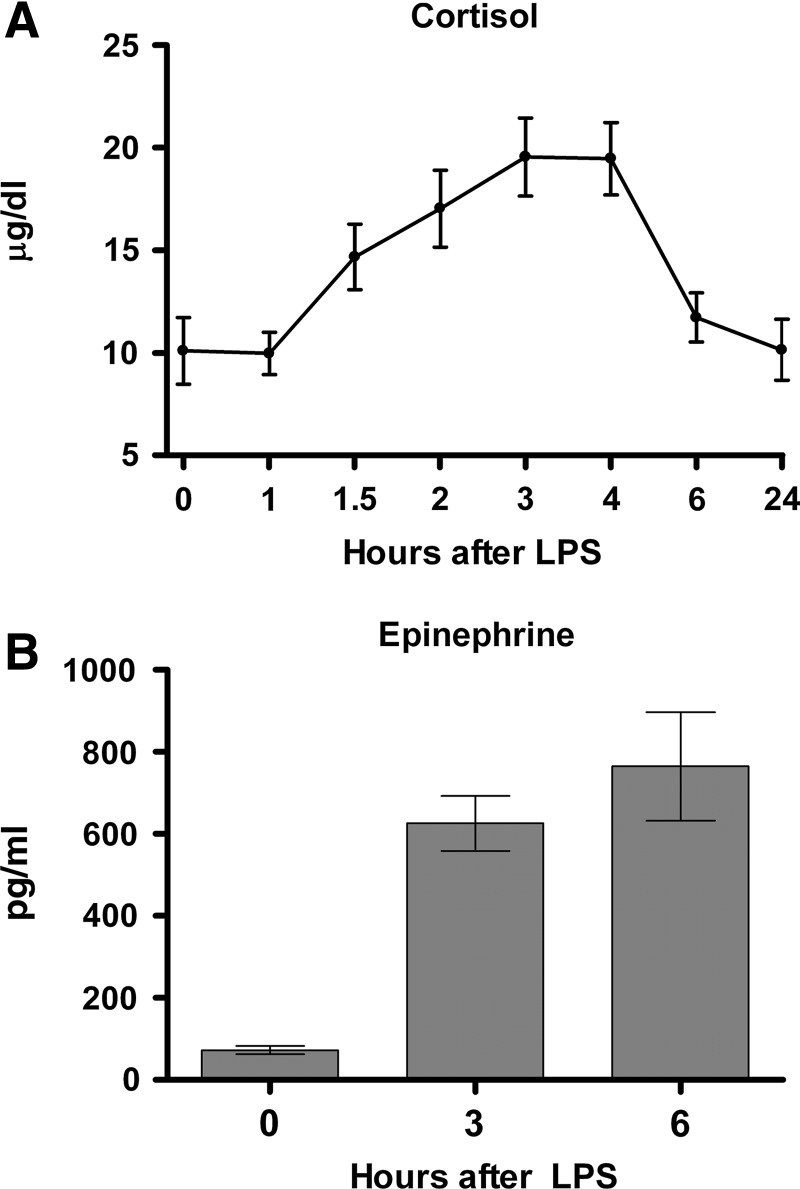
Counter-regulatory hormones
These hormones, including cortisol and epinephrine, have potent and pleiotropic physiological influences (e.g., metabolic, fluid balance, cardiovascular) and additionally have well-established anti-inflammatory effects [16–19]. In Fig. 4A and B, plasma cortisol and epinephrine concentrations are shown. Cortisol concentrations, already at their circadian high point because of the morning (9:00 am) start time of the study, doubled after endotoxin administration. Plasma epinephrine concentrations manifested an even more dramatic, ∼10-fold increase by 6 h after endotoxin administration.
FIG. 4.
Plasma counter-regulatory responses in normal human volunteers receiving intravenous endotoxin (LPS, 2 ng/kg) at time 0. (A) Cortisol (hydrocortisone)(n=22). (B) Epinephrine (n=6). Graphs depict mean±standard error. One-way repeated-measures analysis of variance was performed. For cortisol, endotoxin effect was significant at p<0.0001; for epinephrine, effect was significant at p<0.01.
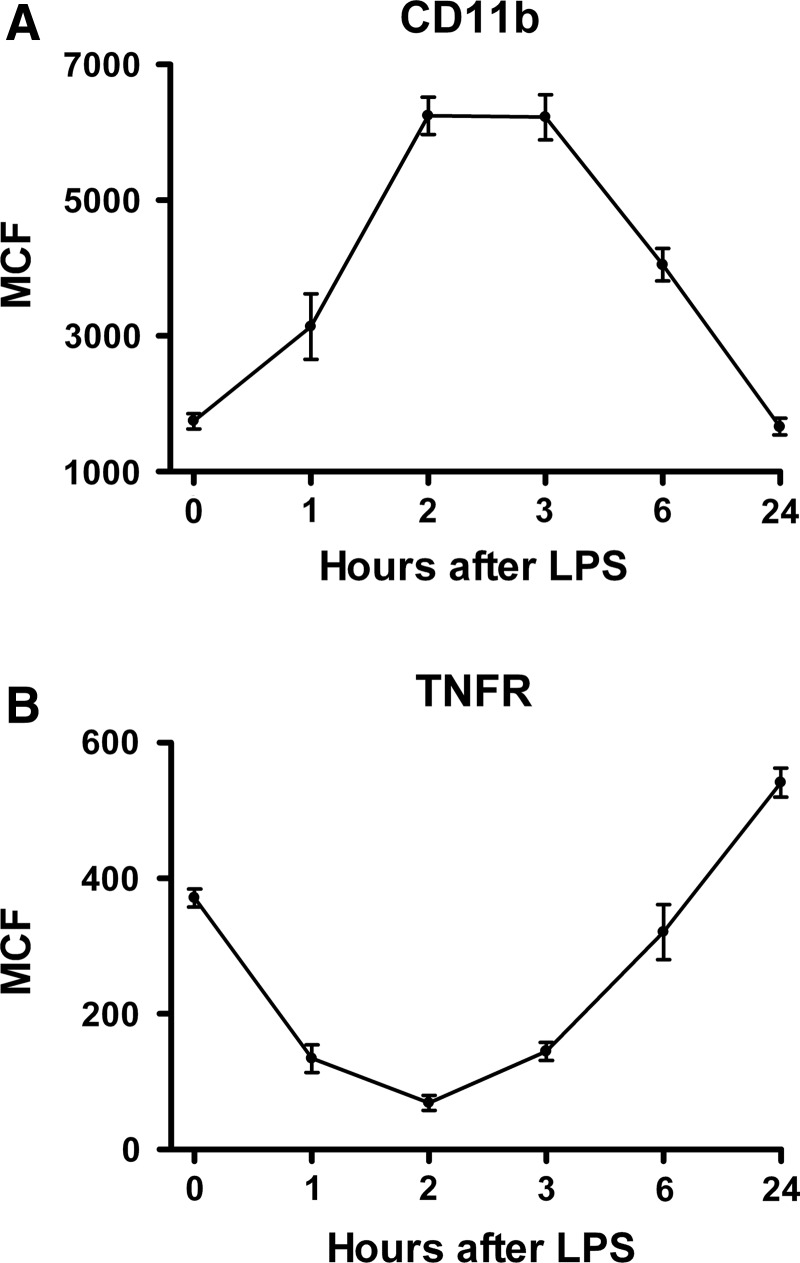
Leukocyte inflammatory receptors
Blood leukocytes respond to inflammatory stimuli by up-regulating or down-regulating certain receptors that participate in the response to inflammation or infection. Two such receptors are CD11b and TNFR-II. The CD11b moiety is the alpha-M subunit of an integrin named macrophage-1 antigen or complement receptor 3. This integrin is expressed on phagocytic cells (neutrophils and monocytes) and is conformationally up-regulated rapidly and robustly in response to inflammation. The TNFR-II protein is expressed weakly by neutrophils and strongly by monocytes and is down-regulated strikingly in response to inflammation [20].
After IV endotoxin administration to human volunteers, an increase in monocyte mean CD11b expression was detected by 1 h, became maximal at 2–3 h, and returned to baseline by 24 h (Fig. 5A). By contrast, blood monocyte TNFR-II expression declined ∼80% from baseline by 2 h after endotoxin administration, returned to baseline by 6 h, and increased by ∼35% over baseline at the 24-h time point (Fig. 5B).
FIG. 5.
Blood monocyte cell-surface inflammatory receptor responses in normal human volunteers given intravenous endotoxin (LPS, 2 ng/kg) at time 0. (A) Cluster of differentiation molecule (CD)11b [n=11]. (B) Tumor necrosis factor receptor-II (n=11). Graphs depict mean±standard error. One-way repeated-measures analysis of variance was performed. For both receptors, endotoxin effect was significant at p<0.0001.
Discussion
In view of these results, generated using an IV 2 ng/kg endotoxin challenge in normal human volunteers, the responses support this model strongly as one of moderate systemic inflammation in which the SIRS criteria [9,10] are met for core temperature (hyperthermia), heart rate, and WBC count (leukocytosis). The respiratory rate approached but perhaps did not satisfy the SIRS criterion, although, because of the relatively small number of subjects, a Type II error cannot be ruled out. Furthermore, the responses to endotoxin occur in a consistent temporal sequence, although the magnitudes may differ among individuals. Most effects resolve by 6–24 h after endotoxin administration. It should be emphasized firmly that, whereas this model appears to simulate systemic inflammation fairly well, it is not a model of sepsis because no infection is present, and patterns of cytokine responses are different in patients with, and animal models of, sepsis [21]. Because endotoxin is a well-characterized pathogen-associated molecular pattern (PAMP) that signals through Toll-like receptor-4 (TLR4), perhaps the most succinct way to describe this model is as a model of TLR4 agonist-induced systemic inflammation.
A major goal of using the human endotoxin model is to understand the etiologic factors of systemic inflammation so that rational clinical therapies to prevent or attenuate amplified or uncontrolled SIRS could be developed and investigated. Indeed, over approximately the last 25 years, Doctor Lowry and his colleagues employed the human endotoxin model to assess the effects of nutrition (enteral vs. parenteral, low lipid vs. high lipid) [22–25], cytokine antagonists (IL-1 receptor antagonist [RA], TNFR constructs) [26–29], endotoxin antagonists (PEGylated polymyxin B) [30], hormones (cortisol, epinephrine, growth hormone, insulin-like growth factor [IGF]) [16–19,31], and modulators of coagulation (e.g., activated protein C) [32,33]. Many of these compounds and approaches tested in the human endotoxin model have graduated to clinical trials in ICU patients (e.g., low-dose cortisol, IL-1-RA, TNF antagonists). One, activated protein C, was approved by the U.S. Food and Drug Administration for use in sepsis for a decade before the manufacturer withdrew it as ineffective [34–36]. Ironically, although these clinical trials were performed in the context of infection and sepsis, the “cytokine antagonist therapies” did not prove to be efficacious in sepsis [37–39], although several have become mainstream therapies for other inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease, and gout [40–45].
This paper has focused on the systemic inflammatory aspects of the human endotoxin model. However, a plethora of other physiological responses have been investigated in the model over the years by Doctor Lowry and others. Hormone effects also include modulation of thyroid and growth hormones [29,31,46]. Metabolic and coagulation effects [7,47–51] are prominent, as are cardiovascular and pulmonary effects, including attenuation of heart rate variability [19,52–55]. Finally, gut barrier function is compromised acutely in the model [8], although, curiously, gastrointestinal distress is not reported commonly by volunteers given IV endotoxin.
Acknowledgment
This work was supported by U.S. Public Health Service grant GM034695. We gratefully acknowledge all of the fellows, students, and staff members who have participated in the human endotoxin studies in the Lowry Laboratory over the past 25 years.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Greisman SE. Hornick RB. Comparative pyrogenic reactivity of rabbit and man to bacterial endotoxin. Exp Biol Med. 1969;131:1154–1158. doi: 10.3181/00379727-131-34059. [DOI] [PubMed] [Google Scholar]
- 2.Wolff SM. Biological effects of bacterial endotoxins in man. J Infect Dis. 1973;128:S259–S264. doi: 10.1093/infdis/128.supplement_1.s259. [DOI] [PubMed] [Google Scholar]
- 3.Greisman SE. Hornick RB. Mechanisms of endotoxin tolerance with special reference to man. J Infect Dis. 1973;128:S265–S276. doi: 10.1093/infdis/128.supplement_1.s265. [DOI] [PubMed] [Google Scholar]
- 4.Elin RJ. Wolff SM. Biology of endotoxin. Annu Rev Med. 1976;27:127–141. doi: 10.1146/annurev.me.27.020176.001015. [DOI] [PubMed] [Google Scholar]
- 5.Suffredini AF. Harpel PC. Parrillo JE. Promotion and subsequent inhibition of plasminogen activation after administration of intravenous endotoxin to normal subjects. N Engl J Med. 1989;320:1165–1172. doi: 10.1056/NEJM198905043201802. [DOI] [PubMed] [Google Scholar]
- 6.Suffredini AF. Fromm RE. Parker MM, et al. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321:280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 7.Michie HR. Spriggs DR. Manogue KR, et al. Tumor necrosis factor and endotoxin induce similar metabolic responses in human beings. Surgery. 1988;104:280–286. [PubMed] [Google Scholar]
- 8.O'Dwyer ST. Michie HR. Ziegler TR, et al. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg. 1988;123:1459–1464. doi: 10.1001/archsurg.1988.01400360029003. [DOI] [PubMed] [Google Scholar]
- 9.Crit Care Med; Members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis; 1992. pp. 864–874. [PubMed] [Google Scholar]
- 10.Crit Care Med; The International Sepsis Definitions Conference Committee. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference; 2001. pp. 1250–1256. [DOI] [PubMed] [Google Scholar]
- 11.Tracey KJ. Beutler B. Lowry SF, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 12.Tracey KJ. Fong Y. Hesse DG, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteremia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 13.Fong Y. Moldawer LL. Marano M, et al. Endotoxemia elicits increased circulating β2-IFN/IL-6 in man. J Immunol. 1989;142:2321–2324. [PubMed] [Google Scholar]
- 14.Van Zee KJ. DeForge LE. Fischer E, et al. IL-8 in septic shock, endotoxemia and after IL-1 administration. J Immunol. 1991;146:3478–3482. [PubMed] [Google Scholar]
- 15.Martich GD. Danner RL. Ceska M. Suffredini AF. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: The effect of anti-inflammatory agents. J Exp Med. 1991;173:1021–1024. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber AE. Coyle SM. Marano MA, et al. Glucocorticoid therapy alters hormonal and cytokine responses of endotoxin in man. J Immunol. 1993;150:1999–2006. [PubMed] [Google Scholar]
- 17.van der Poll T. Barber AE. Coyle SM. Lowry SF. Hypercortisolemia increases plasma interleukin-10 concentrations during human endotoxemia. J Clin Endocrinol Metab. 1996;81:3604–3606. doi: 10.1210/jcem.81.10.8855809. [DOI] [PubMed] [Google Scholar]
- 18.van der Poll T. Coyle SM. Barbosa K, et al. Epinephrine inhibits tumor necrosis factor α and potentiates interleukin-10 production during human endotoxemia. J Clin Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jan BU. Coyle SM. Oikawa LO, et al. Influence of acute epinephrine infusion on endotoxin-induced parameters of heart rate variability: A randomized controlled trial. Ann Surg. 2009;249:750–756. doi: 10.1097/SLA.0b013e3181a40193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Poll T. Calvano SE. Kumar A, et al. Endotoxin induces down-regulation of tumor necrosis factor receptors on circulating monocytes and granulocytes in humans. Blood. 1995;86:2754–2759. [PubMed] [Google Scholar]
- 21.Remick DG. Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24(Suppl 1):7–11. doi: 10.1097/01.shk.0000191384.34066.85. [DOI] [PubMed] [Google Scholar]
- 22.Fong YM. Marano MA. Barber A, et al. Total parenteral nutrition and bowel rest modify the metabolic response to endotoxin in humans. Ann Surg. 1989;210:449–456. doi: 10.1097/00000658-198910000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Poll T. Levi M. Braxton CC, et al. Parenteral nutrition facilitates activation of coagulation, but not of fibrinolysis, during human endotoxemia. J Infect Dis. 1998;177:793–795. doi: 10.1086/517811. [DOI] [PubMed] [Google Scholar]
- 24.Pajkrt D. Doran JE. Koster F, et al. Anti-inflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Poll T. Coyle SM. Levi M, et al. Fat emulsion infusion potentiates coagulation activation during human endotoxemia. Thromb Haemost. 1996;75:83–88. [PubMed] [Google Scholar]
- 26.Van Zee KJ. Coyle SM. Calvano SE, et al. Influence of interleukin-1 receptor blockade on the human response to endotoxemia. J Immunol. 1995;154:1499–1507. [PubMed] [Google Scholar]
- 27.Preas HL., II Reda D. Tropea M, et al. Effects of recombinant soluble type I interleukin-1 receptor on human inflammatory responses to endotoxin. Blood. 1996;88:2465–2472. [PubMed] [Google Scholar]
- 28.Suffredini AF. Reda D. Banks SM, et al. Effects of recombinant dimeric TNF receptor on human inflammatory responses following intravenous endotoxin administration. J Immunol. 1995;155:5038–5045. [PubMed] [Google Scholar]
- 29.van der Poll T. Van Zee KJ. Endert E, et al. Interleukin-1 receptor blockade does not affect endotoxin-induced changes in plasma thyroid hormone and thyrotropin concentrations in man. J Clin Endocrinol Metab. 1995;80:1341–1346. doi: 10.1210/jcem.80.4.7714108. [DOI] [PubMed] [Google Scholar]
- 30.Lin E. Coyle SM. Randhawa S, et al. PMX-622 prevents endotoxin-induced inflammation in humans. Surg Forum. 1998;49:6–8. [Google Scholar]
- 31.Lang CH. Pollard V. Fan J, et al. Acute alterations in growth hormone-insulin-like growth factor axis in humans injected with endotoxin. Am J Physiol. 1997;273:R371–R378. doi: 10.1152/ajpregu.1997.273.1.R371. [DOI] [PubMed] [Google Scholar]
- 32.Derhaschnig U. Reiter R. Knobl P, et al. Recombinant human activated protein C (rhAPC; drotrecogin alfa [activated]) has minimal effect on markers of coagulation, fibrinolysis, and inflammation in acute human endotoxemia. Blood. 2003;102:2093–2098. doi: 10.1182/blood-2003-02-0416. [DOI] [PubMed] [Google Scholar]
- 33.Kalil AC. Coyle SM. Um JY, et al. Effects of drotrecogin alfa (activated) in human endotoxemia. Shock. 2004;21:222–229. doi: 10.1097/01.shk.0000116778.27924.79. [DOI] [PubMed] [Google Scholar]
- 34.Barie PS. The last Xigris® survivor. Surg Infect. 2011;12:423–425. doi: 10.1089/sur.2011.9909. [DOI] [PubMed] [Google Scholar]
- 35.Barie PS. Hydo LJ. Shou J. Eachempati SR. Efficacy of therapy with recombinant human activated protein C of critically ill surgical patients with infection complicated by septic shock and multiple organ dysfunction syndrome. Surg Infect. 2011;12:443–449. doi: 10.1089/sur.2011.133. [DOI] [PubMed] [Google Scholar]
- 36.Ranieri VM. Thompson BT. Barie PS, et al. PROWESS-SHOCK Study Group. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 37.Fisher CJ. Dhainaut JF. Opal SM, et al. Recombinant human interleukin-1 receptor antagonist in the treatment of patients with sepsis syndrome: Results from a randomized, double-blind, placebo-controlled trial. JAMA. 1994;271:1136–1143. [PubMed] [Google Scholar]
- 38.Abraham E. Anzueto A. Gutierrez G, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- 39.Abraham E. Laterre P. Garbino J, et al. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: A randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit Care Med. 2001;29:503–510. doi: 10.1097/00003246-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Feldman M. Maini RN. Anti-TNFα therapy of rheumatoid arthritis: What have we learned? Annu Rev Immunol. 2001:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 41.Blum MA. Koo D. Doshi JA. Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: A systematic review. Clin Ther. 2011;33:901–913. doi: 10.1016/j.clinthera.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 42.van Deventer S. Tumour necrosis factor and Crohn's disease. Gut. 1997;40:443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korzenik JR. Crohn's disease: Future anti-tumor necrosis factor therapies beyond infliximab. Gastroenterol Clin North Am. 2004;33:285–301. doi: 10.1016/j.gtc.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Leso V. Leggio L. Armuzzi A, et al. Role of the tumor necrosis factor antagonists in the treatment of inflammatory bowel disease: An update. Eur J Gastroenterol Hepatol. 2010;22:779–786. doi: 10.1097/MEG.0b013e328331b654. [DOI] [PubMed] [Google Scholar]
- 45.Burns CM. Wortmann RL. Gout therapeutics: New drugs for an old disease. Lancet. 2011;377:165–177. doi: 10.1016/S0140-6736(10)60665-4. [DOI] [PubMed] [Google Scholar]
- 46.van der Poll T. Endert E. Coyle SM, et al. Neutralization of tumor necrosis factor does not influence endotoxin-induced changes in thyroid hormone metabolism in man. Am J Physiol. 1999;276:R357–R362. doi: 10.1152/ajpregu.1999.276.2.R357. [DOI] [PubMed] [Google Scholar]
- 47.Fong YM. Marano MA. Moldawer LL, et al. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990;85:1896–1904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bloesch D. Keller U. Spinas GA, et al. Effects of endotoxin on leucine and glucose kinetics in man: Contribution of prostaglandin E2 assessed by a cyclooxygenase inhibitor. J Clin Endocrinol Metab. 1993;77:1156–1163. doi: 10.1210/jcem.77.5.8077306. [DOI] [PubMed] [Google Scholar]
- 49.Fong Y. Matthews DE. Wei H, et al. Whole body and splanchnic leucine, phenylalanine, and glucose kinetics during endotoxemia in man. Am J Physiol. 1994;266:R419–R425. doi: 10.1152/ajpregu.1994.266.2.R419. [DOI] [PubMed] [Google Scholar]
- 50.Van Deventer SJH. Buller HR. ten Cate JW, et al. Experimental endotoxemia in humans: Analysis of cytokine release and coagulation, fibrinolytic and complement pathways. Blood. 1990;76:2520–2526. [PubMed] [Google Scholar]
- 51.van der Poll T. Levi M. Dentener M, et al. Epinephrine exerts anticoagulant effects during human endotoxemia. J Exp Med. 1997;185:1143–1148. doi: 10.1084/jem.185.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suffredini AF. Shelhamer JH. Neumann RD, et al. Pulmonary and oxygen transport effects of intravenously administered endotoxin in normal humans. Am Rev Respir Dis. 1992;145:1398–1403. doi: 10.1164/ajrccm/145.6.1398. [DOI] [PubMed] [Google Scholar]
- 53.Smith PD. Suffredini AF. Allen JB, et al. Endotoxin administration to humans primes alveolar macrophages for increased production of inflammatory mediators. J Clin Immunol. 1994;14:141–148. doi: 10.1007/BF01541347. [DOI] [PubMed] [Google Scholar]
- 54.Godin PJ. Fleisher LA. Eidsath A, et al. Experimental human endotoxemia increases cardiac regularity: Results from a prospective, randomized, crossover trial. Crit Care Med. 1996;24:1117–1124. doi: 10.1097/00003246-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Alvarez SM. Katsamanis-Karavidas M. Coyle SM, et al. Low-dose steroid alters in vivo endotoxin-induced systemic inflammation but does not influence autonomic dysfunction. J Endotoxin Res. 2007;13:358–368. doi: 10.1177/0968051907086465. [DOI] [PubMed] [Google Scholar]