Abstract
Background
Secondhand smoke (SHS) exposure is higher among lower socioeconomic status (SES) children. Legislation restricting smoking in public places has been associated with reduced childhood SHS exposure and increased smoke-free homes. This paper examines socioeconomic patterning in these changes.
Methods
Repeated cross-sectional survey of 10 867 schoolchildren in 304 primary schools in Scotland, Wales and Northern Ireland. Children provided saliva for cotinine assay, completing questionnaires before and 12 months after legislation.
Results
SHS exposure was highest, and private smoking restrictions least frequently reported, among lower SES children. Proportions of saliva samples containing <0.1 ng/ml (i.e. undetectable) cotinine increased from 31.0 to 41.0%. Although across the whole SES spectrum, there was no evidence of displacement of smoking into the home or increased SHS exposure, socioeconomic inequality in the likelihood of samples containing detectable levels of cotinine increased. Among children from the poorest families, 96.9% of post-legislation samples contained detectable cotinine, compared with 38.2% among the most affluent. Socioeconomic gradients at higher exposure levels remained unchanged. Among children from the poorest families, one in three samples contained >3 ng/ml cotinine. Smoking restrictions in homes and cars increased, although socioeconomic patterning remained.
Conclusions
Urgent action is needed to reduce inequalities in SHS exposure. Such action should include emphasis on reducing smoking in cars and homes.
Keywords: smoking, socioeconomic status, young people
Introduction
Childhood secondhand smoke (SHS) exposure increases the risk of respiratory problems1,2 and arterial damage.3 Indeed, the World Health Organization (WHO) state that ‘scientific evidence has unequivocally established that exposure to tobacco smoke causes death, disease and disability’.4 In all UK countries, childhood SHS exposure is highest amongst children from lower socioeconomic status (SES) families,5–8 representing a key mechanism in the intergenerational reproduction of health inequalities.
In line with WHO calls for increased ‘protection from exposure to tobacco smoke in indoor workplaces, public transport, indoor public places and, as appropriate, other public places’,4 all UK countries have now implemented legislation prohibiting smoking in enclosed public places and workplaces (Scotland March 2006, Wales March 2007, Northern Ireland (NI) April 2007 and England July 2007). Legislation appears to be achieving its aim of protecting workers and the public from SHS.9,10 However, opponents hypothesized that it would displace smoking into the home, increasing childhood SHS exposure. Though displacement has been reported in Hong Kong11 and the USA,12 this hypothesis has found no support in UK studies.8,13–15 While substantial post-legislation declines in exposure have been limited to Scotland, in no country did exposure increase following legislation.
However, although no longitudinal trend data are available elsewhere in the UK, Health Survey for England data indicate that childhood SHS exposure was declining prior to legislation, and doing so most rapidly amongst highly exposed groups (e.g. lower SES children), leading to a narrowing of inequalities.7 While there is no evidence that legislation has harmfully impacted children from lower SES families, evidence is accumulating that greater post-legislation declines in exposure have occurred amongst children at relatively low risk, potentially widening inequalities. In Wales and England, increased proportions of samples containing no detectable cotinine were accompanied by marginal overall reductions.14,15 Reductions in Scotland and Wales have been greatest amongst children of non-smokers;13,14 findings echoed internationally in a recent US study.16 In Scotland, while significant reductions were observed in all SES groups, declines as a proportion of baseline exposure were lowest among children from low SES families.4 In Wales, proportions of samples with no detectable cotinine increased only amongst children from more affluent families.5,6
Recognition of the need to target children at risk from SHS has led to increased emphasis on private spaces, with the Department of Health17 aiming to encourage two-thirds of smoking parents to adopt smoke-free homes by 2020. Jarvis et al.15 suggest that rather than displacing smoking into the home, legislation has contributed to denormalizing smoking near children and growing support for reducing smoking in private spaces. Indeed, post-legislation increases in smoke-free homes have been reported among smoking and non-smoking parents in Scotland, and smoking parents in England.15,18
Although most research into private smoking restrictions focuses on homes, attention is increasingly turning to dangers of smoking in cars, as reflected in the British Medical Association's19 call to ban smoking in all vehicles. One study showed that adolescents regularly exposed to SHS in cars exhibited symptoms of nicotine dependence.20 Whilst not distinguishing between adult and child passengers, one study found that 29% of UK smokers smoked with non-smoking passengers present;21 lower than in Canada (34%) and the USA (44%), but identical to Australia.
Given that smoking is higher among lower SES parents, with smoking restrictions in private spaces less common,18,22 encouraging uptake of smoke-free homes and cars may reduce socioeconomic inequalities. However, little attention has been paid to whether adoption of private smoking restrictions following legislation has been patterned by SES. Negotiation of such restrictions is perhaps easier for parents with fewer smokers in their social network.23 Given the higher prevalence of smoking amongst less affluent adults, denormalizing smoking near children may cause more rapid adoption of smoking restrictions amongst more affluent adults. Hence, patterning in uptake of private smoking restrictions represents a potential mechanism through which trends towards increased inequality following legislation may have occurred.
This paper reports data from the Scottish, Welsh and Northern Irish studies of changes in Child Exposure to Environmental Tobacco Smoke (CHETS), repeated cross-sectional studies examining associations of smoke-free legislation with children's SHS exposure.8,13,14 Through pooling data from three countries, it has enhanced power to examine socioeconomic patterning in SHS exposure and parental restrictions on smoking in private spaces before and after legislation.
Methods
Sample
Participants were 10 867 non-smokers (self-reported non-smokers providing saliva samples containing <15 ng/ml cotinine)24 in their final year at 304 primary schools in Scotland (n = 111), Wales (n = 71) and NI (n = 122).
Measures
Survey year
The year of data collection (pre- vs. post-legislation) was a proxy for smoke-free legislation.
SHS exposure
Salivary cotinine (a metabolite of nicotine) is a well-validated biomarker of SHS exposure in the previous 72 h.25 Anonymous samples were assayed using capillary gas chromatography with a detection limit of 0.1 ng/ml.26
Socioeconomic status
The family affluence scale (FAS)27 asks children whether they have their own bedroom (no = 0, yes = 1), number of family computers and holidays in the past year (none = 0, once = 1, two = 2, more than two = 3) and how many cars their family own (none = 0, one = 1, two or more = 2). The summed scale provides a measure of affluence (from 0, least affluent to 9, most affluent).
Smoking restrictions in the home
Children were asked whether smoking was allowed inside their home. Response options were (i) no, smoking is not allowed at all, (ii) smoking is allowed in certain areas only, (iii) smoking is allowed anywhere in our home, (iv) smoking is only allowed on special occasions in our home, (v) I don't know. Responses (ii) and (iv) represented ‘partial smoking restriction’. Children who selected ‘I don't know’ (n = 952) were excluded from the analysis of smoking restrictions as a dependent variable, though retained as a separate category in cotinine analyses.
Smoking restrictions in cars
Children were asked ‘Are people allowed to smoke in your car, van or truck?’. Options were ‘yes’, ‘no’, ‘I don't know’ or ‘don't have a car, van or truck’. Children whose family did not have a car (n = 941) or did not know whether smoking was allowed (n = 1251) were excluded from the analysis treating car-smoking restrictions as a dependent variable, though retained as a separate category in cotinine analyses.
Parental smoking
Children were asked to indicate how often each parent figure smoked (‘most days’, ‘sometimes’, ‘doesn't’, ‘don't know’ or ‘don't have or see’). Parents were considered smokers if the child indicated that they smoked most days or sometimes. Children were classified as living with no smoking parent figures, smoking father figure only, mother figure only or two smoking parents.
Age
Age was calculated by subtracting dates of birth from survey date.
Procedures
Sampling and data collection are detailed elsewhere.8,13,14 CHETS were repeated cross-sectional studies of schoolchildren in their final year of primary school. As Scottish legislation was introduced 1 year earlier, follow-up coincided with pre-legislation collections in Wales and NI, which replicated the Scottish study. Consent was sought via a letter to the headteacher of each school. Parents or guardians were sent a letter and information sheet and asked to inform the school if they did not wish their child to participate. Students completed questionnaires, with researchers collecting anonymous saliva samples during or after completion of questionnaires, using cotton-wool swabs of a Salivette®. All samples were processed in the same laboratory. The studies received approval from the University of Edinburgh's School of Education Ethics Committee, Cardiff University School of Social Sciences Research Ethics Committee and NI Office of Research Ethics. The pooled data set retained items whose wording and response options were identical in each country, with variables recreated from raw data.
Statistical analysis
As almost 40% of children, and most from some sub-groups, provided samples containing no detectable cotinine, adoption of linear regression would require imputed values to be used for an excessively large proportion of cases. Hence, data were divided into categories, with multinomial regressions, as adopted in Wales,6 favoured over the linear approach adopted in Scotland,13 where fewer concentrations lay <0.1 ng/ml. Concentrations below the limit of detection (0.1 ng/ml) were considered ‘low’, 0.1–0.5 ng/ml ‘medium’ and above 0.5 ng/ml ‘high’ (to create approximately equal tertiles). The terms ‘low’, ‘medium’ and ‘high’ refer to the position of the child's cotinine value in the distribution, rather than associated risk. Medium was the base category, allowing examination of whether movement from the middle of the distribution down was matched by movement from the top to the middle. Proportions of children with each FAS score providing samples above a range of cotinine cut-points before and after legislation are presented graphically to illustrate the change in socioeconomic gradients, and an FAS × survey year interaction term entered into regression models.
Further multinomial regression models examined the change in home-smoking restrictions, with full restriction the base category. Binary logistic regression models examined car-based smoking. Relative risk ratios (multinomial models) and odds ratios (binary models) are presented along with 95% confidence intervals. Models are presented for each country and the combined sample. All models adjust for age. Combined analyses include dummy variables to adjust for country. Percentages drawn from the combined sample are weighted to account for differential sampling fractions across the three countries. Svy settings of Stata 11 were used to account for clustering.
Results
Response rates
Country-specific response rates are reported elsewhere.8,13,14 Of 586 schools approached, 320/304 (54/51%) participated at baseline/follow-up. Participating schools within each country were representative in terms of the percentage of children entitled to free school meals (a common marker of socioeconomic deprivation). Within these schools, 5946/7311 (81%) pupils at baseline and 5803/7044 (82%) at follow-up provided useable saliva samples and completed the survey. After excluding children in lower year groups (n = 100), with cotinine concentration >15ng/ml (n = 80; range 15–383.9 ng/ml), self-reported smokers (n = 157), children who did not complete the smoking question (n = 83), did not report living either with their father and mother, in a single-parent family or in a step-family (n = 215), or from schools who participated at baseline only (n = 245), the data set comprised 10 867 children (5347 baseline/5520 follow-up).
Sample description
Children averaged 11.2 (SD = 0.40) years at both time points, with 50.0% (n = 2672) boys at baseline and 50.3% (n = 2775) at follow-up. Most reported living with their father and mother (73.8%; n = 3942 baseline, 74.0%; n = 4065 at follow-up). The only characteristic varying significantly between survey years was SES, with affluence slightly higher at follow-up.
Cotinine concentrations
Percentages of children with undetectable concentrations increased from 31.0 (n = 1715) to 41.0% (n = 2251) following legislation overall, and from 20.1 to 34.2, 44.9 to 51.0 and from 38.6 to 42.9% in Scotland, Wales and NI, respectively. This increase at the bottom of the distribution was accompanied largely by decreases in samples containing 0.1–0.5 ng/ml of cotinine, with smaller declines observed in the percentages of samples containing higher levels of cotinine (see Fig. 1). Regression analysis indicated that the relative risk of children's samples containing no detectable cotinine increased significantly following legislation (Table 1). The relative risk of providing a sample containing a ‘high’ cotinine concentration also increased significantly, reflecting the failure of higher concentrations to decline to the same extent as more moderate exposure levels. Country-specific models indicate that this non-linear decline occurred in all countries. Increases in the likelihood of children providing samples with no detectable cotinine remain significant after entry of terms for parental smoking and private smoking restrictions (Model 2; Table 1).
Fig. 1.
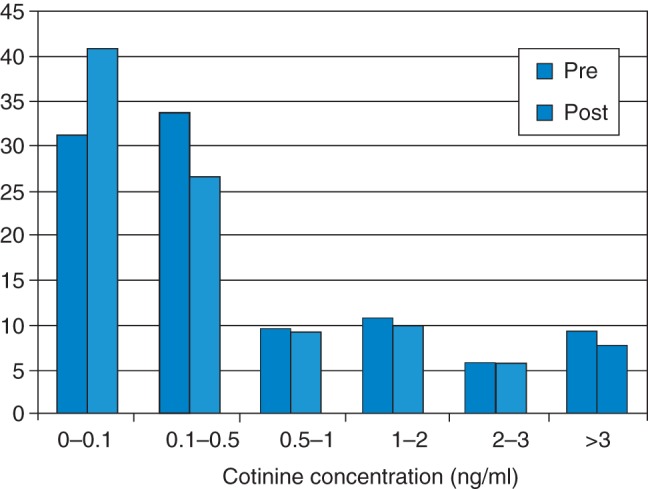
Weighted percentages of children with each level of cotinine (ng/ml), pre- and post-legislation amongst 10–11-year-old children in Scotland, Wales and NI combined (n = 10867).
Table 1.
Relative risk ratios from multinomial logistic regression indicating the change in the risk of providing a sample with no detectable cotinine (low vs. medium cotinine) or >0.5 ng/ml of cotinine (high vs. medium cotinine) relative to the likelihood of a sample containing 0.1–0.5 ng/ml
|
Scotland |
Wales |
NI |
Alla |
|||||
|---|---|---|---|---|---|---|---|---|
| Low vs. medium cotinine | High vs. medium cotinine | Low vs. medium cotinine | High vs. medium cotinine | Low vs. medium cotinine | High vs. medium cotinine | Low vs. medium cotinine | High vs. medium cotinine | |
| Model 1 | n = 4245 | n = 2654 | n = 3712 | n = 10 611 | ||||
| Main effects (Step 1) | ||||||||
| Survey yearb | 2.28 (1.83–2.86) | 1.22 (1.04–1.43) | 1.36 (1.12–1.65) | 1.15 (0.93–1.41) | 1.36 (1.15–1.60) | 1.36 (1.14–1.62) | 1.63 (1.45–1.83) | 1.25 (1.13–1.39) |
| FASc | 1.14 (1.08–1.19) | 0.78 (0.75–0.81) | 1.16 (1.10–1.22) | 0.82 (0.76–0.88) | 1.14 (1.09–1.20) | 0.79 (0.74–0.83) | 1.14 (1.11–1.17) | 0.79 (0.77–0.81) |
| Interaction effects (Step 2) | ||||||||
| FAS by survey year | 1.08 (1.00–1.18) | 1.03 (0.95–1.12) | 1.11 (0.99–1.24) | 1.03 (0.91–1.17) | 1.11 (1.01–1.21) | 1.10 (0.99–1.22) | 1.10 (1.05–1.16) | 1.05 (0.99–1.11) |
| Model 2d | n = 4125 | n = 2520 | n = 3616 | n = 10261 | ||||
| Main effects (Step 1) | ||||||||
| Survey year | 2.34 (1.87–2.93) | 0.95 (0.78–1.16) | 1.38 (1.10–1.73) | 1.18 (0.92–1.52) | 1.42 (1.18–1.70) | 1.41 (1.17–1.70) | 1.70 (1.50–1.93) | 1.16 (1.03–1.31) |
| FAS | 1.09 (1.03–1.15) | 0.92 (0.87–0.97) | 1.11 (1.04–1.18) | 0.89 (0.82–0.97) | 1.11 (1.05–1.17) | 0.85 (0.80–0.91) | 1.10 (1.06–1.13) | 0.89 (0.86–0.92) |
| Parentale smoking | ||||||||
| Father only | 0.51 (0.36–0.73) | 2.53 (1.94–3.30) | 0.34 (0.24–0.48) | 2.13 (1.51–3.02) | 0.35 (0.27–0.44) | 2.22 (1.68–2.93) | 0.39 (0.33–0.47) | 2.39 (2.02–2.82) |
| Mother only | 0.45 (0.29–0.71) | 8.28 (6.46–10.62) | 0.21 (0.13–0.34) | 4.68 (3.28–6.68) | 0.25 (0.18–0.36) | 6.02 (4.57–7.93) | 0.28 (0.22–0.36) | 6.48 (5.51–7.62) |
| Both | 0.24 (0.12–0.48) | 11.81 (8.41–16.59) | 0.21 (0.09–0.47) | 12.09 (8.08–18.08) | 0.16 (0.09–0.28) | 8.79 (6.52–11.83) | 0.19 (0.13–0.27) | 10.78 (8.90–13.05) |
| Interaction effects (Step 2) | ||||||||
| FAS by survey year | 1.09 (1.00–1.20) | 1.01 (0.91–1.12) | 1.16 (1.02–1.33) | 1.04 (0.90–1.20) | 1.14 (1.04–1.26) | 1.06 (0.94–1.19) | 1.13 (1.06–1.20) | 1.02 (0.96–1.09) |
Significant relative risk ratios (at the 95% level) are highlighted in bold. All models are adjusted for age.
aModels from the combined sample are adjusted for country.
bPre-legislation = 0, post-legislation = 1.
cHigher FAS score = higher socioeconomic status.
dModel 2 adjusts also for smoking restriction level in homes and cars (estimates not shown).
eReference category is ‘neither parent figure smokes’.
Patterning in SHS exposure, and post-legislation changes in exposure, by SES
The relative risk of children's samples containing no detectable cotinine increased significantly as SES increased (Table 1), whilst the relative risk of samples containing a ‘high’ cotinine concentration fell. These associations were almost identical in all countries, remaining significant after entry of terms for parental smoking and private smoking restrictions (Table 1; Model 2).
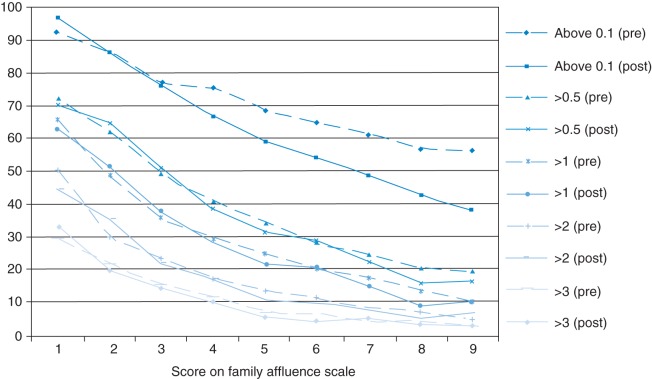
Figure 2 shows the percentage of children with each FAS score providing samples with cotinine levels above a range of thresholds, before and after legislation. Before legislation almost all children from the poorest families provided samples with a detectable level of cotinine (92.7%), whilst substantially fewer children from the most affluent families did (56.6%). However, this inequality appears to have widened following legislation, with percentages of samples above the limit of detection ranging from 96.9 to 38.2% for the least and most affluent children, respectively, after legislation. Gradients for higher exposure levels remained relatively unchanged. For example, at both time points, the percentage of children from the poorest families providing samples containing >3 ng/ml of cotinine, is ∼10 times higher than amongst the most affluent group (33.3 vs. 3.0% post-legislation).
Fig. 2.
Weighted percentage of children providing samples containing cotinine above a range of cutpoints (ng/ml) before and after legislation by score on the family affluence scale (n = 10587).
Entry of an FAS × survey year interaction term into Model 1 (Table 1) indicates significantly increased inequality in the likelihood of a child providing samples with no detectable cotinine for the combined sample. Trends toward widening inequality in the likelihood of a child's sample containing no detectable cotinine were observed for individual countries, though lay near the border of significance (Scotland P = 0.06, Wales P = 0.07, NI P = 0.03). After adjustment for parental smoking and private smoking restrictions, a significant interaction remains (Model 2). There was no significant change in inequality in the relative risk of providing samples with ‘high’ cotinine levels in any country.
Private smoking restrictions
Smoking in the home
Before legislation, 51.9% (n = 2499) of children reported living in homes where smoking was not allowed, while 31.3% (n = 1509) reported partial restrictions and 16.8% (n = 805) that smoking was allowed throughout. Following legislation, the proportion of children reporting no home smoking restrictions fell to12.7% (n = 612) accompanied by slight increases in full (55.1%; n = 2705) and partial (32.2%; n = 1642) restrictions. Percentages reporting no smoking restrictions fell from 18.5 to 14.2, from 14.2 to 11.4 and from 16.4 to 11.0% in Scotland, Wales and NI, respectively, whilst percentages reporting full smoking restrictions increased from 47.3 to 51.2, from 60.0 to 63.2 and from 51.7 to 52.4%. Among children of smokers, 18.3% (n = 370) reported that smoking was not allowed in their home before legislation, with 45.5% (n = 892) reporting partial restrictions and 36.2% (n = 687) that smoking was allowed throughout their home. After legislation, the percentage of these children reporting no restrictions fell to 27.4% (n = 524), accompanied by an increase in partial restrictions to 53.3% (n = 1087), and a small increase in the prevalence of full restrictions increasing (n = 404; 19.3%). Regression analyses indicate that overall, the relative risk of reporting unrestricted smoking, rather than full smoking restrictions, decreased significantly following legislation, whilst the relative risk of partial restrictions did not change (see Table 2). Decreased risk of unrestricted smoking remained significant after adjustment for parents' smoking (Model 2), whilst only in Wales did decreased risk of unrestricted smoking fail to reach significance in either model.
Table 2.
Relative risk ratios and odds ratios (and 95% CIs) from multinomial and binary logistic regression models showing the likelihood of a child reporting partial or no smoking restrictions relative to the likelihood of reporting full restrictions and the odds of smoking being allowed in the car
|
Scotland (n = 3894) |
Wales (n = 2358) |
NI (n = 3391) |
Alla (n = 9643) |
|||||
|---|---|---|---|---|---|---|---|---|
| Partial vs. full | None vs. full | Partial vs. full | None vs. full | Partial vs. full | None vs. full | Partial vs. full | None vs. full | |
| Smoking in the home (Model 1) | ||||||||
| Main effects (Step 1) | ||||||||
| Surveyb year | 0.96 (0.83–1.10) | 0.76 (0.62–0.93) | 0.96 (0.79–1.16) | 0.79 (0.60–1.04) | 1.17 (1.00–1.38) | 0.71 (0.57–0.89) | 1.04 (0.95–1.15) | 0.74 (0.65–0.84) |
| FASc | 0.79 (0.76–0.83) | 0.70 (0.66–0.74) | 0.83 (0.78–0.88) | 0.73 (0.67–0.79) | 0.86 (0.82–0.90) | 0.78 (0.73–0.83) | 0.81 (0.79–0.84) | 0.73 (0.70–0.76) |
| Interaction effects (Step 2) | ||||||||
| FAS × year | 0.93 (0.87–1.00) | 1.00 (0.90–1.10) | 0.96 (0.87–1.06) | 0.99 (0.86–1.14) | 1.07 (0.98–1.17) | 1.06 (0.95–1.19) | 0.98 (0.94–1.03) | 1.02 (0.96–1.09) |
| Scotland (n = 3803) | Wales (n = 2275) | NI (n = 3320) | All (n = 9398) | |||||
| Smoking in the home (Model 2, main effects only) | ||||||||
| Survey year | 0.84 (0.71–0.99) | 0.59 (0.46–0.75) | 0.98 (0.80–1.20) | 0.82 (0.59–1.14) | 1.12 (0.92–1.36) | 0.64 (0.49–0.84) | 0.97 (0.87–1.08) | 0.66 (0.55–0.77) |
| FAS | 0.84 (0.80–0.88) | 0.78 (0.72–0.83) | 0.89 (0.84–0.95) | 0.82 (0.75–0.90) | 0.93 (0.89–0.98) | 0.90 (0.85–0.97) | 0.88 (0.86–0.91) | 0.83 (0.79–0.86) |
| Parentald smoking | ||||||||
| Father only | 5.90 (4.44–7.86) | 20.47 (14.16–29.60) | 6.17 (4.50–8.44) | 17.92 (9.87–32.54) | 4.91 (3.74–6.44) | 8.76 (5.96–12.86) | 5.50 (4.66–6.49) | 14.24 (11.23–18.05) |
| Mother only | 14.24 (10.79–18.79) | 60.90 (42.21–87.84) | 14.97 (10.58–21.19) | 67.68 (37.74–121.38) | 8,91 (6.79–11.70) | 26.02 (17.88–37.88) | 11.82 (9.95–14.05) | 43.66 (34.22–55.69) |
| Both | 16.53 (11.66–23.44) | 92.14 (61.36–138.36) | 22.78 (15.41–33.65) | 140.03 (72.52–270.39) | 12.63 (9.36–17.04) | 31.29 (20.74–47.19) | 16.22 (13.30–19.78) | 68.48 (52.52–89.30) |
| Scotland (n = 3345) | Wales (n = 2066) | NI (n = 3022) | All (n = 8433) | |||||
| Smoking in cars (Model 1) | ||||||||
| Main effects (Step 1) | ||||||||
| Survey year | 0.88 (0.76–1.03) | 0.90 (0.72–1.12) | 0.88 (0.74–1.04) | 0.89 (0.80–0.98) | ||||
| FAS | 0.79 (0.75–0.83) | 0.80 (0.75–0.85) | 0.85 (0.81–0.90) | 0.82 (0.79–0.84) | ||||
| Interaction effects (Step 2) | ||||||||
| FAS × year | 1.04 (0.93–1.15) | 0.93 (0.83–1.05) | 1.04 (0.95–1.14) | 1.02 (0.96–1.08) | ||||
| Scotland (n = 3263) | Wales (n = 1993) | NI (n = 2960) | All (n = 8216) | |||||
| Smoking in cars (Model 2, main effects only) | ||||||||
| Survey year | 0.81 (0.66–0.99) | 0.98 (0.74–1.29) | 0.80 (0.65–0.99) | 0.84 (0.74–0.95) | ||||
| FAS | 0.88 (0.83–0.94) | 0.89 (0.82–0.96) | 0.92 (0.87–0.99) | 0.90 (0.87–0.93) | ||||
| Parental smoking | ||||||||
| Father only | 16.30 (12.27–22.63) | 16.89 (11.72–24.36) | 13.62 (10.44–17.77) | 15.14 (12.80–17.91) | ||||
| Mother only | 18.36 (13.79–24.43) | 23.40 (16.13–33.97) | 11.83 (9.11–15.36) | 16.00 (13.46–19.01) | ||||
| Both | 32.51 (23.87–44.30) | 55.50 (36.24–85.01) | 20.36 (15.35–27.02) | 30.51 (25.30–36.79) | ||||
Significant relative risk ratios and odds ratios (at the 95% level) are highlighted in bold. All models are adjusted for age.
aModels from the combined sample are adjusted for country.
bPre-legislation = 0, post-legislation = 1.
cHigher FAS score = higher socioeconomic status.
dReference category is ‘neither parent figure smokes’.
Smoking in cars
Approximately 28.5% (n = 1225) of children (29.1% in Scotland, 25.4% in Wales and 32.0% in NI) reported that smoking was allowed inside their car before legislation, declining to 25.7% (n = 1148) after legislation (and to 26.1, 22.7 and 29.1% in Scotland, Wales and NI, respectively). Regression analyses indicate that whilst in no individual country did this reduction reach statistical significance, for the combined data set, this was significant (see Table 2). After adjustment for parental smoking, declines in Scotland, NI as well as the combined sample were significant (Model 2; Table 2).
Patterning in private smoking restrictions, and post-legislation changes, by SES
In all countries, and the combined data set, as SES increased, the likelihood of partial or no home smoking restrictions (rather than full smoking restrictions), decreased significantly, whilst the odds of smoking being allowed inside the family car also decreased significantly (Table 2). These trends remained after adjustment for parental smoking (Model 2). Entry of an interaction term for FAS × survey year into models for home and car-based smoking restrictions indicated no change in inequality following legislation. Following legislation, 26.3% of children scoring 1 on FAS reported living in a fully smoke-free home, climbing to 72.0% for those scoring 9. Percentages reporting that smoking was not allowed in their car ranged from 51.7 (least affluent) to 83.0% (most affluent).
Discussion
Main findings of this study
Following legislation, proportions of children providing samples containing no detectable cotinine increased in all countries, though little change occurred at higher ends of the distribution. Furthermore, socioeconomic inequality in the likelihood of a child's sample containing detectable traces of cotinine increased. Hence, declines in exposure occurred predominantly among children with low exposure before legislation, and from more affluent families. Substantial socioeconomic gradients in proportions of children with higher SHS exposure levels remained unchanged.
Overall, declines in the percentage of children reporting that smoking was allowed throughout their home were observed following legislation. However, among children of smokers, full smoking restrictions increased by only 1%, with reductions in the percentage of children reporting no home smoking restrictions matched largely by increases in partial restrictions. Small declines in reports that smoking was allowed in their family's car were also observed, reaching significance in the pooled sample.
Nevertheless, after legislation little more than half of children, including only one in five children living with smoking parents, reported that smoking was not allowed in their home at all, while ∼1 in 4 children whose family owned a car reported that smoking was allowed in their car. While post-legislation changes in smoking restrictions in cars or homes were not patterned by socioeconomic status, with no suggestion of displacement of smoking into the home across the SES spectrum, smoking restrictions in private spaces remain substantially more common among children from higher SES families.
What is already known on this topic
In all UK countries, childhood SHS exposure decreases as socioeconomic status increases.5–8 In no UK countries, and for no SES subgroups, did childhood SHS exposure increase following legislation.8,13–15 However, there is growing evidence that the greatest post-legislation declines in SHS exposure occurred amongst children at least risk.13,14 There is some previous evidence that adoption of smoke-free homes increased following legislation.18
What this study adds
While no longitudinal trend data are available for UK countries in this study, the post-legislation increases in inequality in SHS exposure run counter to trends for greater declines amongst those most exposed reported in the years leading up to legislation in England.15 There was no significant change in inequality in relation to proportions of children with higher levels of SHS exposure. However, following legislation, ∼1 in 12 children provided saliva samples containing more cotinine than samples collected from Scottish bar workers prior to smoke-free legislation (2.94 ng/ml),28 including almost 1 in 3 children from the poorest families (10 times the proportion among children at the opposite end of the socio-economic spectrum). Hence, while the growing proportions of children providing samples with no detectable cotinine are encouraging, sustained emphasis on reducing SHS exposure amongst the sizeable minority continuing to have high levels of exposure remains a priority in reducing health inequalities. As argued by Frohlich and Potvin,29 where unequal responses to whole-population approaches lead to an exacerbation of inequality, there is a strong case for complementary intervention to address the needs of ‘vulnerable’ populations, such as children from lower SES families.
Despite small overall increases in adoption of smoke-free homes, the home remains a key source of SHS exposure for many children, with persistent inequalities in the prevalence of restrictions on smoking in homes. Hence, efforts to encourage smoke-free homes are crucial in attempting to reduce health inequalities. Legislation against smoking in the home is unlikely to prove acceptable,30 and reducing inequalities in exposure may require intervention to support adoption of voluntary smoking restrictions in lower SES households.31,32 However, extending legislation to cars carrying children, as introduced in Australia,33 and for which there appears to be significant public support in the UK,34,35 may contribute to reducing inequalities.
Limitations of this study
The study brings together three large data sets, harmonizing their analyses to allow comparability between three UK countries, benefitting from use of salivary cotinine as a primary outcome; a well-validated indicator of SHS exposure.25 However, our ability to attribute change to smoke-free legislation is weakened by the absence of a counterfactual. A longitudinal study may have facilitated examination of change over time, but would have made it impossible to distinguish between changes due to increased age, or due to legislation. Inclusion of a narrow age group, though enhancing internal validity, limits generalizability. Finally, reliance upon self-reports on some measures is perhaps liable to social desirability biases, although it is hoped that any error would have been equal at both time points.
Authors' roles
G.M. led the development of the paper plan, pooled the data sets, conducted statistical analysis and wrote the manuscript. D.C. and L.M. advised on statistical analysis. All authors contributed to the development of paper plans and provided comments on drafts of the manuscript.
Funding
The work was supported by the Centre for the Development and Evaluation of Complex Interventions for Public Health Improvement (DECIPHer), a UKCRC Public Health Research: Centre of Excellence. Funding from the British Heart Foundation, Cancer Research UK, Economic and Social Research Council (RES-590-28-0005), Medical Research Council, the Welsh Assembly Government and the Wellcome Trust (WT087640MA), under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged. For the original studies, including collection and processing of data samples, CHETS Wales was funded by the Welsh Government (grant 216/2005). CHETS Scotland was funded by NHS Health Scotland and the Scottish Government. CHETS NI was funded by the Department of Health, Social Services and Safety, Northern Ireland.
Acknowledgements
The CHETS Wales team would like to thank: Anna Hamilton and Hayley Collicott (study administrators), Heather Rothwell, Claire Pimm, Nancy West, Ellie Byrne, Rachel Clark, Margaret Humphries, Keith Humphries, Rosie Salazar (Research Assistants); and Janine Hale and Chris Roberts (Welsh Government, Public Health Branch, Social Research Division). The CHETS Northern Ireland team would like to thank Malcolm Buchanan for school recruitment; and the study development/implementation group (Naomi McCay, Lindsay MacDonald and Diana Gossrau-Breen). The CHETS Scotland team would like to thank the study advisory group. We would also like to thank all schools and students who participated in the research in each of the three countries, ABS laboratories (www.abslabs.com) who analysed salivary cotinine collected in all countries, and MVA Consultancy for managing the fieldwork in the Scotland and Northern Ireland arms of the study.
References
- 1.Vork KL, Broadwin RL, Blaisdell RJ. Developing asthma in childhood from exposure to secondhand tobacco smoke: insights from a meta-regression. Environ Health Perspect. 2007;115(10):1394–400. doi: 10.1289/ehp.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pattenden S, Antova T, Neuberger M, et al. Parental smoking and children's respiratory health: independent effects of prenatal and postnatal exposure. Tob Control. 2006;15(4):294–301. doi: 10.1136/tc.2005.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kallio K, Jokinen E, Saarinen M, et al. Arterial intima-media thickness, endothelial function, and apolipoproteins in adolescents frequently exposed to tobacco smoke. Circ Cardiovasc Qual Outcomes. 2010;3(2):196–203. doi: 10.1161/CIRCOUTCOMES.109.857771. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organisation. WHO Framework Convention on Tobacco Control. Geneva: WHO Document Production Services; 2005. [Google Scholar]
- 5.Akhtar PC, Haw SJ, Levin KA, et al. Socioeconomic differences in second-hand smoke exposure among children in Scotland after introduction of the smoke-free legislation. J Epidemiol Community Health. 2010;64(4):341–6. doi: 10.1136/jech.2008.084178. [DOI] [PubMed] [Google Scholar]
- 6.Moore GF, Holliday JC, Moore LAR. Socioeconomic patterning in changes in child exposure to secondhand smoke after implementation of smoke-free legislation in Wales. Nicotine Tob Res. 2011;13(3):903–10. doi: 10.1093/ntr/ntr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sims M, Tomkins S, Judge K, et al. Trends in and predictors of second-hand smoke exposure indexed by cotinine in children in England from 1996 to 2006. Addiction. 2010;105(3):543–53. doi: 10.1111/j.1360-0443.2009.02805.x. [DOI] [PubMed] [Google Scholar]
- 8.Health Promotion Agency. Childhood Exposure to Tobacco Smoke (CHETS) in Northern Ireland. Belfast: Health Promotion Agency for Northern Ireland; 2009. [Google Scholar]
- 9.Haw SJ, Gruer L. Changes in exposure of adult non-smokers to secondhand smoke after implementation of smoke-free legislation in Scotland: national cross sectional survey. BMJ. 2007;335(7619):549–52. doi: 10.1136/bmj.39315.670208.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allwright S, Paul G, Greiner B, et al. Legislation for smoke-free workplaces and health of bar workers in Ireland: before and after study. BMJ. 2005;331(7525):1117–22. doi: 10.1136/bmj.38636.499225.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho SY, Wang MP, Lo WS, et al. Comprehensive smoke-free legislation and displacement of smoking into the homes of young children in Hong Kong. Tob Control. 2010;19(2):129–33. doi: 10.1136/tc.2009.032003. [DOI] [PubMed] [Google Scholar]
- 12.Adda J, Cornaglia FJ. The effect of taxes and bans on passive smoking. 2006. IZA Discussion Paper No. 2191 Available at SSRN http://ssrn.com/abstract=919963 . Last accessed December 2011.
- 13.Akhtar PC, Currie DB, Currie CE, et al. Changes in child exposure to environmental tobacco smoke (CHETS) study after implementation of smoke-free legislation in Scotland: national cross sectional survey. BMJ. 2007;335(7619):545–9. doi: 10.1136/bmj.39311.550197.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holliday JC, Moore GF, Moore L. Changes in child exposure to secondhand smoke after implementation of smoke-free legislation in Wales: a repeated cross-sectional study. BMC Public Health. 2009;9(1):430. doi: 10.1186/1471-2458-9-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarvis MJ, Sims M, Gilmore A, et al. Impact of smoke-free legislation on children's exposure to secondhand smoke: cotinine data from the Health Survey for England. Tob Cont. 2012;21:18–23. doi: 10.1136/tc.2010.041608. [DOI] [PubMed] [Google Scholar]
- 16.Dove MS, Dockery DW, Connolly GN. Smoke-free air laws and secondhand smoke exposure among nonsmoking youth. Pediatrics. 2010;126(1):80–7. doi: 10.1542/peds.2009-3462. [DOI] [PubMed] [Google Scholar]
- 17.Department of Health. A Smokefree Future: A Comprehensive Tobacco Control Strategy for England. London: Department of Health; 2010. [Google Scholar]
- 18.Akhtar PC, Haw SJ, Currie DB, et al. Smoking restrictions in the home and secondhand smoke exposure among primary schoolchildren before and after introduction of the Scottish smoke-free legislation. Tob Control. 2009;18(5):409–15. doi: 10.1136/tc.2009.030627. [DOI] [PubMed] [Google Scholar]
- 19.British Medical Association Board of Science. Smoking in Vehicles: A Briefing from the Board of Science. London, British Medical Association; 2011. [Google Scholar]
- 20.Belanger M, O'Loughlin J, Okoli CTC, et al. Nicotine dependence symptoms among young never-smokers exposed to secondhand tobacco smoke. Addict Behav. 2008;33(12):1557–63. doi: 10.1016/j.addbeh.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitchman SC, Fong GT, Borland R, et al. Predictors of smoking in cars with nonsmokers: findings from the 2007 Wave of the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2010;12(4):374–80. doi: 10.1093/ntr/ntq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borland R, Yong HH, Cummings KM, et al. Determinants and consequences of smoke-free homes: findings from the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15:42–50. doi: 10.1136/tc.2005.012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norman GJ, Ribisl KM, Howard-Pitney B, et al. Smoking bans in the home and car: do those who really need them have them? Prev Med. 1999;29(6):581–9. doi: 10.1006/pmed.1999.0574. [DOI] [PubMed] [Google Scholar]
- 24.West R, Zatonski W, Przewozniak K, et al. Can we trust national smoking prevalence figures? Discrepancies between biochemically assessed and self-reported smoking rates in three countries. Cancer Epidemiol Biomarker Prev. 2007;16(4):820–2. doi: 10.1158/1055-9965.EPI-06-0679. [DOI] [PubMed] [Google Scholar]
- 25.Dolcini MM, Adler NE, Lee P, et al. An assessment of the validity of adolescent self-reported smoking using three biological indicators. Nicotine Tob Res. 2003;5(4):473–83. [PubMed] [Google Scholar]
- 26.Feyerabend C, Russell M. A rapid gas-liquid chromatographic method for the determination of cotinine and nicotine in biological fluids. J Pharm Pharmacol. 1990;42:450–2. doi: 10.1111/j.2042-7158.1990.tb06592.x. [DOI] [PubMed] [Google Scholar]
- 27.Currie C, Molcho M, Boyce W, et al. Researching health inequalities in adolescents: the development of the Health Behaviour in School-Aged Children (HBSC) Family Affluence Scale. Soc Sci Med. 2008;66(6):1429–36. doi: 10.1016/j.socscimed.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Semple S, Maccalman L, Naji AA, et al. Bar workers’ exposure to second-hand smoke: the effect of Scottish smoke-free legislation on occupational exposure. Ann Occup Hyg. 2007;51:571–80. doi: 10.1093/annhyg/mem044. [DOI] [PubMed] [Google Scholar]
- 29.Frohlich KL, Potvin L. The inequality paradox: the population approach and vulnerable populations. Am J Public Health. 2008;98(2):216–21. doi: 10.2105/AJPH.2007.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman S. The future of smoke-free legislation. BMJ. 2007;335(7619):521–2. doi: 10.1136/bmj.39315.616169.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priest N, Roseby R, Waters E, et al. Family and carer smoking control programmes for reducing children's exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD001746.pub2. CD001746. [DOI] [PubMed] [Google Scholar]
- 32.Ritchie D, Amos A, Phillips R, et al. Action to achieve smoke-free homes—an exploration of experts’ views. BMC Public Health. 2009;9(1):112. doi: 10.1186/1471-2458-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman B, Chapman S, Storey P. Banning smoking in cars carrying children: an analytical history of a public health advocacy campaign. Aus NZ J Public Health. 2008;32(1):60–5. doi: 10.1111/j.1753-6405.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 34.Hitchman SC, Fong GT, Zanna MP, et al. Support and correlates of support for banning smoking in cars with children: findings from the ITC Four Country Survey. Eur J Public Health. 2011;21(3):360–5. doi: 10.1093/eurpub/ckq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson G, Wilson N. Public attitudes to laws for smoke-free private vehicles: a brief review. Tob Control. 2009;18(4):256–61. doi: 10.1136/tc.2008.027672. [DOI] [PubMed] [Google Scholar]