Abstract
Background
Tbx2, Tbx3, Tbx4, and Tbx5, members of the Tbx2 subfamily of T-box transcription factor genes, are important for many aspects of embryonic development and mutations in some human TBX2 subfamily genes cause developmental syndromes. In addition, TBX2 and TBX3 are overexpressed in a variety of cancers, including reproductive system cancers. This study characterizes the expression of Tbx2 subfamily genes during development of the reproductive system.
Results
We show that these genes are expressed in both the internal and external reproductive systems. Tbx2 is expressed in gonads and genital ducts, the Wolffian and Müllerian ducts, while Tbx3 is only expressed in genital ducts. Tbx4 is expressed in embryonic and postnatal germ cells. All four genes are expressed in mesenchyme in external genitalia, with Tbx3 and Tbx5 expression in the epithelium as well.
Conclusion
This study lays the foundation for investigation of functional requirements for Tbx2 subfamily genes in development of the mammalian reproductive system.
Keywords: Tbx2; Tbx3; Tbx4; Tbx5; T-box; testis, ovary; Wolffian; Müllerian; genital tubercle
INTRODUCTION
The Tbx2 subfamily of the T-box transcription factor genes plays a critical role in the determination of cell fate decisions during organogenesis and in the development and differentiation of many organ systems (Naiche et al., 2005; Wardle and Papaioannou, 2008; Conlon and Yutzey, 2010). The highly conserved T-box DNA binding motif is common to all members of the T-box gene family. The Tbx2 subfamily consists of Tbx2, Tbx3, Tbx4, and Tbx5. Tbx2 and Tbx3 derive from a common ancestral gene, as do Tbx4 and Tbx5 (Holland et al., 1994; Agulnik et al., 1996; Ruvinsky and Silver, 1997). Mutations in these genes are associated with developmental defects in both mice and humans.
Targeted mutagenesis in mice to produce homozygous disruption of Tbx2 (Harrelson et al., 2004), Tbx3 (Davenport et al., 2003; Mesbah et al., 2008), Tbx4 (Naiche and Papaioannou, 2003), or Tbx5 (Bruneau et al., 2001) results in embryonic lethality. Tbx2 and Tbx5 null embryos die during midgestation due to cardiovascular defects (Bruneau et al., 2001; Harrelson et al., 2004; Suzuki et al., 2004). Heterozygous Tbx3 mutant mice are fertile, but all adult females have a split clitoris and some do not possess a vaginal opening (Davenport et al., 2003). Homozygous null Tbx3 mutants die by embryonic day (E) 16.5 with yolk sac, limb, mammary gland, and heart abnormalities (Davenport et al., 2003). In homozygous null Tbx4 mutants, lack of chorio-allantoic fusion, which prevents formation of the umbilical vessels, results in death by E10.5 (Naiche and Papaioannou, 2003).
While there are no documented human developmental syndromes associated with mutations in TBX2, mutations causing heterozygous loss of TBX3, TBX4, or TBX5 in humans result in ulnar-mammary (Bamshad et al., 1995, 1997), small patella (Bongers et al., 2004), or Holt-Oram (Basson et al., 1997; Li et al., 1997) syndromes, respectively. The phenotype of humans affected with ulnar-mammary syndrome (UMS) is similar to, although less severe than, that observed in homozygous null Tbx3 mutant embryos (Davenport et al., 2003). Humans with UMS have abnormal development of the ulnar aspect of the hand and/or forearm, breasts, teeth, and external genitalia. UMS may also result in delayed onset of puberty in males (Schinzel, 1987; Schinzel et al., 1987; Franceschini et al., 1992; Davenport et al., 2003). The similarities between mice and humans underlie the importance of the Tbx2 subfamily of T-box genes in mammalian development and strongly suggest conservation of function among different species.
In addition to critical roles in development, two members of the TBX2 subfamily, TBX2 and TBX3, are overexpressed in human cancers, including those of the reproductive system. Increased TBX2 and TBX3 expression was detected in ovarian, uterine, cervical, and breast cancers (Jacobs et al., 2000; Sinclair et al., 2002; Adem et al., 2004; Lomnytska et al., 2006; Lyng et al., 2006; Liu et al., 2010a,b). Although TBX2 protein was detectable in only 2% of normal uterine endometrial or cervical tissue samples, more than 50% of endometrioid endometrial adenocarcinoma and squamous cell cervical carcinoma samples expressed TBX2 (Liu et al., 2010a,b). Increased expression of TBX3 was detected in plasma protein extracts from ovarian and breast cancer patients (Lomnytska et al., 2006), and TBX3 was detected in gene expression microarrays of metastatic squamous cell cervical carcinoma (Lyng et al., 2006).
The central role of Tbx2 subfamily genes in mammalian development coupled with the overexpression of TBX2 and TBX3 in tumors of the reproductive system strongly suggests that T-box genes may also be essential for development of the reproductive system and sexual differentiation. It was noted that Tbx2 and Tbx3 are expressed in the murine genital ridge and that all four Tbx2 subfamily genes are expressed in the genital papilla (Chapman et al., 1996); however detailed expression of Tbx2 subfamily genes during development of the mammalian reproductive system has not been previously reported.
In this study, we examine the spatio-temporal expression patterns of Tbx2 subfamily genes in the internal and external reproductive systems throughout organogenesis and postnatal, pre-pubertal differentiation using in situ hybridization (ISH), immunofluorescence (IF), and comparison with cell-type specific markers. Our results show that Tbx2, Tbx3, and Tbx4 are expressed in the internal reproductive system. Tbx5 expression was not detected in the internal reproductive system by ISH or RTPCR (data not shown) at any stage. Tbx2 is expressed in the interstitium of the testis and Tbx4 is expressed in germ cells in the testis and ovary. Tbx2 and Tbx3 are expressed in the genital ducts, the Wolffian (mesonephric) ducts and Müllerian (paramesonephric) ducts, and their derivatives from E9.5 to postnatal day (P) 16, with overlapping, yet distinct expression patterns. In addition, we show that all four members of the Tbx2 subfamily are expressed in the genital tubercle (GT) mesenchyme during both androgen-independent and androgen-dependent developmental phases.
RESULTS AND DISCUSSION
Expression of Tbx2 Subfamily Genes in the Urogenital Ridge
The urogenital ridges, comprised of bipotential gonads and mesonephroi, arise from the intermediate mesoderm by E9.5. Primordial germ cells, which arise in the epiblast between E7 and E7.5, migrate to and enter the gonads. By E10.5, the bipotential gonads, with germ cells and somatic cells, are detected as thickenings on the ventromedial surface of the mesonephroi (Kaufman and Bard, 1999; Staack et al., 2003).
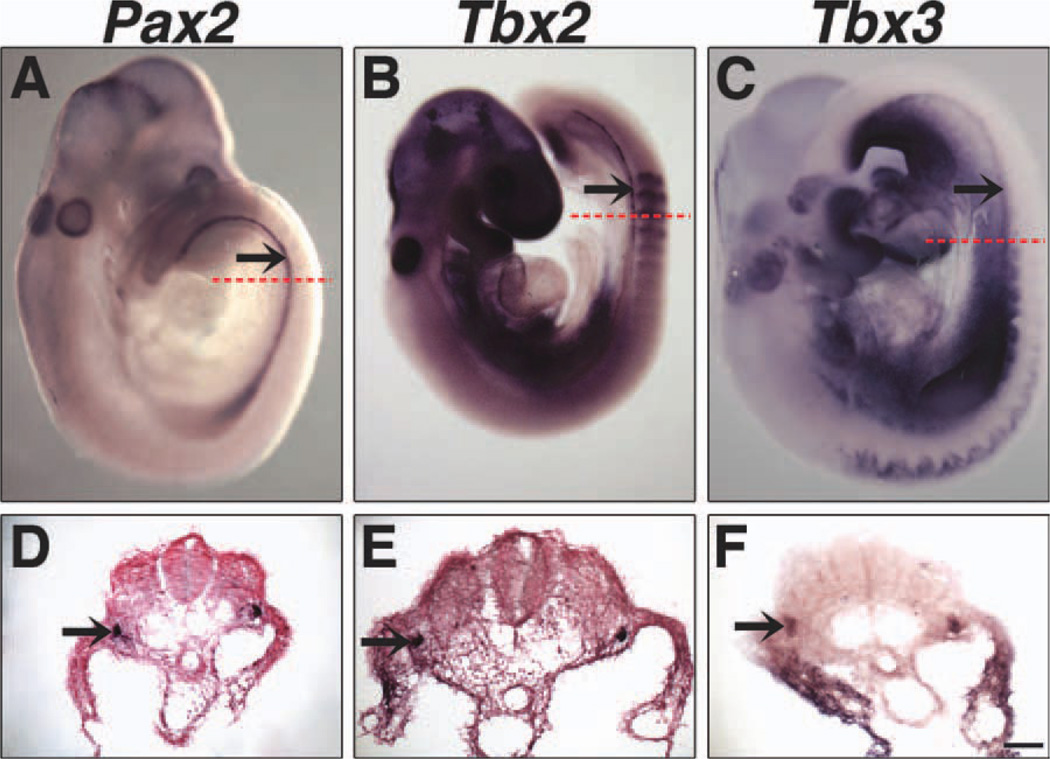
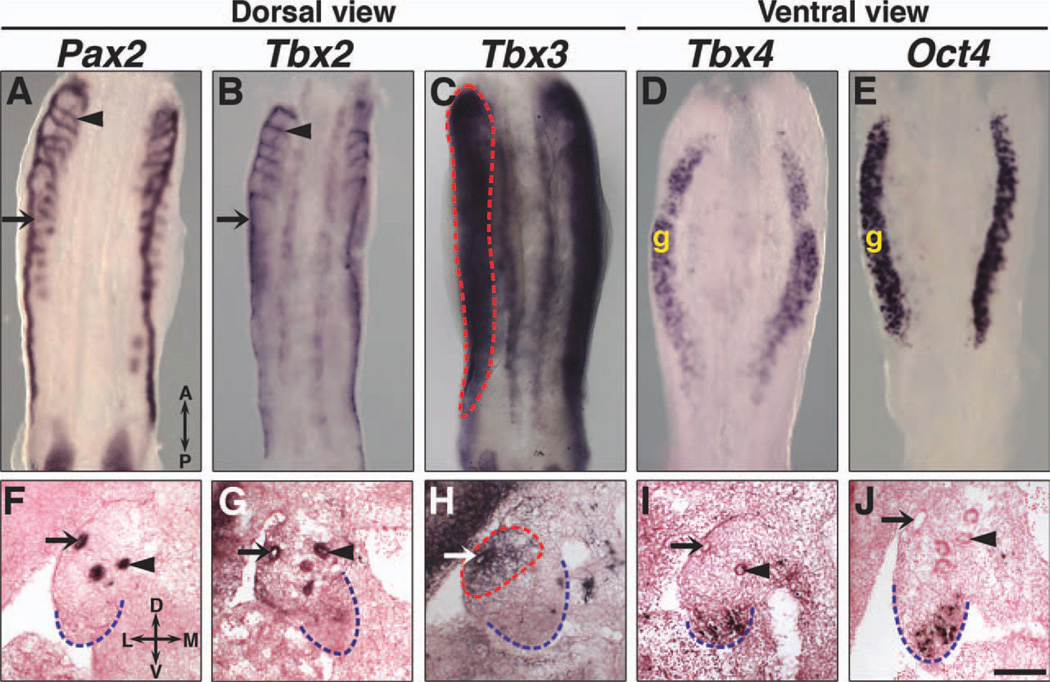
Tbx2, Tbx3, Tbx4, but not Tbx5 (data not shown) are expressed in the urogenital ridge. Tbx2 and Tbx3 expression was first detected in the epithelium of the developing Wolffian ducts at E9.5 (Fig. 1), which are marked by Pax2 expression (Torres et al., 1995). At E11.5, Tbx2 is expressed in both Wolffian ducts and cranial mesonephric tubules (Fig. 2B, G), which later become the efferent ductules between the testis and epididymis (Sainio et al., 1997). Tbx3 is expressed in the Wolffian duct epithelium and mesonephric mesenchyme (Fig. 2C, H), but not in the mesonephric tubules (data not shown). Pax2 expression in Wolffian ducts, cranial and caudal mesonephric tubules (Sainio et al., 1997) is shown for comparison (Fig. 2A, F). Whereas neither Tbx2 nor Tbx3 is expressed in the bipotential gonad (Fig. 2G, H), ventral views of urogenital ridges and transverse sections at E11.5 reveal Tbx4 expression in gonads (Fig. 2D, I) in a pattern similar to that of Oct4, which is expressed in germ cells (Urven et al., 1993) (Fig. 2E, J). The unique expression patterns of Tbx2, Tbx3, and Tbx4 in the urogenital ridges suggest that these Tbx2 subfamily genes have non-redundant roles in the development of the internal reproductive system.
Fig. 1.
Expression of Tbx2 and Tbx3 in Wolffian ducts at E9.5. Dissected whole embryos and transverse sections were hybridized with antisense Pax2 (A, D), Tbx2 (B, E), and Tbx3 (C, F) riboprobes. Pax2 expression highlights the Wolffian duct. Both Tbx2 and Tbx3 are expressed in the Wolffian duct. Arrows identify Wolffian ducts and dashed red lines indicate the level of the corresponding transverse sections. Nuclear Fast Red counter-stain was not used in F. Scale bar in F = 50 µm (D–F).
Fig. 2.
Tbx2, Tbx3, and Tbx4 expression in urogenital ridges at E11.5. Whole-mount ISH on dissected urogenital ridges, dorsal views (A–C) and ventral views (D, E), and section ISH on transverse sections are shown. Tbx2 is expressed in the epithelium of the Wolffian duct and mesonephric tubules (B, G). Tbx3 is expressed in the Wolffian duct epithelium and mesonephric mesenchyme, highlighted by dashed red lines (C, H). Tbx4 is expressed in gonads but not the Wolffian ducts or mesonephroi (D, I). Pax2 expression in the epithelium of the Wolffian duct and mesonephric tubules (A, F) and Oct4 expression in germ cells (E, J) are shown for comparison. Arrows identify Wolffian ducts and arrowheads identify mesonephric tubules. Dashed blue lines outline gonads in transverse sections. A, anterior; P, posterior; D, dorsal; L, lateral; M, medial; V, ventral; g, gonad. Scale bar in J = 100 µm (F–J).
Expression of Tbx2 Subfamily Genes in the Internal Reproductive System After Sexual Differentiation
Gonads
Bipotential gonads differentiate into testes or ovaries. Expression of Sry, the Y-linked testis-determining gene, in somatic cells of XY gonads at E10.5 results in Sox9-mediated differentiation of these bipotential cells into Sertoli cells, which then organize the testes into two main compartments, the epithelial testis cords and the mesenchymal interstitium between the testis cords. Testis cords consist of Sertoli cells and germ cells enclosed by peritubular myoid cells. The interstitium between the testis cords contains androgen-producing Leydig cells, vascular endothelial cells, fibroblasts, and hematopoietic cells (McLaren, 2000; Wilhelm et al., 2007; Sekido and Lovell-Badge, 2009). In contrast to what is seen in testes, embryonic ovaries, which also contain germ, somatic, and vascular endothelial cells, are morphologically similar to bipotential gonads. Thus, by E13.5, male and female gonads are morphologically distinct.
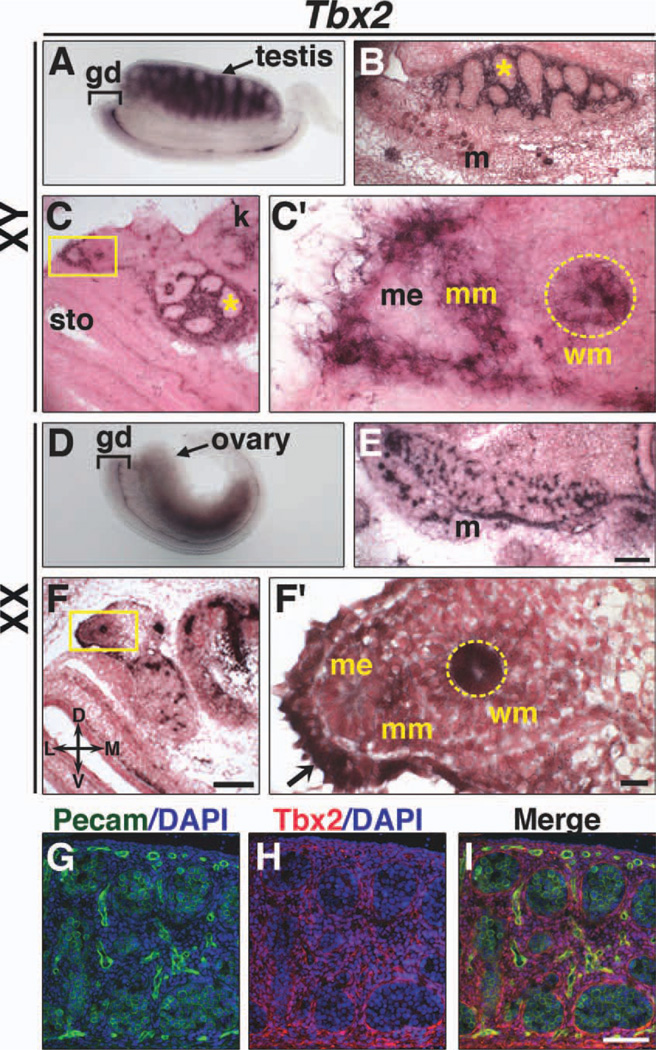
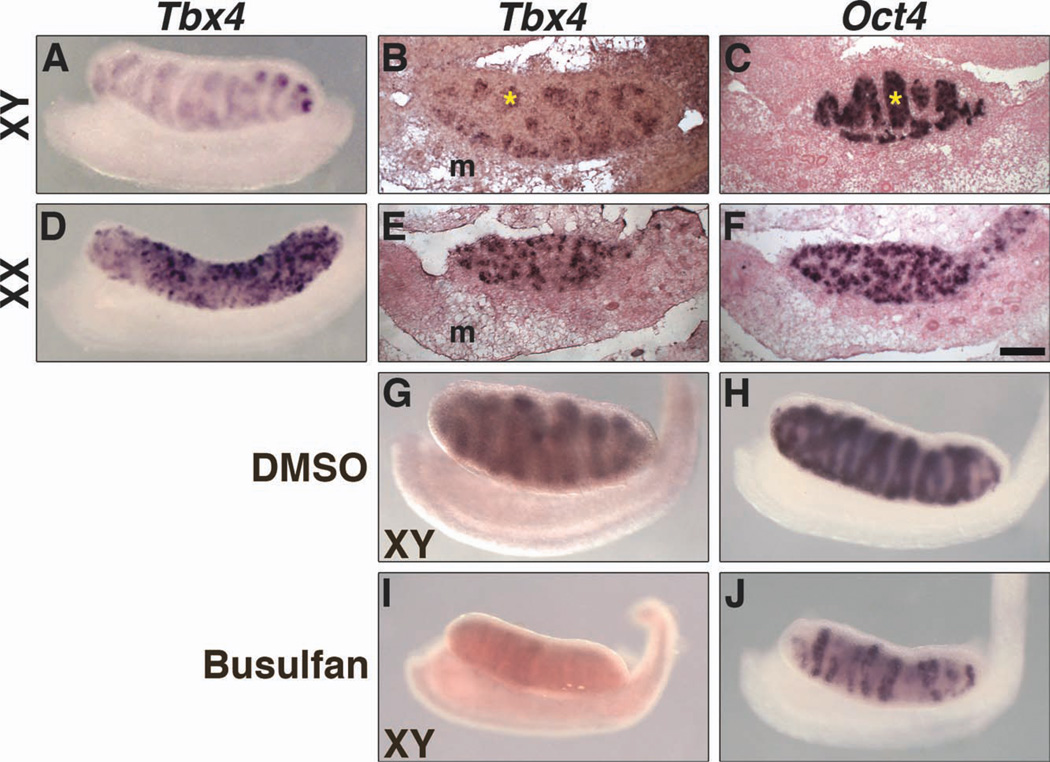
Tbx2 expression in the interstitium around testis cords (Fig. 3A–C) was observed at E13.5. Double immunofluoresence (IF) with antibodies to Tbx2 and Pecam, which labels germs cells and vascular endothelial cells (Wakayama et al., 2003), shows Tbx2 protein expressed throughout the interstitium (Fig. 3G–I). Tbx2 expression was also observed in ovaries at E13.5 (Fig. 3D–F). Neither Tbx3 (Fig. 4) nor Tbx5 (data not shown) is expressed in testes or ovaries at E13.5. Tbx4 expression was observed in both XY and XX gonads (Fig. 5). Tbx4 is expressed within testis cords (Fig. 5A, B) and throughout the ovaries (Fig. 5D, E), similar to Oct4 (Fig. 5C, F), which is expressed in germ cells. To determine if Tbx4 is expressed in embryonic germ cells, we treated pregnant female mice with busulfan and analyzed Tbx4 and Oct4 expression in the gonads of embryos from busulfan-treated mice. Busulfan is an alkylating agent that has been used to deplete germ cells and the study of gene expression in gonads from mice treated with busulfan has been previously described (Ross et al., 2007). A reduction in Oct4 expression in testis cords of E13.5 XY gonads (Fig. 5H, J) confirms depletion of germ cells after busulfan administration. Tbx4 expression was not detected in XY and was greatly reduced in XX gonads after germ cell depletion (Fig. 5G, I, data not shown for XX). These data are consistent with Tbx4 expression in XY and XX germ cells at E13.5. Expression of Tbx2 and Tbx4 does not overlap in testes. Thus, our results suggest unique roles for Tbx2 and Tbx4 in sexual differentiation of the gonads.
Fig. 3.
Expression of Tbx2 in isolated internal reproductive systems at E13.5. Whole mount ISH (A, D), section ISH on sagittal (B, E) and transverse (C, F) sections of isolated gonads, and IF (G–I) are shown. In XY embryos, Tbx2 is expressed in the interstitium around testis cords (asterisk) (A–C), Müllerian duct mesenchyme (C′), and Wolffian duct epithelium (C′, dashed yellow circle). In XX embryos, Tbx2 expression is observed throughout ovaries (D–F). Tbx2 is expressed in Wolffian duct epithelium (F′, dashed yellow circle) and coelomic epithelium (F′, arrow). Tbx2 is not expressed in Müllerian duct mesenchyme in females or Müllerian duct epithelia of either sex. Double IF (G–I) staining of sagittal sections shows Tbx2 expression in the interstitium around testis cords and not in Pecam-positive germ cells. Boxed areas in C and F are shown at higher magnification in C′ and F′. D, dorsal; L, lateral; M, medial; V, ventral; gd, genital ducts; k, kidney; m, mesonephros; me, Müllerian duct epithelium; mm, Müllerian duct mesenchyme; sto, stomach; wm; Wolffian duct mesenchyme. Scale bars in E and F = 100 µm (B, C, E, F); scale bar in F′ = 10 µm (C′, F′); scale bar in I = 50 µm (G–I).
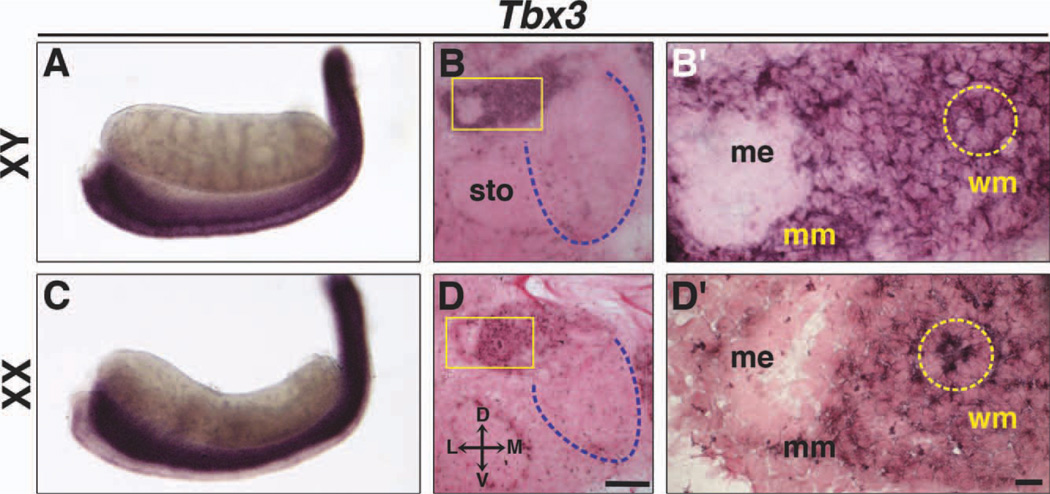
Fig. 4.
Expression of Tbx3 in Wolffian and Müllerian ducts at E13.5. Whole-mount ISH and section ISH on transverse sections of XY (A, B, B′) and XX (C, D, D′) embryos. In XY and XX embryos, Tbx3 is expressed in the Wolffian duct epithelium (dashed yellow circles) and mesenchyme around Wolffian and Müllerian ducts, but not in testes (A, B) or ovaries (C, D). Boxed areas in B and D are shown at higher magnification in B′ and D′. Dashed blue lines outline gonads in transverse sections. D, dorsal; L, lateral; M, medial; V, ventral; me, Müllerian duct epithelium; mm, Müllerian duct mesenchyme; sto, stomach; wm; Wolffian duct mesenchyme. Scale bar in D = 100 µm (B, D); scale bar in D′ = 10 µm (B′, D′).
Fig. 5.
Expression of Tbx4 in gonads at E13.5. Whole-mount and section ISH on sagittal sections are shown. In XY embryos, Tbx4 is expressed in testis cords (asterisk) (A, B). In XX embryos, Tbx4 is expressed throughout ovaries (D, E). Oct4 expression in XY and XX germ cells (C, F) is shown for comparison. Tbx4 and Oct4 are expressed in control XY gonads (G, H). After administration of busulfan to deplete germ cells, expression of both Tbx4 and Oct4 is reduced in XY gonads (I, J). Tbx4 is not expressed in genital ducts of either sex. m, mesonephros. Scale bar in F = 100 µm (B, C, E, F).
Genital ducts
The Wolffian and Müllerian ducts, anlagen of XY and XX genital ducts, respectively, are unipotential structures that develop in both male and female embryos. Whereas Wolffian ducts are present by E10.5, Müllerian ducts arise between E11.5 and E13.5 (Kobayashi and Behringer, 2003; Staack et al., 2003; Hannema and Hughes, 2007). After sexual differentiation of the gonads, Wolffian ducts regress in females and Müllerian ducts regress in males by E16.5 (Kobayashi et al., 2004).
Both Tbx2 and Tbx3 are expressed in the Wolffian duct epithelium and Müllerian duct mesenchyme in XY embryos at E13.5 (Figs. 3C, C′, 4B, B′). In addition, Tbx3, but not Tbx2 expression was observed in the Wolffian duct mesenchyme (Figs. 3C, C′, B, B′). Tbx2 and Tbx3 are expressed in the Wolffian duct epithelium in XX embryos at E13.5 (Figs. 3F, F′, 4D, D′), and, as seen in males, Tbx3 is expressed in mesenchyme around both Wolffian and Müllerian ducts (Fig. 4D, D′). Tbx2 and Tbx3 are also expressed in the coelomic epithelium (Fig. 3F′, arrow; data not shown for Tbx3). The coelomic epithelium proliferates and undergoes an epithelial-to-mesenchymal transition to give rise to mesenchymal cells that contribute to connective tissue in the internal reproductive tracts (Moore et al., 1998; Guioli et al., 2007). Thus, Tbx2- and Tbx3-positive cells in the Müllerian duct mesenchyme may reflect their origin from coelomic epithelium.
In summary, our results show that Tbx2 and Tbx3 are expressed in the genital ducts from E9.5 to E13.5 in both unique and overlapping expression domains (Table 1) and sexually dimorphic Tbx2 expression is observed in the Müllerian ducts. The patterns of Tbx2 and Tbx3 expression in the genital ducts suggest a role for Tbx2 -, Tbx3-mediated cellular interactions in the sex-specific differentiation of the Wolffian and Müllerian ducts. For example, Tbx2 and Tbx3 may interact to induce Wolffian duct differentiation and/or Müllerian duct regression in male embryos. Additionally, Tbx2 and Tbx3 may interact to mediate Wolffian duct regression in female embryos.
TABLE 1.
Summary of Tbx2 Subfamily Gene Expression in the Embryonic and Postnatal, Pre-Pubertal Internal Reproductive Systema
| E13.5 | |||||||
|---|---|---|---|---|---|---|---|
| P0 | P14 | P16 | |||||
| Gene | E9.5 | E11.5 | XY | XX | XY | XY | XX |
| Tbx2 | we | we | Testis interstitiumb, we, mm | Ovary, we | Testis interstitiumb, epididymis | Epididymis, seminal vesicle | Oviduct |
| Tbx3 | we | we, wm | we, wm, mm | we, wm, mm | Epididymis | Epididymis, vas deferens, seminal vesicle | Uterus |
| Tbx4 | Absent | Gonad | gc in testis cords | gc in ovary | Absent | Absent | Oocytes |
gc, germ cells; we, Wolffian duct epithelium; wm, Wolffian duct mesenchyme; mm, Müllerian duct mesenchyme.
Mesenchymal compartment.
Epithelial compartment.
Expression of Tbx2 Subfamily Genes in Postnatal, Pre-Pubertal Male and Female Internal Reproductive Systems
Elongation and coiling of the testis cords to form mature seminiferous tubules occurs before birth. Wolffian duct differentiation into the epididymides, vas deferentia, and seminal vesicles starts at E15.5 and is completed within the first 2 weeks of postnatal life (Archambeault et al., 2009). In contrast, primordial follicles, the first stage of ovarian folliculogenesis, form between P1 and P3 (Wilhelm et al., 2007), and Müllerian ducts differentiate into the oviducts, uterus, cervix, and upper vagina during the first 2 weeks of postnatal life.
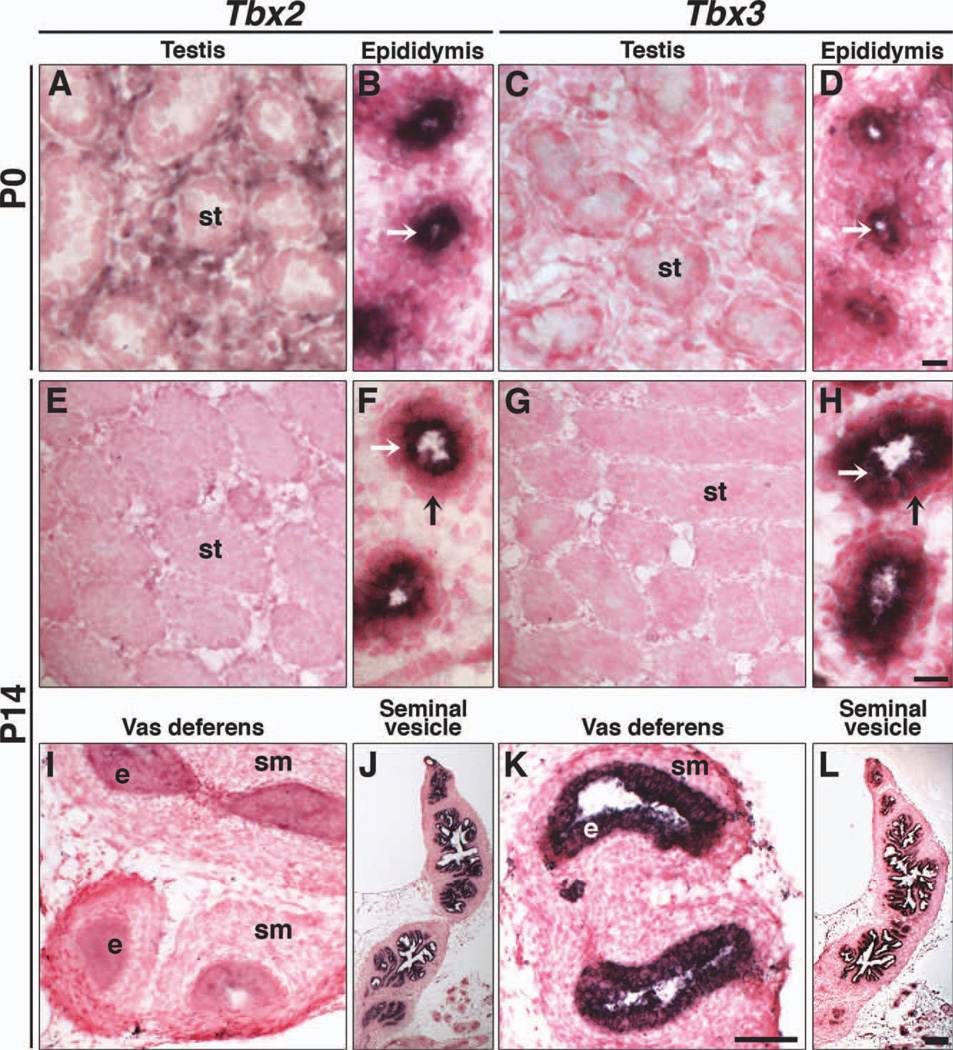
Of the four Tbx2 subfamily genes, only Tbx2 and Tbx3 are expressed in postnatal, pre-pubertal males (Fig. 6). Tbx2 is expressed in the testicular interstitium at P0 (Fig. 6A), but not at P14 (Fig. 6E). Tbx3 is not expressed in postnatal testes (Fig. 6C, G), similar to E13.5. Both Tbx2 and Tbx3 are expressed in the epithelium of the epididymis (Fig. 6B, D, F, H white arrows) and seminal vesicles (Fig. 6J, L). Tbx3, but not Tbx2, is expressed in the vas deferens (Fig. 6K, I). Neither Tbx2 nor Tbx3 expression was observed in mesenchymal cells around epididymal coils (Fig. 6F, H, black arrows), although Tbx3 was expressed in the embryonic Wolffian duct mesenchyme (Figs. 2 and 4). Expression of Tbx2 and Tbx3 during Wolffian duct formation and differentiation suggests a role for these two genes in sperm maturation, transport, and/or storage.
Fig. 6.
Tbx2 and Tbx3 expression in postnatal testes and differentiated Wolffian ducts. ISH on sagittal sections of testes, epididymides, vas deferentia, and seminal vesicles. At P0, Tbx2 is expressed in interstitial cells that surround seminiferous tubules (A) and epithelial cells of the epididymis (B, white arrow). Tbx3 is not observed in testes (C), but is expressed in epithelial cells of the epididymis (D, white arrow). At P14, neither Tbx2 nor Tbx3 is expressed in testes (E, G). Both are expressed in epithelial cells (white arrows) of epididymal tubules and not mesenchymal cells (black arrows) surrounding epididymal tubules (F, H). Tbx3, but not Tbx2, is expressed in epithelial cells of the vas deferens (I, K) and both are expressed in seminal vesicles (J, L). e, epithelium; st, seminiferous tubules; sm, smooth muscle. White arrows indicate epithelial cells of epididymal tubules. Black arrows indicate mesenchymal cells surrounding epididymal tubules. Scale bar in D = 10 µm (A–D); scale bar in H = 10 µm (F, H); scale bar in K = 50 µm (E, G, I, K); scale bar in L = 100 µm (J, L).
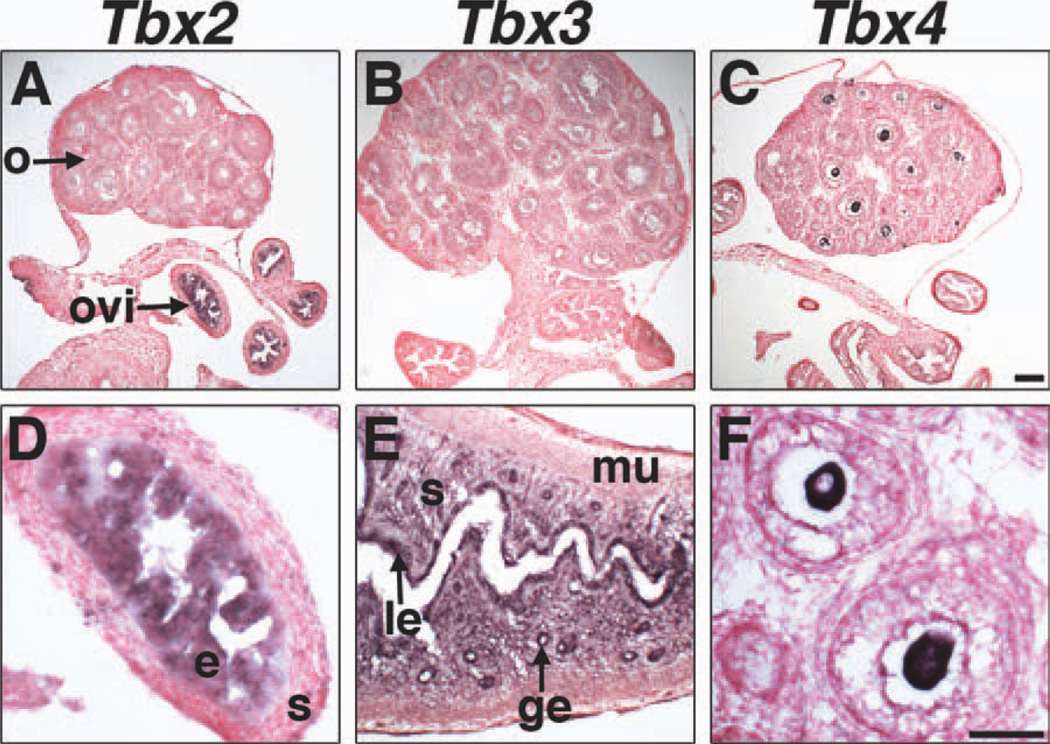
In postnatal, pre-pubertal females at P16, non-overlapping expression of Tbx2, Tbx3, and Tbx4 was observed (Fig. 7). Tbx2 is expressed in epithelial cells in oviducts (Fig. 7A, D). Tbx3 was not detected in ovaries or oviducts, but was observed in glandular and luminal uterine epithelium and uterine stroma (Fig. 7B, E), which contain cell types, such as fibroblasts, of mesenchymal origin. Tbx4 is the only member of the Tbx2 subfamily expressed in postnatal, pre-pubertal ovaries. Tbx4 is expressed in oocytes of primary and secondary ovarian follicles (data shown for secondary follicles) (Fig. 7C, F). Taken together, our data support unique, non-overlapping expression of Tbx2 and Tbx4 in sexually differentiated gonads; Tbx2 is expressed in testicular and ovarian somatic cells at E13.5, while Tbx4 is expressed in embryonic male and female germ cells and postnatal oocytes.
Fig. 7.
Tbx2, Tbx3, and Tbx4 expression in the postnatal female internal reproductive system by section ISH on sagittal sections at P16. Tbx2 is expressed in the epithelium, but not the stroma of the oviduct, and not in ovaries (A, D). Tbx3 is expressed in the uterine luminal epithelium, glandular epithelium, and stroma (E), but not in ovaries or oviducts (B). Tbx4 is expressed in oocytes, but not oviducts or in uterus (C, F). F shows expression of Tbx4 in oocytes of two secondary follicles. ge, glandular epithelium; e, epithelium; le, luminal epithelium; m, muscle; o, ovary; ovi, oviduct; s, stroma. Scale bar in C = 100 µm (A–C); scale bar in F = 50 µm (D–F).
Expression of Tbx2 Subfamily Genes in the External Genitalia
The GT, a bipotential embryonic structure that is morphologically identical in males and females prior to E16.5, gives rise to the penis and foreskin, scrotum, clitoris, and labia. Androgen-independent outgrowth of the GT, ventral to the cloacal membrane, starts after E10.5 (Perriton et al., 2002; Yamada et al., 2003, 2006). However, differentiation of the GT mesenchyme, which results in corpora cavernosum with functional erectile tissue and chondrogenesis and osteogenesis in the os penis (Murakami and Mizuno, 1986; Murakami, 1987), is androgen dependent.
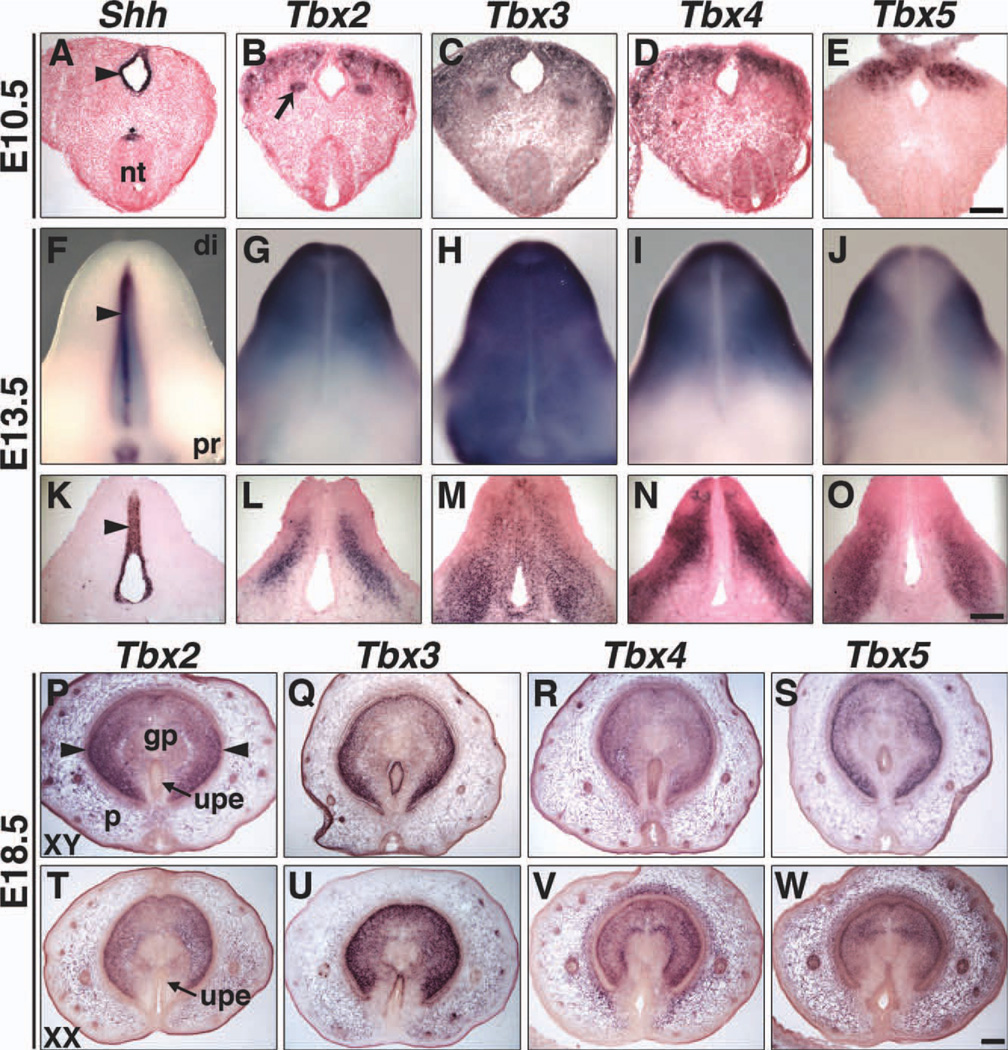
All four Tbx2 subfamily genes are expressed in the GT at E10.5 and E13.5 and in the sexually dimorphic GT at E18.5 (Fig. 8). At E10.5 (Fig. 8B–E), these T-box genes are expressed in mesenchyme lateral to the cloacal epithelium, which expresses Shh (Perriton et al., 2002) (Fig. 8A). At E13.5, whole mounts and sections reveal overlapping, yet unique expression domains of Tbx2, Tbx3, Tbx4, and Tbx5 in the GT mesenchyme (Fig. 8G–J, L–O). Expression of Shh in the urethral plate epithelium (UPE) is shown for comparison (Fig. 8F, K). Tbx3 is the only member of the Tbx2 subfamily expressed in the UPE at E10.5 (Fig. 8C) and E13.5 (Fig. 8H, M).
Fig. 8.
Expression of Tbx2, Tbx3, Tbx4, and Tbx5 in genital tubercles at E10.5, E13.5, and E18.5 by ISH. E10.5 coronal sections (A–E), E13.5 ventral views of whole mounts (F–J) and longitudinal sections through GT (K–O), and E18.5 XY and XX transverse sections through GT (P–W) are shown. Shh expression in the epithelium of the cloaca (A, arrowhead) and UPE (F, K, arrowheads) is shown for comparison. Tbx2 is expressed in the GT mesenchyme, but not epithelium (B, G, L). The arrow in B highlights Tbx2 expression in the Wolffian duct. Tbx3 is expressed in the epithelium and mesenchyme of the GT (C, H, M). Tbx4 (D, I, N) and Tbx5 (E, J, O) are both expressed in the GT mesenchyme, but their patterns of expression differ. Neither Tbx4 nor Tbx5 are expressed in the epithelium at E10.5 and E13.5. At E18.5, the dorsal surface of the GT is at the top of each section (P–W). Tbx2, Tbx3, Tbx4, and Tbx5 are expressed in mesenchyme, including the glans penis and prepuce, but not the glans lamellae (arrowheads) of XY (P–S) and XX (T–W) GT. Neither Tbx2 (P, T) nor Tbx4 (R, V) is expressed in urethral plate epithelial cells of XY or XX embryos. Tbx3 is expressed in the UPE of both XY (Q) and XX (U) GT. Tbx5 is expressed in XY (S), but not XX (W) UPE. di, distal; gp, glans penis; nt, neural tube; p, prepuce; pr, proximal; upe, urethral plate epithelium. Scale bars in E, O, and W = 100 µm (A–E, K–O, and P–W). Nuclear Fast Red counter-stain was not used at E18.5 (P–W).
At E18.5, Tbx2, Tbx3, Tbx4, and Tbx5 are expressed in mesencyhme that gives rise to the glans penis, glans clitoris, and foreskin (derived from the prepuce) (Fig. 8P–W). In addition, Tbx3 is expressed in the UPE of males (Fig. 8Q) and females (Fig. 8U) and Tbx5 is expressed in the male UPE (Fig. 8S). Signals from the epithelium control external genital development. Thus, Tbx3 and Tbx5 may interact with epithelial genes like Shh (Perriton et al., 2002) or mesenchymal genes like Fgf10 (Haraguchi et al., 2000), which are integral to normal external genitalia development.
The pattern of Tbx2 subfamily gene expression in the embryonic and postnatal, pre-pubertal internal reproductive system is summarized in Table 1. Tbx2 subfamily gene expression was observed from initiation of organ development to sexual differentiation in both the internal and external reproductive systems. Thus, our study lays the foundation for investigation of functional requirements for Tbx2, Tbx3, Tbx4, and Tbx5 in the development, differentiation, and post-pubertal function of the mammalian reproductive system.
EXPERIMENTAL PROCEDURES
Animals, In Situ Hybridization, and Immunofluorescence
Wild type adult ICR mice (Taconic, Germantown, NY) were bred to generate embryos from E9.5 to E13.5 and pups at P0, P14, and P16. All mice were housed in a temperature-controlled facility with a 12-hr light/12-hr dark cycle. Noon on the day a mating plug was observed was designated E0.5. The sex of each embryo or pup was determined by staining the amnion with 2% orcein in 60% acetic acid to detect sex chromatin (Papaioannou and Behringer, 2005), by PCR of yolk sac lysates for the Sry gene, or by morphological differences in the gonads at later stages. Embryos, urogenital ridges, and gonads were dissected in cold phosphate buffered saline (PBS), fixed in 4% paraformaldehyde, washed in PBS containing Tween-20, dehydrated in a methanol series, and stored at −20°C until use. For section ISH and IF, whole embryos and internal reproductive organs from postnatal mice were isolated and fixed in 4% paraformaldehyde. Following fixation, samples were infiltrated with sucrose, embedded in Tissue-Tek® O.C.T.™ Compound (Sakura Fine Technical Co, Ltd, Tokyo, Japan), snap-frozen on dry ice in ethanol, and stored at −80°C. Animal protocols were approved by the Institutional Animal Care and Use Committee of Columbia University.
Whole mount and section ISH were performed using standard protocols (Wilkinson and Nieto, 1993; Grieshammer et al., 2004). Antisense and sense digoxigenin–labeled RNA probes were transcribed from linearized plasmids in the presence of digoxigenin-labeled dUTP (Roche, Nutley, NJ). For section ISH, frozen sections at 20 µm were made for E9.5 and E18.5 embryos, 16 µm for E10.5 embryos, and 10 µm for E11.5 and E13.5 embryos and postnatal organs. Sections were counter-stained with Nuclear Fast Red unless otherwise indicated.
ISH was performed on a minimum of 5 male and 5 female samples. ISH results for XY and XX embryos (E9.5–E11.5) and GT (E10.5–E13.5) were identical. Data are shown for XY embryos. IF was performed on 10-µm frozen sections at E13.5 using standard protocols (Wilhelm et al., 2005). The following antibodies were used: rat anti-Pecam (BD Pharmingen, San Diego, CA), mouse monoclonal anti-Tbx2 (C. R. Goding, unpublished data), Alexa fluor488 donkey anti-rat (Molecular Probes Inc., Eugene, OR), Cy3-conjugated goat anti-mouse (Jackson ImmunoResearch, West Grove, PA). Sections were stained with 4′, 6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO) for nuclear visualization.
Images of whole-mount samples were taken under bright field on a Nikon SMZ1500 microscope (Nikon, Japan). Section ISH and IF staining was examined with a Nikon MICROPHOT-FXA microscope (Nikon, Japan) and images were captured using NIS-Elements D3.10 software.
Depletion of Embryonic Germ Cells
Intraperitoneal (IP) administration of busulfan (Sigma-Aldrich) was utilized to deplete embryonic gonads of germ cells. Pregnant female mice were injected with 150 µl of busulfan solution (20 mg/ml in 50% DMSO) or 50% DMSO (control) at E11.5. Gonads were removed at E13.5 and processed for ISH with probes for Tbx4 and Oct4 as described above.
Key findings.
This is the first study to characterize the expression of multiple T-box genes, the four closely related members of the Tbx2 subfamily, in both the internal and external reproductive systems of males and females.
We show that Tbx2 and Tbx3 have both unique and overlapping expression domains in the internal reproductive system during initial stages of gonad and genital duct formation, after establishment of sexual dimorphism and at postnatal, pre-pubertal stages.
β-Tbx4 is expressed in embryonic male and female germ cells and postnatal oocytes.
All four Tbx2 subfamily genes, Tbx2, Tbx3, Tbx4, and Tbx5, are expressed in mesenchyme in external genitalia, with Tbx3 and Tbx5 expression in the epithelium as well.
ACKNOWLEDGMENTS
We thank Dr. Colin R. Goding (Oxford, UK) for his generous gift of anti-Tbx2 antibody. This work was supported by the National Institutes of Health grant HD033082 (to V.E.P.) and the Reproductive Scientist Development Program grant NIH K12 HD000849 (to N.C.D.) and Robert Wood Johnson Foundation grant (N.C.D.).
Grant sponsor: National Institutes of Health; Grant sponsor: Robert Wood Johnson Foundation.
REFERENCES
- Adem C, Soderberg CL, Hafner K, Reynolds C, Slezak JM, Sinclair CS, Sellers TA, Schaid DJ, Couch F, Hartmann LC, Jenkins RB. ERBB2, TBX2, RPS6KB1, and MYC alterations in breast tissues of BRCA1 and BRCA2 mutation carriers. Genes Chromosomes Cancer. 2004;41:1–11. doi: 10.1002/gcc.20057. [DOI] [PubMed] [Google Scholar]
- Agulnik SI, Garvey N, Hancock S, Ruvinsky I, Chapman DL, Agulnik I, Bollag R, Papaioannou V, Silver LM. Evolution of mouse T-box genes by tandem duplication and cluster dispersion. Genetics. 1996;144:249–254. doi: 10.1093/genetics/144.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambeault DR, Tomaszewski J, Joseph A, Hinton BT, Yao HH. Epithelial-mesenchymal crosstalk in Wolffian duct and fetal testis cord development. Genesis. 2009;47:40–48. doi: 10.1002/dvg.20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Krakowiak PA, Watkins WS, Root S, Carey JC, Jorde LB. A gene for ulnar-mammary syndrome maps to 12q23-q24.1. Hum Mol Genet. 1995;4:1973–1977. doi: 10.1093/hmg/4.10.1973. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Lin RC, Law DJ, Watkins WC, Krakowiak PA, Moore ME, Franceschini P, Lala R, Holmes LB, Gebuhr TC, Bruneau BG, Schinzel A, Seidman JG, Seidman CE, Jorde LB. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet. 1997;16:311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, Soults J, Grayzel D, Kroumpouzou E, Traill TA, Leblanc-Straceski J, Renault B, Kucherlapati R, Seidman JG, Seidman CE. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- Bongers EM, Duijf PH, van Beersum SE, Schoots J, van Kampen A, Burckhardt A, Hamel BC, Losan F, Hoefsloot LH, Yntema HG, Knoers NV, van Bokhoven H. Mutations in the human TBX4 gene cause small patella syndrome. Am J Hum Genet. 2004;74:1239–1248. doi: 10.1086/421331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE. Expression of the T-box family genes, Tbx1–Tbx5, during early mouse development. Dev Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Yutzey KE. T-box factors. In: Rosenthal N, Harvey RP, editors. Heart development and regeneration. Burlington, MA: Elsevier Science and Technology; 2010. [Google Scholar]
- Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- Franceschini P, Vardeu MP, Dalforno L, Signorile F, Franceschini D, Lala R, Matarazzo P. Possible relationship between ulnar-mammary syndrome and split hand with aplasia of the ulna syndrome. Am J Med Genet. 1992;44:807–812. doi: 10.1002/ajmg.1320440618. [DOI] [PubMed] [Google Scholar]
- Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- Guioli S, Sekido R, Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Dev Biol. 2007;302:389–398. doi: 10.1016/j.ydbio.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Hannema SE, Hughes IA. Regulation of Wolffian duct development. Horm Res. 2007;67:142–151. doi: 10.1159/000096644. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M, Sekine K, Kawano H, Kato S, Ueno N, Yamada G. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development. 2000;127:2471–2479. doi: 10.1242/dev.127.11.2471. [DOI] [PubMed] [Google Scholar]
- Harrelson Z, Kelly RG, Goldin SN, Gibson-Brown JJ, Bollag RJ, Silver LM, Papaioannou VE. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the out-flow tract during heart development. Development. 2004;131:5041–5052. doi: 10.1242/dev.01378. [DOI] [PubMed] [Google Scholar]
- Holland PW, Garcia-Fernandez J, Williams NA, Sidow A. Gene duplications and the origins of vertebrate development. Development (Suppl) 1994:125–133. [PubMed] [Google Scholar]
- Jacobs JJ, Keblusek P, Robanus-Maandag E, Kristel P, Lingbeek M, Nederlof PM, van Welsem T, van de Vijver MJ, Koh EY, Daley GQ, van Lohuizen M. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19ARF) and is amplified in a subset of human breast cancers. Nat Genet. 2000;26:291–299. doi: 10.1038/81583. [DOI] [PubMed] [Google Scholar]
- Kaufman MH, Bard JBL. The anatomical basis of mouse development. London: Academic Press; 1999. [Google Scholar]
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Shawlot W, Kania A, Behringer RR. Requirement of Lim1 for female reproductive tract development. Development. 2004;131:539–549. doi: 10.1242/dev.00951. [DOI] [PubMed] [Google Scholar]
- Li QY, Newbury-Ecob RA, Terrett JA, Wilson DI, Curtis AR, Yi CH, Gebuhr T, Bullen PJ, Robson SC, Strachan T, Bonnet D, Lyonnet S, Young ID, Raeburn JA, Buckler AJ, Law DJ, Brook JD. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet. 1997;15:21–29. doi: 10.1038/ng0197-21. [DOI] [PubMed] [Google Scholar]
- Liu WK, Jiang XY, Zhang ZX. Expression of PSCA, PIWIL1 and TBX2 and its correlation with HPV16 infection in formalin-fixed, paraffin-embedded cervical squamous cell carcinoma specimens. Arch Virol. 2010a;155:657–663. doi: 10.1007/s00705-010-0635-y. [DOI] [PubMed] [Google Scholar]
- Liu WK, Jiang XY, Zhang ZX. Expression of PSCA, PIWIL1, and TBX2 in endometrial adenocarcinoma. Onkologie. 2010b;33:241–245. doi: 10.1159/000305098. [DOI] [PubMed] [Google Scholar]
- Lomnytska M, Dubrovska A, Hellman U, Volodko N, Souchelnytskyi S. Increased expression of cSHMT, Tbx3 and utrophin in plasma of ovarian and breast cancer patients. Int J Cancer. 2006;118:412–421. doi: 10.1002/ijc.21332. [DOI] [PubMed] [Google Scholar]
- Lyng H, Brovig RS, Svendsrud DH, Holm R, Kaalhus O, Knutstad K, Oksefjell H, Sundfor K, Kristensen GB, Stokke T. Gene expressions and copy numbers associated with metastatic phenotypes of uterine cervical cancer. BMC Genomics. 2006;7:268. doi: 10.1186/1471-2164-7-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol. 2000;163:3–9. doi: 10.1016/s0303-7207(99)00234-8. [DOI] [PubMed] [Google Scholar]
- Mesbah K, Harrelson Z, Theveniau-Ruissy M, Papaioannou VE, Kelly RG. Tbx3 is required for outflow tract development. Circ Res. 2008;103:743–750. doi: 10.1161/CIRCRESAHA.108.172858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AW, Schedl A, McInnes L, Doyle M, Hecksher-Sorensen J, Hastie ND. YAC transgenic analysis reveals Wilms’ tumour 1 gene activity in the proliferating coelomic epithelium, developing diaphragm and limb. Mech Dev. 1998;79:169–184. doi: 10.1016/s0925-4773(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Murakami R. A histological study of the development of the penis of wild-type and androgen-insensitive mice. J Anat. 1987;153:223–231. [PMC free article] [PubMed] [Google Scholar]
- Murakami R, Mizuno T. Proximal-distal sequence of development of the skeletal tissues in the penis of rat and the inductive effect of epithelium. J Embryol Exp Morphol. 1986;92:133–143. [PubMed] [Google Scholar]
- Naiche LA, Harrelson Z, Kelly RG, Papaioannou VE. T-box genes in vertebrate development. Annu Rev Genet. 2005;39:219–239. doi: 10.1146/annurev.genet.39.073003.105925. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Papaioannou VE. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development. 2003;130:2681–2693. doi: 10.1242/dev.00504. [DOI] [PubMed] [Google Scholar]
- Papaioannou VE, Behringer RR. Mouse Phenotypes: A Handbook of Mutation Analysis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Ross A, Munger S, Capel B. Bmp7 regulates germ cell proliferation in mouse fetal gonads. Sex Dev. 2007;1:127–137. doi: 10.1159/000100034. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Silver LM. Newly identified paralogous groups on mouse chromosomes 5 and 11 reveal the age of a T-box cluster duplication. Genomics. 1997;40:262–266. doi: 10.1006/geno.1996.4591. [DOI] [PubMed] [Google Scholar]
- Sainio K, Hellstedt P, Kreidberg JA, Saxen L, Sariola H. Differential regulation of two sets of mesonephric tubules by WT-1. Development. 1997;124:1293–1299. doi: 10.1242/dev.124.7.1293. [DOI] [PubMed] [Google Scholar]
- Schinzel A. Ulnar-mammary syndrome. J Med Genet. 1987;24:778–781. doi: 10.1136/jmg.24.12.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel A, Illig R, Prader A. The ulnar-mammary syndrome: an autosomal dominant pleiotropic gene. Clin Genet. 1987;32:160–168. doi: 10.1111/j.1399-0004.1987.tb03347.x. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25:19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Sinclair CS, Adem C, Naderi A, Soderberg CL, Johnson M, Wu K, Wadum L, Couch VL, Sellers TA, Schaid D, Slezak J, Fredericksen Z, Ingle JN, Hartmann L, Jenkins RB, Couch FJ. TBX2 is preferentially amplified in BRCA1-and BRCA2-related breast tumors. Cancer Res. 2002;62:3587–3591. [PubMed] [Google Scholar]
- Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: a practical approach. Differentiation. 2003;71:402–413. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Takeuchi J, Koshiba-Takeuchi K, Ogura T. Tbx genes specify posterior digit identity through Shh and BMP signaling. Dev Cell. 2004;6:43–53. doi: 10.1016/s1534-5807(03)00401-5. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Urven LE, Weng DE, Schumaker AL, Gearhart JD, McCarrey JR. Differential gene expression in fetal mouse germ cells. Biol Reprod. 1993;48:564–574. doi: 10.1095/biolreprod48.3.564. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Hamada K, Yamamoto M, Suda T, Iseki S. The expression of platelet endothelial cell adhesion molecule-1 in mouse primordial germ cells during their migration and early gonadal formation. Histochem Cell Biol. 2003;119:355–362. doi: 10.1007/s00418-003-0528-1. [DOI] [PubMed] [Google Scholar]
- Wardle FC, Papaioannou VE. Teasing out T-box targets in early mesoderm. Curr Opin Genet Dev. 2008;18:418–425. doi: 10.1016/j.gde.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, Bowles J, Mizusaki H, Koopman P. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev Biol. 2005;287:111–124. doi: 10.1016/j.ydbio.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yamada G, Suzuki K, Haraguchi R, Miyagawa S, Satoh Y, Kamimura M, Nakagata N, Kataoka H, Kuroiwa A, Chen Y. Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn. 2006;235:1738–1752. doi: 10.1002/dvdy.20807. [DOI] [PubMed] [Google Scholar]