Abstract
The phosphatidylinositolphosphate phosphatase PTEN is the second most frequently mutated protein in human tumors. Its membrane association, allosteric activation and membrane dissociation are poorly understood. We recently reported PTEN binding affinities to membranes of different compositions and a preliminary investigation of the protein-membrane complex with neutron reflectometry (NR). Here we use NR to validate molecular dynamics (MD) simulations of the protein and study conformational differences of the protein in solution and on anionic membranes. NR shows that full-length PTEN binds to such membranes roughly in the conformation and orientation suggested by the crystal structure of a truncated PTEN protein, in contrast with a recently presented model which suggested that membrane binding depends critically on the SUMOylation of the CBR3 loop of PTEN’s C2 domain. Our MD simulations confirm that PTEN is peripherally bound to the bilayer surface and show slight differences of the protein structure in solution and in the membrane-bound state, where the protein body flattens against the bilayer surface. PTEN’s C2 domain binds phosphatidylserine (PS) tightly through its CBR3 loop, and its phosphatase domain also forms electrostatic interactions with PS. NR and MD results show consistently that PTEN’s unstructured, anionic C-terminal tail is repelled from the bilayer surface. In contrast, this tail is tightly tugged against the C2 domain in solution, partially obstructing the membrane-binding interface of the protein. Arresting the C-terminal tail in this conformation by phosphorylation may provide a control mechanism for PTEN’s membrane binding and activity.
Keywords: PTEN phosphatase, PI3K pathway, phosphatidylserine, protein-membrane interaction, C2 domain
Introduction
The cytosolic enzyme, phosphatase and tensin homologue deleted on chromosome 10 (PTEN),1 is the major antagonist to phosphoinositide-3-kinase at the origin of the PI3K/Akt pathway that controls many important cellular events, including proliferation and apoptosis (Di Cristofano and Pandolfi, 2000; Song et al., 2012). PTEN has also been observed in the nucleus, but the cell signaling functions investigated here occur at the inner leaflet of the plasma membrane where it hydrolyzes PI(3,4,5)P3 with high specificity for the 3-position of the inositol ring to produce PI(4,5)P2 (Maehama and Dixon, 1998). In its role as a PI3K antagonist, PTEN limits the basal level of PI(3,4,5)P3. Because elevated PI(3,4,5)P3 levels lead to unconditional cell survival and growth, PTEN inactivation is associated with multiple disease states, including many types of cancer (Stiles, 2009), and in fact, PTEN is the second most frequently mutated protein observed in sporadic human tumors (Simpson and Parsons, 2001). To initiate its phosphatase activity, PTEN is recruited to a membrane surface through interactions with anionic lipids. Selectivity for the plasma membrane is provided by specific interaction of PTEN’s N-terminal PIP-binding module (PBM) with PI(4,5)P2, i.e., its dephosphorylation product, while a C2 domain binds anionic lipids in a Ca2+-independent way. The phosphatase domain (PD), located between the PBM and C2, recognizes and hydrolyzes the substrate, PI(3,4,5)P3 (Campbell et al., 2003; Redfern et al., 2008). In conjunction with electrostatic attraction to more abundant membrane components such as phosphatidylserine (PS), the complex interactions between PTEN and its set of specific lipid targets results in compartment specific, temporally and spatially structured membrane enrichment of the protein. Understanding these complex interactions, how they are regulated in the cellular context and affected by mutations are the long-term goals of this work.
As an interesting new mechanism for the regulation of PTEN activity in the PI3K/Akt pathway, it was recently reported that the membrane binding of the protein depends on SUMOylation of the C2 domain (Huang et al., 2012). In that work, cell biological studies and molecular dynamics (MD) simulations suggested that SUMO1 is required as a adhesion promoter that imparts membrane affinity to the protein via electrostatic interactions, and that mutation of the SUMO1 target sites impairs down-regulation of phosphoryl-Akt. This would suggest that mutations of the main SUMO1 target sites, K266 and K254 in the CBR3 loop of PTEN’s C2 domain (Huang et al., 2012), should be particularly conspicuous for tumorigenesis, which is, however, not observed in the known spectrum of somatic mutations (http://www.sanger.ac.uk/genetics/CGP/cosmic/).
Beyond the two core domains, PD and C2, and the PBM, PTEN has a 50 amino-acid (AA) long C-terminal tail. This tail encompasses two PEST motifs and one PDZ motif. It may be unstructured, as it was found to impede the crystallization of full-length (403 AA) PTEN (Lee et al., 1999). While therefore no structural information is available for this C-terminal tail, it has important regulatory functions. A construct that lacks the C-terminus, PTEN1-353, was observed to have a reduced lifetime and increased enzymatic activity (Vazquez et al., 2000). This suggests that full-length PTEN assumes a stabilized state with reduced activity, which undergoes accelerated degradation once activated. Phosphorylation is a likely mechanism for this regulatory function. About 30% of the C-terminal tail consists of Ser or Thr residues which provide potential phosphorylations sites. This includes the two consecutive PEST sites at residues 350-375 and 379-385 (Georgescu et al., 1999), and indeed, S370, S380, T382, T383, and S385 were reported to be simultaneously phosphorylated (Vazquez et al., 2000). Replacement of these sites decreased the steady-state levels of PTEN dramatically and increased its catalytic activity (Vazquez et al., 2000). In particular, PTEN-S385A showed increased membrane binding and activity (Odriozola et al., 2007). Phosphorylation of S229, T232, T319, and T321 on the C2 domain by the rho-associated protein kinase (ROCK), on the other hand, resulted in enhanced localization of PTEN to the membrane (Li et al., 2005).
Here we use atomistic MD simulations to augment structural investigations with neutron reflectometry (NR) in which we studied PTEN adsorbed to solid-supported, planar phospholipid membranes, i.e., sparsely-tethered bilayer lipid membranes (stBLMs) (Shenoy et al., 2012). The stBLM membrane model is a physically relevant representation of biomembranes. It has been shown to be virtually de-fect-free and impermeable to ions (McGillivray et al., 2007). The lipid bilayer is in-plane fluid with free diffusion of phospholipids in the leaflet adjacent to the aqueous phase (Shenoy et al., 2010). stBLMs are sufficiently malleable to permit reconstitution of transmembrane proteins (McGillivray et al., 2009) and enzymatic activity (Valincius et al., 2006). Yet, they are also sufficiently resilient for experimental manipulation (Nanda et al., 2010; Valincius et al., 2008; Vockenroth et al., 2008). Among other probes, they permit extensive studies with NR, which in connection with isotopic contrast variation can provide out-of-plane resolution of the laterally averaged structure that approaches 1 Ångstrom (McGillivray et al., 2009). Such resolution can be achieved when a single sample is studied after multiple consecutive exchanges of the adjacent buffer for contrast variation, which requires stability of the sample for at least a day. Structural information is provided in the form of neutron scattering length density (nSLD) profiles, proportional to an overall mass density distribution across the interface. It is then a matter of isotopic contrast engineering and the use of complementing information, such as volumetric data (Schalke and Lösche, 2000; Schalke et al., 2000), chemical connectivity (Schalke et al., 2000; Shekhar et al., 2011), and protein crystal or NMR structures (McGillivray et al., 2009; Nanda et al., 2010) to interpret such nSLD profiles in terms of supramolecular aggregate structures. Using Monte-Carlo resampling of data, one can systematically assess the composite resolution, confidence limits on the results and parameter correlations (Heinrich et al., 2009). We show that MD simulations are an ideal complement to NR. By combining these two methods one gains insight into partially disordered molecular complexes that cannot be attained by other structural techniques.
It was previously reported that PTEN-membrane interactions have a significant electrostatic component (Redfern et al., 2008). Indeed, PTEN binds with moderate affinity (Kd ~ 10 μ;M) to model membranes that contain only PC and PS, and the amount of bound protein depends on the amount of PS in binary bilayers (Shenoy et al., 2012). In the absence of PS, PTEN affinity to bilayers containing PIPs in low concentration is increased by an order of magnitude (Kd < 1 μ;M), and if both PS and PIP are present in the membrane, PTEN binding affinity is rather high (Kd ~ 50 nM) (Shenoy et al., 2012). PTEN variants without the C-terminal tail, such as the truncated PTEN that was crystallized by Lee and coworkers (Lee et al., 1999), bind PS-containing membranes with higher affinity than full-length PTEN (Shenoy et al., 2012). In addition, PTEN membrane affinity depends on lipid fluidity within the bilayer. Taken together, these findings paint a picture in which PTEN association with membranes depends critically on local membrane composition and the ability of lipids to rearrange into patterns conducive to protein binding. In turn, there is the expectation that the interaction of the bound protein with anionic lipids restricts the mobility of these binding partners much more than that of the zwitterionic membrane components.
We recently measured the neutron scattering of stBLMs in the absence and presence of wt PTEN and reported that the protein bound peripherally to the membrane surface without any significant penetration into the bilayer (Shenoy et al., 2012). Because the protein was unlabeled, these measurements reported only an overall nSLD distribution across the interface, with no direct evidence for the protein’s internal organization. In particular, such measurements do not provide any direct information on the protein conformation at the interface, or on changes of that conformation upon membrane binding. Yet, such conformational changes are a relevant concern. For the PTEN system, Gericke and coworkers reported changes in the IR spectra upon binding to membranes that contained PI(4,5)P2. On the other hand, PTEN binding to membranes that contained only PC and PS was not accompanied by spectral changes (Redfern et al., 2008). In any case, the overall nSLD contribution of wt PTEN on PC/PS stBLMs suggested the formation of homogeneous, monomolecular protein layers on the membrane. The lack of IR-spectroscopic changes may then indicate that PTEN doesn’t undergo major structural rearrangements of its two core domains, PD and C2, upon membrane binding, but the resolution of NR does not provide rigorous conclusions. Fortunately, a crystal structure was reported on a truncated PTEN construct which, however, lacks major portions of the protein: 13 and 50 AAs at the N-terminus and the C-terminus, respectively, which are presumed to be structurally disordered, as well as 31 AAs on a connecting loop within the C2 domain were removed to achieve crystallization or were not resolved in the resulting structure (Lee et al., 1999). Nevertheless, aligning the truncated crystal structure on the membrane surface reconstructed prominent features of the nSLD profiles of PTEN on PC/PS and on PC/PI(4,5)P2 membranes, thereby suggesting an interpretation of these profiles (Shenoy et al., 2012). For example, differences between the experimental nSLD profiles and the model in which the truncated crystal structure was aligned on the membrane suggested that the C-terminal tail, which accounts for the major part of the truncation, is displaced away from the bilayer surface when PTEN is membrane-bound (Shenoy et al., 2012).
In order to interpret the NR results more rigorously, we subsequently undertook all-atom MD simulations of wt PTEN on a PC/PS bilayer, reported here, and validated their outcome against the NR results. An intriguingly close correspondence between the simulation results and the nSLD profiles shows convincingly that the in silico model provides a realistic representation of the interfacial structure, providing confidence in atomistic details gleaned from the simulations that are beyond the resolution of the neutron scattering experiments. We also proceeded with MD studies of wt PTEN in buffer. A comparison with the simulations of the membrane-bound state showed adjustments of the organization of the core domains to better accommodate the membrane surface. We also observed striking differences in the organization of the C-terminal tail in the absence and the presence of the membrane, which for PTEN in the membrane-bound state is again consistent with the NR results.
Results
Neutron Scattering
NR experiments were performed on an stBLM composed of DOPC and DOPS (7:3), in which we first measured the as-prepared membrane in 3 isotopic buffers (based on H2O, D2O, and a 1:2 mixture of both designated as CM4), and subsequently incubated with full-length PTEN (20 μ;M) in H2O-based and CM4-based buffers to study the protein on the membrane (Shenoy et al., 2012). For an initial interpretation of the nSLD profiles of the protein-containing samples, we placed the neutron scattering length distribution computed from the PTEN crystal structure (Lee et al., 1999) on top of the nSLD distribution of the neat stBLM (Shenoy et al., 2012). The PTEN scattering length distribution was determined for the most likely orientation of the protein on the membrane, as suggested from the crystallization work, and was appropriately smoothed (McGillivray et al., 2009; Nanda et al., 2010) to match the resolution of the NR experiments. However, the crystal structure describes a truncated PTEN protein, from which parts of the PBM (residues 1-6 at the N-terminal tail), an interconnecting loop on the surface of the C2 domain (residues 285-309) and the C-terminal tail (residues 353-403) were removed. In addition, AAs 7-13, 282-284, 310-312 and 352 and were not resolved in the structure (Lee et al., 1999). Therefore, about 20% of the full-length PTEN are missing in the crystal structure. Nevertheless, after scaling of the amplitude and adjusting the distance of the PTEN crystal structure from the membrane surface for optimal correspondence with the experimentally derived nSLD distribution, its contribution to the overall nSLD profile matched a certain region of the envelope profile exceedingly well (Shenoy et al., 2012). Details are shown below after a discussion of the corresponding MD results.
Molecular Dynamics Simulations: PTEN on a DOPC/DOPS bilayer
Membrane Structure and Protein Orientation on the Membrane
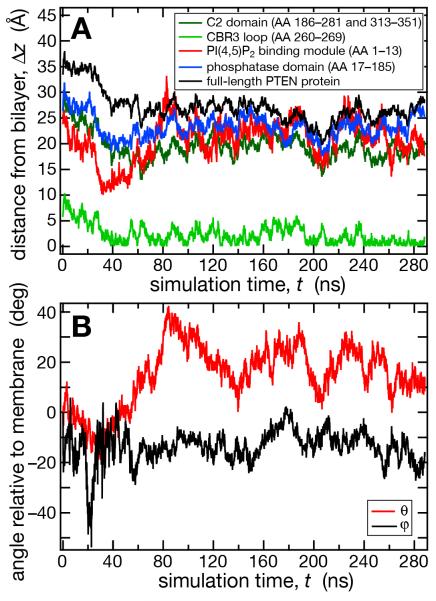
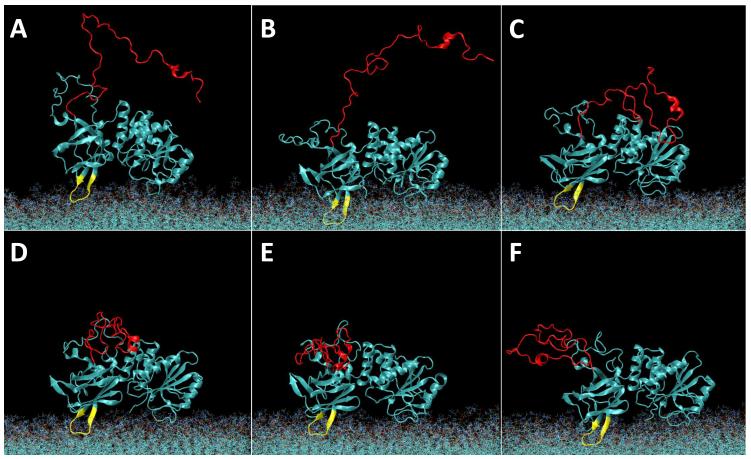
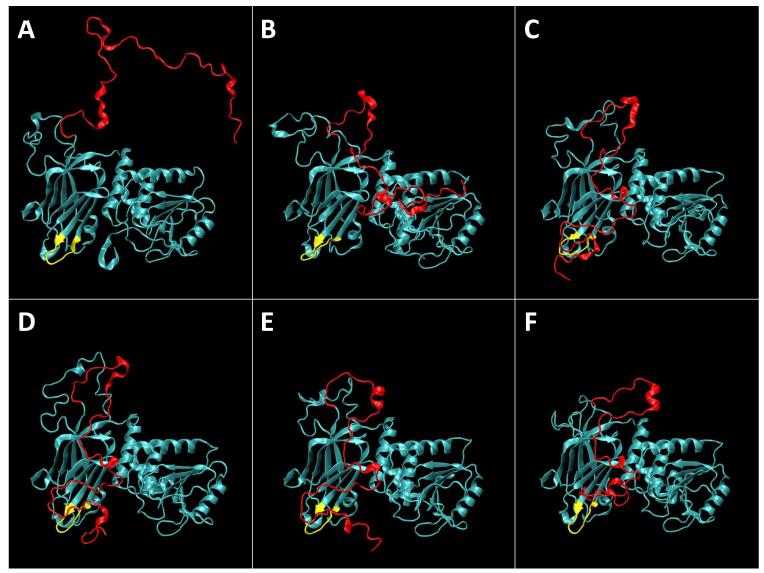
Equilibration of a neat PC/PS (2:1) membrane (details, see Methods section) was achieved within approx. 10 ns of simulation time (Fig. 1A,B) after which the area per lipid and the hydrophobic bilayer thickness had stabilized at A = 64.6 Å2 and d = 30.4 Å, respectively. Equilibration of the PTEN/membrane system took considerably longer (Fig. 1C,D). The protein was initially placed at ~10 Å separation from the membrane with its putative membrane-binding interface oriented as suggested by the crystal structure (Lee et al., 1999). Because of the periodic boundary condition in the z direction, the placement of the protein created two distinct bilayer surfaces of which one is designated as proximal and the other as distal to the protein. Once fully released, PTEN rapidly docked onto the proximal membrane surface. After ~ 60 ns of equilibration, the protein had moved considerably closer to the membrane (Fig. 2A). This configuration is shown in Fig. 3A and its development in the simulation is depicted in equidistant snapshots in the subsequent panels of Fig. 3. However, its insertion depth did not stabilize for another 60 ns of simulation time.
Figure 1.
Equilibration of the neat PC/PS (2:1) membrane and the PTEN/membrane system. (A) Area per lipid of the neat bilayer. (B) Hydrophobic thickness of the neat bilayer. (C) Area per lipid of the PTEN/membrane system. (D) Hydrophobic thickness of the PTEN/membrane system.
Figure 2.
PTEN equilibration on a PC/PS (2:1) membrane. (A) Vertical distances, Δz, between the average lipid phosphate position and the centers of mass of the full-length protein and various protein domains. (B) The two relevant Euler angles θ and φ of PTEN on the membrane surface. θ is the angle between the polar axes of the protein and the bilayer. φ of is the rotation angle of the protein about its polar axis. Both angles are zero in that protein orientation where PTEN’s putative membrane binding interface, proposed from the crystal structure (Lee et al., 1999), is oriented parallel to the membrane surface.
Figure 3.
Evolution of the wt PTEN structure on a PC/PS (2:1) membrane in the course of the MD simulation. Water molecules are omitted for clarity. The initial snapshot (A) at t = 0 shows the starting configuration of PTEN with its core domains oriented with respect to the plane of the bilayer as suggested from the crystal structure (Lee et al., 1999) and placed ca. 10 Å further away from the membrane surface than in the equilibrated state. The disordered protein trains (in particular, PTEN’s C-terminal tail, indicated in red) were initialized in an artificially extended conformation taken from a Monte-Carlo simulation. The CBR3 loop on the C2 domain is shown in yellow. Subsequent panels show equidistant snapshots at t = (B) 55 ns, (C) 110 ns, (D) 165 ns, (E) 220 ns and (F) 275 ns.
Figure 2A shows the development of the distance between the PTEN core domains and the lipid phosphate groups proximal to the protein. In the first ca. 60 ns of simulation time, the entire protein moved by about 10 Å toward the membrane surface. Residues 260-269, a Lys-rich CBR3 motif (Perisic et al., 1998) in the C2 domain (yellow loop in Fig. 3), implicated as the PS binding domain (Lee et al., 1999), led the insertion and centered itself in the vicinity of the phosphate groups. The N-terminal PBM remained away from membrane during the entire run (note the absence of PI(4,5)P2 in this simulation). The C2 and phosphatase domains were initially placed parallel to the membrane surface, see Fig. 2B. After the initial 60 ns of simulation time, however, the protein stabilized in a distinct orientation in which the PD was only surface-associated while the C2 domain was more deeply inserted into the bilayer. This orientation deviated consistently from the one expected from the crystal structure. It drifted by as much as 40° from the initial protein placement and deviated from the predicted orientation by θ = 20 ± 8° and φ = −13 ± 5° about the two principal in-plane axes (for a definition of θ and φ, see Materials and Methods and Figure 2) with fluctuations that decreased in the last 80 ns of simulation time. PTEN protein binding exerted only a minor impact on the density and thickness of the bilayer. As Fig. 1C shows, the area per lipid of the PC/PS membrane (64.6 ± 0.4 Å2 without protein vs. 63.8 ± 0.5 Å2 with PTEN) and the hydrophobic thickness of the membrane (30.4 ± 0.2 Å vs. 30.8 ± 0.2 Å) are essentially unaltered, see Fig. 1D.
In the assessment of PTEN interactions with the membrane and protein-membrane aggregate structure, below, only the final 240 ns of the 300+ ns total simulation time were analyzed.
Cross-Validation of the NR and MD results
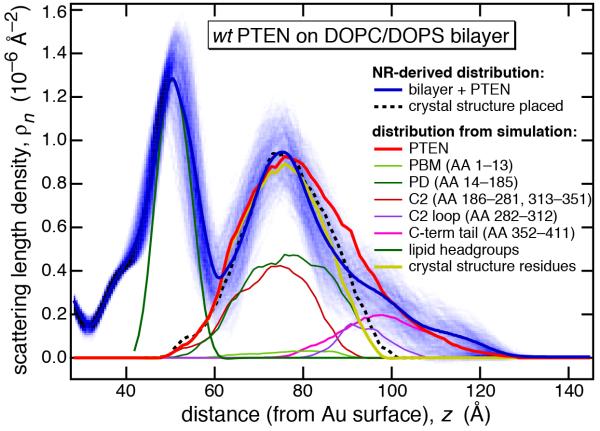
Figure 4 shows a comparison of the experimental nSLD profiles with calculated profiles based on the averaged structure from the MD production run for wt PTEN bound to the PS-bearing bilayer. Alignment of the proximal lipid headgroup profiles from experiment (blue line) and MD simulation (green) and adjustment of the protein-to-lipid ratio resulted in an excellent agreement between the experimental and simulated protein density envelopes. Not only was the MD profile (red) located within the confidence interval of the experimental nSLD distribution (shaded blue), but even more, it almost perfectly retraced the trial placement of the truncated x-ray structure (dashed black line) in the region close to the membrane (z ~ 0 to 40 Å). This suggests strongly that the two PTEN core domains are indeed interfacially adsorbed to the membrane lipid headgroups, as proposed in an initial interpretation of the NR data, which lacked the MD simulation (Shenoy et al., 2012). Vice versa, the close resemblance between the independently determined scattering length density distributions validates the MD simulations and provides confidence that more detailed structural features, which are beyond the resolution of NR, are also realistically assessed in the simulations.
Figure 4.
Comparison of nSLD profiles derived from experimental data (Shenoy et al., 2012) and computed from time averaged MD trajectories. The horizontal axis has its origin at the gold surface of the experimental system. Shown in this plot is a region that spans the bilayer center (“methyl trough” near z = 30 Å) to the buffer phase (z > 130 Å). Profiles from the MD simulations have been adjusted such that the center of the lipid headgroup distribution (green) matches the nSLD peak due to the lipid headgroups near z = 50 Å in position and in height. The solid blue line shows the most probable nSLD distribution given the experimental data and the shaded blue band shows 1σ confidence limits for the nSLD derived from Monte-Carlo resampling (Heinrich et al., 2009) of the NR data. The dashed line shows the tentative placement of the crystal structure of a truncated PTEN (Lee et al., 1999) optimized to retrace the experimental nSLD profile around its peak near z = 75 Å, which has been attributed to the membrane-bound protein (Shenoy et al., 2012). Based on the placement of the headgroups in the simulation results, the remaining traces show the distributions of various portions of the protein, as indicated: full-length PTEN protein, PI(4,5)P2 binding module (PBM), phosphatase domain (PD), C2 domain (truncated to those AA residues included in the crystal structure), the unstructured loop of the C2 domain that was not included in the crystal structure, the C-terminal tail, and the composite profile of the PD and the truncated C2 domain, i.e., the crystal structure.
Comparison of nSLD Profiles Determined in Silico and Experimentally in Vitro
According to our earlier evaluation of the NR results, the phosphatase and C2 domains were both located in close proximity to the membrane surface, but could not be distinguished within the nSLD profile. Here, their slightly distinct contributions to the nSLD envelope can be determined in the averaged MD trajectories, as shown by brown and dark green lines in Fig. 4, which add up to the trace shown in yellow. Amazingly, this yellow compound profile retraces almost perfectly the putative profile of the truncated crystal structure (dashed black line), indicating how closely experimental scattering results and MD simulations agree. Minor discrepancies between these two profiles indicate that the MD PTEN core structure on the bilayer does in fact have a slightly distinct organization from the crystal structure (see below), with differences that are again beyond the resolution of the scattering experiments. Both core domains have residues that interact with the lipid headgroups. In the absence of PIP lipid in the bilayer, the N-terminal PBM is a highly disordered peptide train that shows minimal interaction with the bilayer. Its time-averaged profile extends over 30 Å along the bilayer normal indicating a high degree of dynamic flexibility. In conjunction with the C-terminal tail and a loop on the surface of the C2 domain (residues 282-312), the N-terminus forms the disordered segments of the protein which were not included in the crystal structure. These three disordered segments therefore account for the difference between the red and yellow profiles derived from the MD simulation and the blue and dashed-black profiles in the experimental nSLD distributions. Moreover, there is no direct experimental information on their conformation in solution, or their role in membrane binding. (In this argument, we assume that the crystal structure closely represents the conformation of the truncated protein in solution.) The MD-derived nSLD profile suggests strongly that both the C-terminal train and the disordered C2 loop, with a net negative charge of −10 and −4, respectively, are distally located to the anionic bilayer surface. Hence they do not contribute to the membrane association of the full-length protein. As suggested earlier,(Shenoy et al., 2012) the C-terminus is therefore primarily responsible for the discrepancy between the experimental nSLD profile and the hypothetical profile of the truncated protein. This is consistent with an overall structure in which the C-terminal pe ptide tail is repelled from the bilayer interface by electrostatic interactions.
Protein-Membrane Interactions
To identify PS binding sites on the protein and compare their relative affinities, we analyzed the lipid mean-square displacements as a function of interval length, τ, along the trajectories for different lipid classes (Fig. 5) as well as residence times of PC and PS with PTEN AAs (Fig. 6) over the production run. As expected, PS has more extended interactions with the protein, as is therefore significantly less mobile. For example, the diffusion coefficient D for both DOPC and DOPS in the distal bilayer leaflet is about 7.3 μ;m2/s (Table 1), close to experimentally observed values in stBLM outer leaflets (Shenoy et al., 2010) and in giant unilamellar vesicles (Kahya et al., 2004; Przybylo et al., 2006). For PS within the footprint of the bound protein, we find a stark reduction of D to ~ 1.5 μ;m2/s while the diffusion for PC is only reduced to D to ~ 3 μ;m2/s, see Fig. 5 and Table 1. The fact that both lipid species are significantly reduced in their mobility by PTEN even if they do not directly interact indicates how lipid immobilization by the protein transgresses through lipidlipid contacts to slow down diffusive motion of lipids. Overall, these analyses of the simulation data suggest that PS plays multiple key roles in the initial anchoring of the PTEN protein at the membrane surface: The charged membrane surface repels the C-terminal tail electrostatically and steers the protein toward the bilayer surface in the correct orientation of the substrate binding pocket. Moreover, PS lipids make bind the protein initially, thus localizing it at the membrane surface, before the binding of PIP lipid, which resides in the membrane at much lower concentration, locks the protein in place.
Figure 5.
Lipid mean-square displacements in the membrane observed in the MD simulations. MSD values were determined by averaging over multiple overlapping time intervals of length τ along the trajectories of distinct classes of lipids as specified. Diffusion constants D derived from the MSD data are given in Table 1.
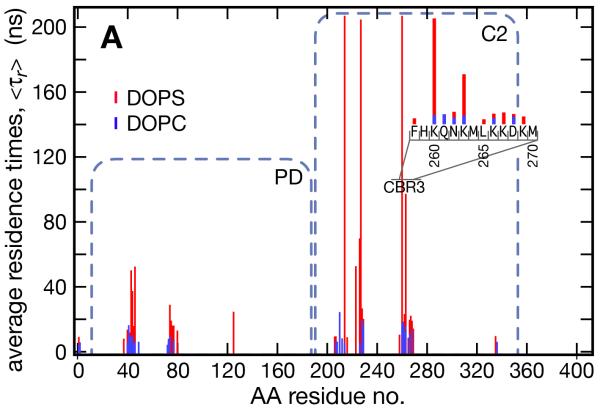
Figure 6.

Interactions of the PTEN protein with PS in the proximal membrane leaflet and comparison with interactions with PC. (A) Residence times of membrane lipids (defined as consecutive time spent within 5 Å of an AA residue) at each AA of wt PTEN, averaged over the course of the production run. PS is shown in red and PC in blue. The inset shows an expanded view across the CBR3 loop of C2. (B,C) Exemplary snapshots illustrate the binding geometry of wt PTEN to PS by the two core domains. The 200 ns residence binding site on the C2 domain is shown in (B) with Q214, S227, K260 and K263 making a combination of hydrogen bonding and salt bridge interactions with the PS headgroup, along with the hydrophobic L265 residues which “snorkels” through the headgroup region and binds to the lipid backbone. M264 also makes intermittent contacts with the phospholipid backbone. Contrasting the long-lasting (200+ ns) electrostatic interactions with C2, (C) shows interactions of residues E43, Y46 and 74R of the PD that are not as well coordinated and have comparatively short binding times (~ 50 ns).
Table 1.
Diffusion constants, D, derived from the mean-square displacements shown in Fig. 5.
| lipid class | diffusion constant, D (μm2/s) |
|---|---|
| DOPC in distal leaflet | 7.3 ± 0.5 |
| DOPS in distal leaflet | 7.3 ± 0.5 |
| DOPC in proximal leaflet | 4.3 ± 0.5 |
| DOPS in proximal leaflet | 3.8 ± 0.3 |
| DOPC in contact with protein | 2.9 ± 0.2 |
| DOPS in contact with protein | 1.5 ± 0.1 |
The latter point is substantiated by a more detailed analysis of PS residence times at specific PTEN AA residues, depicted in Fig. 6. On the C2 domain, multiple AA residues coordinated PS for longer than 100 ns. The PD also showed a cluster of AAs that bound PS for 40 ns or longer, although with significantly shorter residence times than the AAs on the C2 domain. In comparison, residence of PC on any of these AAs was limited to 20 ns or less and free diffusion is expected to locate lipids less than 5 ns on average at a particular AA. C2 domain residues 260-269 constitute a Lys-rich loop on the protein surface in the putative membrane binding interface that encompasses a CBR3 motif and has been shown to be the primary PS-binding site of PTEN (Lee et al., 1999). Indeed, an analysis of the binding kinetics showed that the CBR3 loop was the first to interact with the membrane. It penetrated the headgroup layer, thus allowing the hydrophobic residues M264 and L265, located at its apex, to bury into the bilayer, as suggested by Lee and coworkers. This brought secondary PS binding sites on C2 sufficiently close to the PS headgroups to interact, anchoring the domain firmly at the membrane surface. M264-L265 on the PTEN C2 domain are equivalent to Y96-V97-M98 on the CBR3 loop of cytosolic phospholipase A2 where the hydrophobic AA residues have been shown to contribute significantly to membrane binding (Chapman and Davis, 1998). On synaptotagmin, the CBR3 loop has been directly shown to insert into the membrane by means of fluorescence quenching of the F231W and F234W mutants upon adsorption to liposomes (Bittova et al., 1999). While in PTEN there are no single a mutation, M-CBR3 PTEN, on which all charged or mutations reported in the CBR3 loop that were identified as tumor relevant (http://www.sanger.ac.uk), hydrophobic AAs are replaced with Ala or Gly (Lee et al., 1999), had a similarly reduced growth suppression activity as the tumor-derived L345Q mutant although it remained catalytically active with soluble, short-chained PI(3,4,5)P3 (Lee et al., 1999; McConnachie et al., 2003). This suggested that the CBR3 loop is indeed essential for PTEN membrane binding.
We also observed that K260, N262/K263 and residues 266-269 (KKDK) partook in electrostatic PS binding and that K260 and K263 exhibited significant PS residence times. The CBR3 loop localized at a cluster of PS lipids and two other AA stretches assisted PS binding to C2. One consisted of Q214 and V216 and the other of K223 and the polar residues 226-229 (SSNS), both adjacent to residues 260-269 in the 3D structure. Three of these AAs (residues Q214, S227 and K260) coordinated a single PS lipid early in the simulation and bound it continuously for the remaining 200+ ns. These residues engaged in several interactions that all played a composite role in the attraction of one single PS lipid (shown in Fig. 6B), including salt bridges formed by both K260 and the side chain amine of Q214 with the PS headgroup carboxyl. Hydrogen bonding occurred between the backbone carbonyl of S227 and the headgroup amine. In addition, L265 dipped its apolar side chain into the membrane, making frequent hydrophobic contacts with the acyl chain of the immobilized lipid. These results suggested that tight membrane binding of the C2 domain involved several orchestrated interactions between basic, polar and nonpolar amino acids and the PS lipids.
Two patches on the PD also bound to PS, albeit weaker than those on the C2 domain (Fig. 6C). The first consisted of residues 40-46 (ERLEGVY) and the second of residues 73-77 (ERHYD) and K80. In these sites, multiple coordinated interactions were not observed as often as on C2 and as a result, the associated PS lipids had markedly shorter residence times.
Molecular Dynamics Simulations: PTEN Solution Structure and Comparison With the Membrane-Bound State
Two key aspects of the protein conformation were addressed in comparative simulations of PTEN in solution and in the membrane-bound state and validated by comparison with the x-ray structure of the truncated protein and with NR results for full-length PTEN. (1) Rearrangement of the core domains, C2 and PD, against each other and internally, assuming that the truncated PTEN crystal structure is a good reference for the solution structure. (2) The organization of the disordered protein stretches – in particular that of the C-terminal tail – where NR provides a reference for the membrane-bound protein (Shenoy et al., 2012). Figure 7 shows the initial configuration and a selection of equidistant structural snapshots from an all-atom MD simulation of PTEN in solution. In comparison with Fig. 3, which presents similar snapshots of PTEN in the membrane-bound state, two distinctions catch the eye: profound differences in the orga nization of the C-terminal tail (red) and a change in protein gesture in which PTEN flattens against the membrane surface if compared with the solution structure.
Figure 7.
Evolution of the wt PTEN structure in solution in the course of the MD simulation. Water molecules are omitted for clarity. The initial snapshot (A) at t = 0 shows the starting configuration. The disordered protein trains (in particular, the C-terminal tail, indicated in red) were initialized in an artificially extended conformation taken from a Monte-Carlo simulation. The CBR3 loop on the C2 domain is shown in yellow. Subsequent panels show equidistant snapshots at t = (B) 70 ns, (C) 140 ns, (D) 210 ns, (E) 280 ns and (F) 350 ns.
In the course of the simulations, the disordered PTEN regions underwent significant conformational rearrangement from their initial, artificially elongated states, but they developed into distinct directions in different molecular environments. Because these regions are missing in the crystal structure, it cannot be directly inferred from experiments if membrane interactions, for example the electrostatic surface charge of the PC/PS bilayer, exert a distinct influence on PTEN dynamics and structure. Fortunately, the MD simulations fill this gap of knowledge, as described below.
Structure of the C-terminal Tail
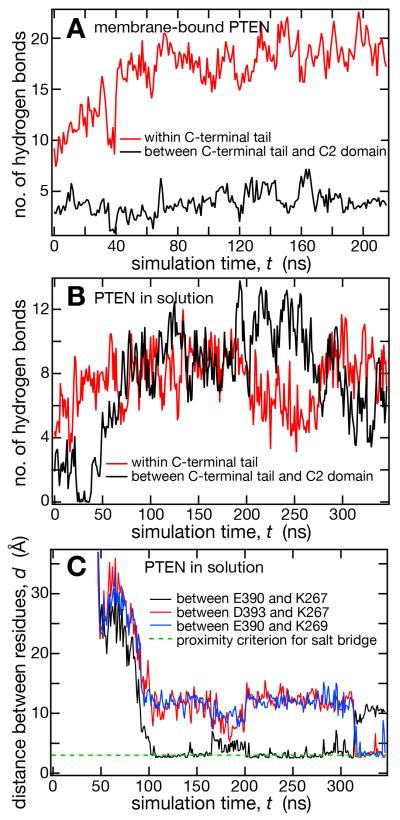
In Fig. 7, and also earlier in Fig. 3, the C-terminal tail is high-lighted in red to facilitate a visual analysis of the development of its conformation. The starting configuration in Figs. 3 and 7 were similar in terms of tail expansion; in both cases, the tail pointed away from the surface of the protein opposite to the membrane binding face and was detached from the PTEN core domains, with little or no interaction with the protein body. The initial localization of the peptide train away from the membrane, although artificial, is not unexpected as the tail has a net charge of −10 and is therefore electrostatically repelled by an anionic membrane, such as the one simulated here with ~ 30% PS. In both simulations, the peptide train contracts rapidly. In the membrane-bound structure (Fig. 3), this compaction is due to the formation of several loops along the length of the tail and of a short α-helical motif at the very end of the tail (residues 396-403). These conformational changes decreased the tail’s radius of gyration from > 20 Å initially to 14-15 Å. This peptide coil was internally stabilized primarily through hydrogen bonds with minor contributions from electrostatic interactions in the form of salt bridges. Figure 8A shows counts of hydrogen bonds formed between AAs within the C-terminal tail (red) and between the tail and the C2 domain (black) over the course of the simulation. Within the first 50 ns of simulation time, we observed the formation of ~10 hydrogen bonds as the peptide train collapsed into a coil. Fluctuations indicate that hydrogen bonds were repeatedly broken and re-formed, consistent with the disordered nature of the tail conformation. There were only few hydrogen bonding interactions with the C2 domain, and these occurred primarily with the disordered loop (residues 285-309) that is also missing from the crystal structure.
Figure 8.
Interactions within the C-terminal tail and between the tail and the C2 domain of PTEN. Total count of hydrogen bonds within the C-terminal tail (red) and between the tail and the C2 domain (black) in membrane-bound (A) and in dissolved (B) wt PTEN. (C) Distances between AA residues, as indicated, that are engaged in salt bridge formations between the C-terminal tail (E390, D393) and the CBR3 loop of the C2 domain near the membrane binding interface of wt PTEN.
The C-terminal tail organization developed very differently in solution (Figs. 7 and 8B) than in the membrane-bound state. A loop formed between T350 and S370 within the first 50 ns of simulation time which brought the remainder of the tail into close proximity to the C2 domain (Fig. 7B). Subsequently, several contacts formed between the tail and the C2 domain, with the result that the C-terminal tail remained always extended throughout the final 250+ ns of the simulation, due to substantial interactions with the protein core. This initial contraction was assisted by a surface-exposed hydrophobic patch on C2, formed by residues 191-195 (VALLF), I224, and 246-249 (PLPV), which is located on the face opposite to the domain’s membrane-binding interface. Shortly after the tail collapsed on the C2 domain, this patch was shielded from solvent by tightly bound tail residues. In distinction to the membrane-bound state, presumably due to a lack of electrostatic repulsion between the tail and the membrane, these contacts and salt bridges formed between the C-terminus and the C2 domain allowed the tail to wrap around the protein body and partially obstruct its membrane-binding interface.
The transition of the C-terminal tail from a dissociated, polymer-like state to its C2-associated state followed a clearly structured sequence of salt-bridge formation events. Acidic AA residues further upstream on the C-terminal peptide train first interacted with the protein core, followed by downstream acidic AA residues (Fig. 7), essentially forming a “zipper” that closed the gap between the peptide train and the protein domain by moving in the direction of the C-terminal end, while the remaining length of the unstructured portion of the tail became progressively shorter. This closing of the zipper, again, happens surprisingly quickly, within the first 100 ns of simulation time (Fig. 8C). In the remaining 200+ ns of simulation, interactions between acidic AA residues near the C-terminus and basic residues between AAs 260-269 in the CBR3 loop of C2 (yellow) formed and persisted almost continuously, i.e., the same residues that coordinated PS lipids in the simulation of membrane-bound PTEN were integrated into a stabilizing network of interconverting salt-bridges with the C-terminal tail of the protein in solution.
Differences in structural organization of the C-terminus in the membrane-bound and the dissolved states of the PTEN protein were also clearly evident in counts of hydrogen bonds formed by the C-terminal tail. Figure 8B (solution structure) is a direct comparison with Fig. 8A (membrane-bound state) and depicts total numbers of hydrogen bonds formed within the C-terminal tail (red) and between the tail and the C2 domain (black). After the relaxation from the artificial initial state, approx. 100 ns into the simulation, the number of internal hydrogen bonds within the tail fluctuated around 18 while the number of hydrogen bonds between the tail and C2 lingered at ca. 4 in the membrane-bound state. In distinction, hydrogen bonds occurred with a similar probability within the tail and between tail and C2 in the dissolved state of the protein, see Fig. 8B. However, the total number of hydrogen bonds was slightly smaller in solution than in the membrane-bound state (16.8 vs. 18 hydrogen bonds on average over the final 150 ns of the simulation), so that it cannot be argued that hydrogen bonding drives the reorganization of the tail. Rather, the binding of the tail against C2 was assisted by shielding the hydrophobic patch on the surface of the C2 domain, followed by a sequence of salt bridge formations that occurred very fast. Exemplified by following the K267/E390 distance in the simulation is solution, Fig. 8C visualizes how quickly the “zipper” closed within the first 100 ns of simulation time. Also displayed in this figure are distances between the K267/D393 and K269/E390 pairs, which were pulled into closer vicinity of each other by other salt bridges. These two distances lingered at a stable value, d ~ 10 Å, within the first 200 ns after closure of the “zipper”, i.e., up to the 300 ns timestamp of the simulation. Beyond the 300 ns mark, the trajectories of these distance recordings then showed a sudden jump of K267/D393 and K269/E390 into contact while K267/E390 jumped off, demonstrating fluctua-tions in the network of sa lt bridges that stabilizes the C-terminal tail against the body of the PTEN protein.
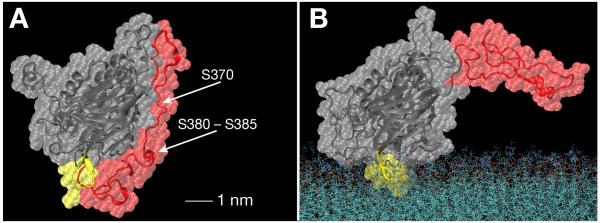
Figure 9 shows a comparison of the solution and membrane-bound PTEN structures that highlights the differences in C-terminal organization and shows how closely the tail hugged the surface of the C2 domain (Fig. 9A). This view shows the protein rotated by ca. 90° around a vertical axis from the views shown in Figs. 3 and 7. Comparison of panels A and B, and the position of the membrane surface in Fig. 9B suggests that the location of the C-terminal end in the solution structure obstructs membrane binding of the protein significantly.
Figure 9.
Head-on view of the PTEN protein onto the C2 domain in the dissolved (A) and membrane-bound state (B) that shows the distinct organizations of the C-terminal tail (red). The core domains are shown in grey, and the CBR3 loop on C2 is shown in yellow. Volume-filling representations overlay the peptide backbone trains. The model in (A) shows clearly how the C-terminal tail wraps around the C2 domain while associating tightly with that domain’s surface. The tip of the tail is compact, yet flexible, and forms salt bridges with CBR3 loop residues, see Fig. 8C, thereby obstructing the membrane-binding interface of the core protein. AA residues on the tail that have been shown to be phosphorylated and prevent membrane association of the dissolved protein are also indicated in (A).
Domain Reorganization
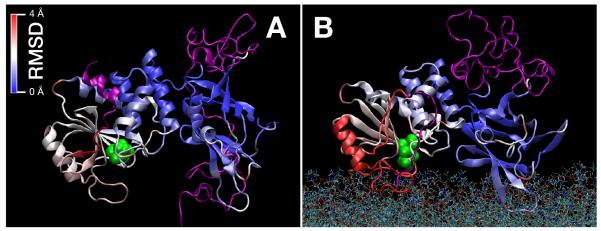
Figure 10 visualizes structural differences of the protein core in the dis-solved (Fig. 10A) and the membrane-bound states (Fig. 10B). The two views show clearly how the solution structure of the protein was more compact and the membrane-bound state was more flattened against the membrane surface. The following information is color-encoded onto these representations. The unstructured protein trains, removed in the crystallized protein, are shown in purple and the Cys residues which mark the substrate binding pocket is shown in green. The two crystallized core domains are shown in a heat map that encodes displacement of the core AA residues from their positions in the crystal structure. We identified a region in the protein core as least distorted, shown in blue in the heat map, and aligned the two structural models on this protein region. Overall, both models show deviations from the crystal structure, but the solution structure is less distorted than the membrane-bound state of the protein (with RMS displacements averaged over the entire core protein of 0.7 Å and 1.0 Å, respectively). There was no clear hinge identified in the adjustment of the protein structure to the membrane surface. Specifically, the interface between the two domains remained rather rigid, as the protein regions bordering this interface were only marginally displaced in the two models. The largest displacements from the crystal structure was observed at the far side of the PD in the membrane-bound state, shown in red in Fig. 10B, where the α-helix designated as pα1 (AAs 50-60) (Lee et al., 1999), is collectively displaced to flatten the membrane-binding interface against the membrane. The lack of gross reorganization of protein structure was also observed in the conservation of the secondary structure content (Table 2) which was identical in the two models. This close correspondence between the secondary structural compositions is consistent with infrared spectroscopy studies that showed spectral changes upon wt PTEN binding to PI(4,5)P2-containing membranes but did not detect such changes upon binding to PC/PS bilayers (Redfern et al., 2008). In terms of dynamics, both models showed similar flexibility (time-averaged root-mean-square displacements of the individual AA residues) in the simulations. The only conspicuous difference was the flexibility of the CBR3 loop, which was observed to be rather dynamic in solution, but considerably more constrained in the membrane-bound state, where it is ligated to PS.
Figure 10.
Differences of the wt PTEN protein structure in solution (A) and in the membrane-bound state (B). Unstructured segments not included in the crystal structure, are shown in purple. The catalytic site is indicated by its two Cys residues, shown in green. The remainder of the protein, i.e., the PTEN core domains, are color encoded according to the deviations of their Cα positions from those observed in the crystal structure. While both the solution and the membrane-bound models deviate somewhat from the crystal structure (Lee et al., 1999), major portions of the C2 domain and, less so, of the PD are rigid. This is also true for the interface between the two core domains. In the membrane-bound state, the single major adjustment of the structure concerns the region around the pα1 helix, to the far left of the model, and leads to a flattening of the overall protein structure against the membrane.
Table 2.
Secondary structure content of PTEN. core relates to PTEN’s core domains, PD and C2, i.e., those stretches of the full-length protein that are included in the crystal structure (Lee et al., 1999). unstructured indicates the remainder of the full-length protein, i.e., those stretches that were not included in the crystal structure.
| system | α helix (%) | β sheet (%) | random coil (%) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| PTEN | core | unstructured | core | unstructured | core | unstructured |
| crystal structure (truncated) |
21.5 | n.a. | 31.3 | n.a. | 14.7 | n.a. |
| in solution | 24 ± 1 | 13 ± 1 | 30 ± 1 | 0 | 17 ± 2 | 46 ± 6 |
|
| ||||||
| membrane-bound | 23 ± 1 | 8 ± 3 | 30 ± 1 | 0 | 16 ± 2 | 52 ± 5 |
Discussion
High-resolution structures of PTEN bound to the lipid membrane are urgently needed to resolve even the most straightforward questions about the functioning of this phosphatase in cell signaling. Is PTEN autonomously capable of membrane binding or does its membrane association depend on an adhesion promoter? A recent study suggests that SUMO1 modification of the CBR3 loop is essential for the membrane binding of the protein (Huang et al., 2012). Huang and coworkers show an impressive body of cell biological evidence that points to a critical role for SUMO1 to promote membrane binding. However, direct structural evidence in that work is weak. Short all-atom MD simulations of PTEN-SUMO1 in solution were analyzed for potential membrane binding interfaces of the hybrid protein, and it was concluded that SUMO1 in conjunction with basic surfaces areas on the C2 domain may electrostatically interact with the anionic bilayer. In this new model, the proposed structure of the membrane-bound PTEN-SUMO1 hybrid protein has the PTEN associated with the membrane in an orientation that is essentially perpendicular to the protein’s membrane orientation suggested by the crystal structure of the truncated protein (Lee et al., 1999). In the two contradictory models, therefore, the orientations of PTEN at the membrane differ by about 90 degrees, and it is crucial to determine which is the correct one in order to establish how the substrate gains access to the catalytic site. As we showed experimentally (Shenoy et al., 2012) and confirmed in this work with extensive MD simulations that include the lipid membrane, PTEN binds in vitro to anionic bilayers without SUMOylation. In the absence of SUMO, the orientation of the PTEN protein is close to that suggested by the crystal structure. This is consistent with our earlier SPR work, in which we determined a binding constant, Kd ≈ 12 μ;M/L of wt PTEN to mixed PC/PS bilayers (Shenoy et al., 2012).
The resolution achieved in NR is not sufficient to decide whether or not the PTEN core under-goes a structural reorganization upon transfer from solution to the membrane. Nevertheless, the corre-spondence between the NR and MD results can be read in two ways: (1) The excellent agreement serves as an experimental validation of the MD simulations. Refinement of the parameterization for MD simulations of lipids and proteins in the past two decades has established progressively more realistic parameter sets. Yet, there has been a history of persisting issues (Benz et al., 2005; Benz et al., 2006; Mihailescu et al., 2011), often resulting in improvements of the consensus potentials (Klauda et al., 2010; MacKerell et al., 2004; Mallajosyula et al., 2012; Patel et al., 2004). The correspondence between experiment and simulation results obtained here suggests that our use of the latest parameter sets leads to a realistic description of the system in silico. (2) Vice versa, the MD simulation also verifies the experimental result that PTEN’s association with PS-bearing membranes is strictly interfacial, with virtually no penetration of the protein into the hydrophobic core of the bilayer. It also supports the tentative interpretation of recent NR results that the C-terminal tail is repelled from the membrane surface and organized on the cytosolic side of the protein in the membrane bound state (Shenoy et al., 2012). However, the simulations provide additional detail on protein-membrane binding and can reveal inter-actions, based upon persistent atomic-scale localizations, that occur on a strictly local level between hydrophobic AA residues and the lipid backbone region. Evidently, such detail could not be identified from scattering experiments because of insufficient resolution.
Reference Structure for a Membrane-Bound Phosphatase
Figure 10 provides a comparison of the equilibrium structures of wt PTEN in solution and in the membrane-bound state. High-resolution structures of proteins in their near-natural membrane environment are still hard to obtain. Here, we determined a reference structure for the membrane-bound phosphatase PTEN on a mixed phospholipid bilayer composed of zwitterionic and anionic lipids. Details of the adjustment of the protein structure to its membraneous environment upon binding from the cytosol are thus becoming clear. We provided independent evidence from NR and MD simulations that the membrane-bound enzyme scoots on the membrane peripherally in its active state. Only the CBR3 loop snorkels through the headgroup region of the bilayer and interacts with membrane lipid backbones. Beyond this hydrophobic anchor, the interactions between the protein and the bilayer are dominantly electrostatic.
In broad strokes, this picture already emerged from the analysis of recent NR studies (Shenoy et al., 2012), in which we measured the shallow penetration of the PTEN protein into the bilayer and identified the organization of its C-terminal tail tentatively by comparing the envelope nSLD with that of the crystal structure, appropriately placed on the bilayer. Scattering techniques offer an inherent strength that they can provide structural information for intrinsically disordered systems – a feat that is particularly valuable for membrane proteins in their thermally disordered membraneous environment and for proteins, such as PTEN, with major unstructured segments. However, NR does not provide the resolution required to distinguish between slightly different protein conformations nor does it reveal inplane structural information. MD simulations are a powerful complementing tool to overcome these shortcomings. In Fig. 4, we demonstrate the near-perfect correspondence between the nSLD distribution across the membrane-bound PTEN protein, as deduced from NR, its tentative interpretation utilizing the crystal structure of the truncated PTEN, and the detailed, entirely independently determined structural model from all-atom simulations. Not only does this close agreement justify the interpretation of the NR data, but it also provides validation and a strong endorsement for the MD results, thereby paving the way to analyze the simulations for further insights into the supramolecular structure, the interactions that drive its formation, and its molecular-scale dynamics. This achievement also sets the stage for more in-depth analyses of the interaction of membrane-bound PTEN with its substrate, PI(3,4,5)P3, and its product, PI(4,5)P2, which plays a major role in the recruiting of the phosphatase to the membrane (Das et al., 2003; Iijima et al., 2004; Shenoy et al., 2012; Vazquez et al., 2006). It will further facilitate studies of the mechanisms by which phosphorylation of the protein and functional mutations modify these interactions (Redfern et al., 2010; Shenoy et al., 2012).
Comparison with the Proposed PTEN-SUMO1 Membrane Structure
By invoking SUMOylation as a critical element of PTEN membrane binding (Huang et al., 2012), Huang and coworkers presented a model that is grossly different from that deduced from the crystal structure (Lee et al., 1999). Our earlier NR studies (Shenoy et al., 2012) ascertained that PTEN binds to anionic membranes on its own, i.e. without the need for an auxiliary SUMO adhesion promoter. Moreover, the NR results confirm unquestionably without any detailed model that the PTEN membrane orientation is consistent with that suggested by the crystal structure. The approximately perpendicular protein orientation on the membrane in the alternate PTEN-SUMO1 model (Fig. 4a in Huang et al., 2012) has PTEN extend from the bilayer surface by ≈ 65 Å, which is in sharp contrast to the nSLD profile derived from neutron scattering (Fig. 4 of this work). Because the MD simulation work reported here was completed well before the publication of the SUMOylation work, we did not consider a PTEN-SUMO1 construct in our own study. Nevertheless, our MD results confirm that PTEN binds the membrane on its own and associates with the bilayer in an orientation that brings the catalytic site close to its membrane-bound substrate. Indeed, since our own explicit-membrane simulations suggest that the CBR3 loop of the C2 domain drives membrane association of PTEN with the anionic bilayer, it is hard to comprehend that an obstruction of this loop by SUMOylation at K266 might enhance membrane binding. Moreover, in the PTEN-SUMO1 model structure, the catalytic site is located ≈ 30 Å away from the lipid headgroups of the bilayer and direct access of the substrate to this site is obstructed by the membrane-bound SUMO1, such that a significant reorganization of the protein-membrane complex from the structure proposed by Huang and coworkers is apparently required for catalytic activity. We also comment that functional evidence showed that PTEN K266 mutants retain their catalytic activity in vivo and in vitro (Liu et al., 2005; Redfern et al., 2008) which raises further doubts about the suggested role of the proposed SUMOylation site. Finally, while there are currently > 2,000 tumor-relevant mutations documented for PTEN, none of those has been observed at K266, the putative SUMOylation site (see http://www.sanger.ac.uk/perl/genetics/CGP/cosmic).
Adjustment of the PTEN Structure to the Membrane Surface Upon Membrane Binding
The small reorganizations that occur on the PTEN protein upon binding to the membrane from the cytosol are clearly beyond the resolution of NR measurements. Yet, with MD simulations validated by the close correspondence of structural features that NR can determine with confidence, we assume that the finer details revealed by these simulations are also realistic descriptions of the supramolecular structure. The PTEN crystal structure showed the substrate binding pocket deeply buried in the PTEN protein and tugged away from the putative membrane binding surface of the protein (Lee et al., 1999). This raised the question whether the protein might dig into the membrane surface to gain access to its substrate or, alternatively, might pull the PI(3,4,5)P3 lipid out of the bilayer by a short distance to gain access. Our results suggest that neither is strictly true. The phosphatase scoots the membrane superfi-cially, with only the CBR3 loop pene trating the bilayer surface, and at the same time adjusts its structure slightly to the membrane surface by flattening its membrane binding interface. This small rearrangement brings the substrate binding pocket closer to the membrane surface. One major caveat is that we do not yet have a full picture of the protein membrane interactions: It is clear that binding to PI(4,5)P2 is a major factor that controls the membrane association of the enzyme (Redfern et al., 2008; Shenoy et al., 2012; Vazquez and Devreotes, 2006; Vazquez et al., 2006), and that the structural changes of the protein upon binding to PI(4,5)P2-containing membranes are more pronounced than upon binding to PC/PS membranes (Redfern et al., 2008). However, in this study we have not yet included any PIP lipids into the model system, and it remains to be seen how the presence of PI(4,5)P2 modifies the structural picture developed here.
The MD results suggest that there are quantifiable differences between the PTEN crystal structure and both its structures in solution and on the membrane. In fact, the deviation of the solution structure from the crystal coordinates is almost as large as that of the protein in the membrane-bound state. Overall, however, the adjustments of the PTEN solution structure to the membrane are subtle and do not invoke major reorganization of secondary structure elements (Table 2 and Fig. 10). This is consistent with the lack of evidence for rearrangement of secondary structure in IR data (Redfern et al., 2008). In particular, a close examination of the inter-domain interface does not give indications for any major rearrangements of the domains against each other. Nevertheless, there is a change in protein gesture upon membrane binding which flattens the membrane binding interface of the protein against the membrane surface. So, how does this adjustment to the membrane come about? It is well established that the membrane surface is a chemical environment prolific with donor/acceptor moieties for molecular interactions (White and Wimley, 1999; White et al., 2001). Replacement of hydration water on the protein surface with lipid headgroups and the formation of electrostatic interactions may lower the total energy of the system and induce changes in the region around of the pα1 helix, shown to the far left in Fig. 10A,B. The speed by which the protein model settled into a stable structure after the initial placement near the bilayer hints that a coordinated action of multiple interactions, each minor, adjust the PTEN structure to the membrane and indicates that the combined free energy driving these adjustments must be substantial. Similar to the case of HIV Gag matrix domain, which we have recently studied with NR (Nanda et al., 2010), it is apparently the electrostatic interactions with the membrane surface that steer the protein into the correct orientation for membrane binding. This is suggested by the major readjustment of orientation of the protein with respect to the membrane observed in the MD trajectories (Fig. 3B). Later on, it is then the binding of PS through the CBR3 loop and, to a lesser extent, PS binding to the PD that recruits the phosphatase to the membrane, even in the absence of PI(4,5)P2. It remains to be seen in future work how the highly charged phosphoinositide adds specificity and even more stable anchoring to this general picture.
Functional Role of the Disordered C-terminal Tail of wt PTEN
Electrostatic interactions also provide the molecular basis for the functioning of the C-terminal tail as a regulator of PTEN-membrane interactions. It was shown that the occupancy of phosphorylation sites, in particular that of S370, S380, T382, T383, and S385, impacts on the activity of PTEN in vivo (Vazquez et al., 2000) and that control of PTEN activity is exerted through regulation of its membrane association (Rahdar et al., 2009). Furthermore, co-expression of truncated PTEN and the C-terminal tail (PTEN352-403) rescued the regulatory features, indicating that the isolated C-terminal tail binds the PTEN core independent of whether or not it is linked to the protein (Rahdar et al., 2009). This is strong evidence that the regulatory function of the tail is achieved by switching the full-length pro-tein between a membrane-binding competent (“open”) and an incompetent (“closed”) state (Ross and Gericke, 2009; Song et al., 2012). Here, we observe dramatic differences between the organization of the protein-bound tail in the presence and absence of an anionic membrane surface: In solution, the C-terminal tail wraps around the C2 domain, forming a sequence of salt bridges with the surface of the core protein, and obstructing its membrane binding interface with the tip of the tail. In distinction, the tail forms a disordered cytoplasmic peptide coil, internally stabilized through multiple, fluctuating hydrogen bonds, in the membrane-bound state, presumably because of electrostatic repulsion of the strongly acidic peptide stretch by acidic lipids in the membrane.
When analyzing the swift reorganization of the tail from its initial, artificially extended conformation to its equilibrium state in which it is bound against the C2 domain, we observed the closing of a zipper of salt bridges that stabilized this state, which suggests a pathway by which the protein transitions from its membrane-bound organization to its solution conformation. It was previously reported that a PTEN variant without the C-terminal tail binds to PC/PS membranes with higher affinity than wt PTEN, Kd ~ 4 μ;M and ~ 12 μ;M, respectively (Shenoy et al., 2012). The outcome of this MD simulation explains this result as an auto-inhibitory effect that is due to the tail’s association with the C2 domain and the formation of salt bridges with its PS-binding CBR3 loop (Fig. 8C). The simulation also indicated that this solution conformation of the full-length protein is subject to fluctuations of the interaction network on the time scale of hundreds of ns, as indicated by the observed interchanges between stabilizing salt bridges. This suggests that the tail can release its grip on the membrane-binding surface if the protein enters the electrostatic potential of an anionic membrane.
It remains to be seen how phosphorylation impedes such fluctuations of the interaction pattern. One hint on tail phosphorylation as a regulatory mechanism is that the AA residues in the PEST motif, which have been experimentally demonstrated to be phosphorylated (Vazquez et al., 2000), are positioned at kink points of the C-terminal tail as it runs in loops along the C2 domain in the structure predicted by the simulation, such that they are solvent exposed and should be very well susceptible to external modification. For example, phosphorylation near the end of the C-terminal tail may results in a much stronger binding between the tail end and the PS-binding site on the CBR3 loop of the C2 domain, thereby preventing membrane association by locking the protein into a closed state in solution (Gericke et al., 2006). Such a regulation of PTEN activity may be critical for maintaining various pools of the protein, of which only a small fraction of the total PTEN population may be competent of membrane association (Ross and Gericke, 2009).
Conclusions
We provided a reference structure for an active phosphatase, PTEN, docked onto a disordered phospholipid bilayer by combining MD simulations and neutron reflectometry of the protein bound to an artificial membrane model. MD simulations of PTEN on the membrane were validated by the NR results and provided confidence that the simulations provide realistic models for details on the protein structure and its association with membranes that are beyond the resolution of the NR experiments.
No evidence was revealed for a dependence of PTEN membrane binding on its SUMOylation, as the unmodified wt protein binds perfectly well to anionic membranes in an orientation that is close to the one deduced from the crystal structure. We observed that the PTEN protein hugs the membrane tightly but strictly superficially, with only the leading part of its CBR3 loop on the C2 domain snorkeling through the lipid headgroup layer. Persistent electrostatic interactions anchor the CBR3 motif tightly to the bilayer surface, and two hydrophobic AA sidechains, M264 and L265, dock to the lipid backbone. PTEN’s PD also engages in electrostatic interactions with membrane lipids which are, however, more short lived. Upon membrane binding, the protein flattens against the bilayer, thus bringing its catalytic binding pocket closer to the substrate. However, these conformational changes are minute and well beyond the resolution of the NR experiments. Overall, the membrane binding of the PTEN protein reduces lipid diffusion in the proximal bilayer leaflet significantly.
The reorganization upon membrane binding of the major unstructured peptide train on the PTEN protein, the 50 AA C-terminal tail, is more prominent, such that its conformation can be independently characterized by NR. In the membrane-bound state, the strongly anionic tail is repelled by the anionic membrane surface and forms an unstructured coil on the cytoplasmic side of the protein that is characterized by fluctuating internal hydrogen bonds. In distinction, this same protein segment is bound to, and wraps around, the C2 domain in solution where it is stabilized by multiple salt bridges that fluctuate on a slower time scale (hundreds of ns). This brings the tip of the C-terminus onto the membranebinding interface of the PTEN protein where it interferes with membrane binding, engaging in salt bridges with the CBR3 loop. The transition from the extended state to C2-bound state of the tail is surprisingly fast, ca. 100 ns, and follows a path that resembles the closing of a zipper. Phosphorylation of the tail in this bound state could lock in this configuration, thus providing a plausible mechanism for regulation of the membrane-binding competence of the PTEN phosphatase.
Materials and Methods
Sample Preparation and Neutron Reflection Measurements
Full detail of the sample preparation and NR experiments have been recently published (Shenoy et al., 2012). stBLMs were prepared on [100]-cut Si wafers (Silicon Quest International) onto which a ~ 150 Å thick Au layer was sputtered on top of a ~ 20 Å Cr bonding layer. stBLMs were prepared by rapid solvent exchange (Cornell et al., 1997; McGillivray et al., 2007) by transferring freshly goldcoated wafers into a 3:7 (mol:mol) 0.2 mM (total concentration) ethanolic solution of Z 20-(Z octadec-9-enyloxy)-3,6,9,12,15,18,22-heptaoxatetracont-31-ene-1-thiol (HC18) and β-mercaptoethanol (βME), followed by assembly into the neutron sample cell (Dura et al., 2006), incubation of the surface with an ethanolic solution of the phospholipids at the desired mixing ratio (DOPC/DOPS 7:3 with 3% of cholesterol added; lipids from Avanti Polar Lipids) and the rapid displacement of this lipid solution with aqueous buffer. The sample was placed on the sample stage of the AND/R reflectometer (Dura et al., 2006) at the NIST Center for Neutron Research and the neat stBLM measured in different isotopic buffers. PTEN protein was then injected at ~ 20 μ;M concentration and the protein/membrane sample again measured at various solvent contrasts. The results were analyzed by modeling the membrane as a continuous distribution of submolecular fragments (CD model) (Shekhar et al., 2011). The NR measured for the protein-containing samples was modeled by describing the mass distribution of the protein at the interface with Catmull-Rom splines (Catmull and Rom, 1974) and accounting for the proportional exchange of hydrogens on PTEN in isotopically distinct buffers when determining nSLD distributions. With this procedure, five individual NR curves (3 for the neat stBLM and 2 for the stBLM with protein) were simultaneously evaluated. Monte-Carlo resampling then provided the most probable nSLD distributions (Heinrich et al., 2009), as well as a band of nSLD that indicates a specified confidence level. Shown in the comparison of the experimental NR results with the MD results (Fig. 4), is the 1σ confidence level.
MD Simulations
The MD simulations were set up using the NAMD 2.8 software package (Phillips et al., 2005) with the CHARMM22 (MacKerell et al., 1998) and CHARMM36 (Klauda et al., 2010) force field parameters to describe protein and lipids (DOPC and DOPS), respectively. The simulations were performed using periodic boundary and NPT conditions at a constant temperature of 300 K using a Langevin thermostat with a damping coefficient of 1 ps-1 and a constant pressure of 1 atm achieved by a Langevin piston barostat. The non-bonded van der Waals potential energy was smoothly truncated between 10 Å and 12 Å with non-bonded pairs calculated within a 13.5 Å cutoff. Full electrostatic interactions were implemented using the Particle Mesh Ewald method with a grid spacing of 1 Å. The simulations were then performed by integrating every 2 fs and the trajectories analyzed using the VMD 1.9.1 software package (Humphrey et al., 1996) and th e MDAnalysis toolkit (Michaud-Agrawal et al., 2011).
Lipid Bilayer
A 50 Å × 50 Å equilibrated DOPC bilayer patch that consisted of 36 lipids per leaflet was obtained from prior work (Klauda et al., 2010), and 9 copies stitched together to form a 150 Å × 150 Å patch. The bilayer was immersed in TIP3 water and sodium chloride added to establish a total concentration of 100 mM to match experimental conditions. The system energy was minimized for 1,000 steps, followed by 2 ns of equilibration. The psfgen structure building tool in VMD was then used to mutate 1 out of 3 lipids in the equilibrated DOPC bilayer to provide a DOPC/DOPS = 2:1 membrane. Extra salt was added to neutralize the bilayer and to readjust the total salt concentration to 100 mM. The system energy was minimized for 1,000 steps, forces were applied to keep water out of the membrane for 0.5 ns followed by a 14 ns equilibration run free of external forces.
wt PTEN in Solution
The truncated PTEN crystal structure (PDB ID: 1D5R) (Lee et al., 1999) was obtained and AA residues 1-13, 282-312 and 352-411 added to the structure along with a C-terminal His6-tag (used to purify the protein for NR experiments) to simulate the experimental conditions as closely as possible. The SASSIE software (Curtis et al., 2012) was used to generate extended conformations of the unstructured protein stretches as starting configurations for the all-atom MD runs. Simulated annealing (SA) was used to equilibrate the added residues. For the SA protocol, PTEN was simulated in implicit solvent using a dielectric constant of 80. Amino acids resolved in the crystal structure were held fixed while MD simulations were run using a temperature ramp from 300 K to 600 K in 15 K increments over 300 ps. The system was then held at 600 K for 600 ps and ramped back to 300 K in 300 ps. The SA cycle was repeated three times for a total of 3.6 ns of equilibration. Finally, the protein was placed in a 110 Å × 200 Å × 145 Å solvation box (protein dimensions plus 25 Å in each direction of space) with sodium chloride salt to neutralize the system and establish a total concentration of 100 mM. The simulation box then contained approx. 74,000 water molecules. With the PTEN protein core held fixed, the system energy was minimized for 1,000 steps. Subsequently, a 3 kcal/mol harmonic force constraint was applied for one ns, followed by a 1 kcal/mol constraint for another ns. The fixation of the protein core was then released and a 1 kcal/mol constraint was applied for a further 1 ns before initiating a 350 ns production run without constraints.
Placement of wt PTEN on the Membrane
The equilibrated PTEN protein structure obtained in solution at the 9 ns simulation time point was taken and combined with the equilibrated DOPC/DOPS membrane obtained at the 14 ns simulation time point of that run. The protein was centered with respect to the bilayer and shifted along the bilayer normal to create a ~ 10 Å layer of water that separated the protein from the lipid headgroups. An extra 40 Å depth of water was added along the bilayer normal to account for flexibility of the C-terminal tail. The simulation box then contained approx. 104,000 water molecules. Sodium chloride was added to neutralize the system and to establish the total concen-tration at 100 mM. The entire protein was held fixed for 5 ns, followed by a 3 kcal/mol force harmonic constraint applied for 5 ns and a 1 kcal/mol constraint for further 5 ns. Finally, all constraints on the protein were released and a 300 ns production run was initiated.
Analysis of Lipid Diffusion
Einstein’s equation relates the self diffusion constant, D, to an observed mean square displacement as 〈 x2 〉 = 2d · Dτ where τ is the observation time and d is the dimensionality of the system. This is implemented as
with d = 2 for diffusion in a lipid bilayer. r(t) is the x,y position of the center of mass of a lipid at time t. Angled brackets indicate an ensemble average over all lipid molecules of a certain class and over multiple time origins, t0. Every 100 ps time step in the production run of the simulation was taken as t0 and the mean square displacement calculated as a function of τ from that point. We used τmax = 60 ns as a cutoff for τ because at larger correlation times fewer independent origins are available which results in poor statistical averaging of the mean square displacement. The diffusion constant was then calculated from a linear regression of the mean square displacement as a function of the correlation time, τ.
Analysis of Protein Orientation on the Bilayer and of Molecular Interactions
The principal axes of the protein were calculated for the crystal structure residues and for the bilayer lipid phosphates on the the proximal side of the membrane at 100 ps intervals. We then determined the Euler angles θ and φ of the protein with respect to the bilayer by measuring the angle between the polar axes of protein and bilayer, resulting in θ, and the rotation of the protein about its polar axis, resulting in φ.
A hydrogen bond was considered to be formed between a donor atom (bearing the bonded hydrogen) and an acceptor atom if the distance between the two was less than 3 Å and the angle between the donor, the hydrogen involved in bonding and the acceptor was less than 60°. A salt bridge was considered to be formed between an oxygen atom of an acidic residue and a nitrogen of a basic residue if the two were within 3.2 Å of each other. Residence times of membrane lipids were defined as consecutive time spent within 5 Å of an AA residue.
Supplementary Material
Acknowledgments
We thank Dr. A. H. Ross for PTEN protein used in the NR experiments and Dr. D. J. Vanderah for the HC18 membrane anchor used in the stBLMs. We also thank Drs. A. Gericke and A. H. Ross for insightful discussions and for critically reading the manuscript. This work was supported by the NIH (1P01 AG032131) and the Department of Commerce (70NANB8H8009 and 70NANB11H8139). Beamtime at the NIST Center for Neutron Research is gratefully acknowledged. This study utilized computational capabilities of the Biowulf Linux cluster at the NIH (http://biowulf.nih.gov), the Extreme Science and Engineering Discovery Environment (XSEDE), supported by the NSF (OCI-105357) with computations performed at the NICS (http://www.nics.tennessee.edu/) and the Pittsburgh Supercomputing Center (BIO110004P).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: PTEN, phosphatase and tensin homologue deleted on chromosome 10; PI3K, phosphatidylinositol-3-kinase; AA, amino acid; PD, phosphatase domain; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; DOPS, 1,2-dioleoylsn-glycero-3-phosphoserine; PIP, phosphatidylinositolphosphate; PBM, PIP binding module; PI(4,5)P2, phosphatidylinositol-4,5-diphosphate; PI(3,4,5)P3, phosphatidylinositol-3,4,5-triphosphate; βME, β-mercaptoethanol; HC18, Z 20-(Z octadec-9-enyloxy)-3,6,9,12,15,18,22-heptaoxatetracont-31-ene-1-thiol; MD, molecular dynamics; stBLM, sparselytethered bilayer lipid membrane; NR, neutron reflection; nSLD, neutron scattering length density.
Electronic Supplementary Material
The following Electronic Supplementary Material is available for this paper:
(1) Production run of the PTEN simulation in solution (file name: <PTEN in Solution.mpg>)
(2) Production run of the PTEN simulation on a DOPC/DOPS (2:1) bilayer (file name: <PTEN on PC-PS.mpg>)
(3) Animation of the structural differences of the PTEN core domains in solution and on the membrane (file name: <ConfChange.gif>), prepared using the Morph Server (W.G. Krebs and M. Gerstein, 2000. Nucleic Acids Res. 28, 1665).
Bibliography
- Benz R, Castro-Román F, Tobias DJ, White SH. Experimental validation of molecular dynamics simulations of lipid bilayers: A new approach. Biophys. J. 2005;88:805–817. doi: 10.1529/biophysj.104.046821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz RW, Nanda H, Castro-Roman F, White SH, Tobias DJ. Diffraction-based density restraints for membrane and membrane-peptide molecular dynamics simulations. Biophys. J. 2006;91:3617–3629. doi: 10.1529/biophysj.106.084483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittova L, Sumandea M, Cho W. A structure-function study of the C2 domain of cytosolic phospholipase A2. Identification of essential calcium ligands and hydrophobic membrane binding residues. J. Biol. Chem. 1999;274:9665–9672. doi: 10.1074/jbc.274.14.9665. [DOI] [PubMed] [Google Scholar]
- Campbell RB, Liu F, Ross AH. Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 2003;278:33617–33620. doi: 10.1074/jbc.C300296200. [DOI] [PubMed] [Google Scholar]
- Catmull E, Rom R. A class of local interpolating splines, p. 317-326. In: Barnhill RE, Reisenfeld RF, editors. Computer-Aided Geometric Design. Academic Press; New York: 1974. pp. 317–326. [Google Scholar]
- Chapman ER, Davis AF. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 1998;273:13995–14001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- Cornell BA, Braach-Maksvytis VLB, King LB, Osman PDJ, Raguse B, et al. A biosensor that uses ion-channel switches. Nature. 1997;387:580–583. doi: 10.1038/42432. [DOI] [PubMed] [Google Scholar]
- Curtis JE, Raghunandran S, Nanda H, Krueger S. SASSIE: A program to study intrinsically disordered biological molecules and macromolecular ensembles using experimental scattering constraints. Comp. Phys. Commun. 2012;183:382–389. [Google Scholar]
- Das S, Dixon J, Cho W. Membrane-binding and activation mechanism of PTEN. Proc. Natl. Acad. Sci. USA. 2003;100:7491–7496. doi: 10.1073/pnas.0932835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–90. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Dura JA, Pierce D, Majkrzak CF, Mali szewskyj N, McGillivray DJ, et al. AND/R: A neutron diffractometer/reflectometer for investigation of thin films and multilayers for the life sciences. Rev. Sci. Instrum. 2006;77:074301. doi: 10.1063/1.2219744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc. Natl. Acad. Sci. USA. 1999;96:10182–10187. doi: 10.1073/pnas.96.18.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke A, Munson M, Ross AH. Regulation of the PTEN phosphatase. Gene. 2006;374:1–9. doi: 10.1016/j.gene.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Heinrich F, Ng T, Vanderah DJ, Shekhar P, Mihailescu M, et al. A new lipid anchor for sparsely tethered bilayer lipid membranes. Langmuir. 2009;25:4219–4229. doi: 10.1021/la8033275. [DOI] [PubMed] [Google Scholar]
- Huang J, Yan J, Zhang J, Zhu S, Wang Y, et al. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat. Commun. 2012;3:911–922. doi: 10.1038/ncomms1919. [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Iijima M, Huang YE, Luo HR, Vazquez F, Devreotes PN. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J. Biol. Chem. 2004;279:16606–16613. doi: 10.1074/jbc.M312098200. [DOI] [PubMed] [Google Scholar]
- Kahya N, Scherfeld D, Bacia K, Schwille P. Lipid domain formation and dynamics in giant unilamellar vesicles explored by fluorescence correlation spectroscopy. J. Struct. Biol. 2004;147:77–89. doi: 10.1016/j.jsb.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Klauda JB, Venable RM, Freites JA, O’Connor JW, Tobias DJ, et al. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B. 2010;114:7830–7843. doi: 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, et al. Crystal structure of the PTEN tumor suppressor: Implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- Li Z, Dong X, Dong X, Wang Z, Liu W, et al. Regulation of PTEN by Rho small GTPases. Nat. Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- Liu F, Wagner S, Campbell RB, Nickerson JA, Schiffer CA, et al. PTEN enters the nucleus by diffusion. J Cell Biochem. 2005;96:221–34. doi: 10.1002/jcb.20525. [DOI] [PubMed] [Google Scholar]
- MacKerell A, Bashford D, Bellott M, Dunbrack RL, Evanseck J, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- MacKerell AD, Feig M, Brooks C. Extending the treatment of backbone energetics in protein force fields: Limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem. 2004;25:1400–1415. doi: 10.1002/jcc.20065. [DOI] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Mallajosyula SS, Guvench O, Hatcher E, MacKerell AD. CHARMM additive all-atom force field for phosphate and sulfate linked to carbohydrates. J. Chem. Theory Comput. 2012;8:759–776. doi: 10.1021/ct200792v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnachie G, Pass I, Walker SM, Downes CP. Interfacial kinetic analysis of the tumour suppressor phosphatase, PTEN: Evidence for activation by anionic phospholipids. Biochem. J. 2003;371:947–55. doi: 10.1042/BJ20021848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillivray DJ, Valincius G, Vanderah DJ, Febo-Ayala W, Woodward JT, et al. Molecular-scale structural and functional characterization of sparsely tethered bilayer lipid membranes. Biointerphases. 2007;2:21–33. doi: 10.1116/1.2709308. [DOI] [PubMed] [Google Scholar]
- McGillivray DJ, Valincius G, Heinrich F, Robertson JWF, Vanderah DJ, et al. Structure of functional Staphylococcus aureus α-hemolysin channels in tethered bilayer lipid membranes. Biophys. J. 2009;96:1547–1553. doi: 10.1016/j.bpj.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud-Agrawal N, Denning EJ, Woolf TB, Beckstein O. MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011;32:2319–2327. doi: 10.1002/jcc.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihailescu M, Vaswani RG, Jardon-Valadez E, Castro-Roman F, Freites JA, et al. Acylchain methyl distributions of liquid-ordered and -disordered membranes. Biophys. J. 2011;100:1455–1462. doi: 10.1016/j.bpj.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda H, Datta SAK, Heinrich F, Lösche M, Rein A, et al. Electrostatic interactions and binding orientation of HIV-1 matrix, studied by neutron reflectivity. Biophys. J. 2010;99:2516–2524. doi: 10.1016/j.bpj.2010.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odriozola L, Singh G, Hoang T, Chan AM. Regulation of PTEN activity by its carboxylterminal autoinhibitory domain. J. Biol. Chem. 2007;282:23306–23315. doi: 10.1074/jbc.M611240200. [DOI] [PubMed] [Google Scholar]
- Patel S, MacKerell AD, Brooks C. CHARMM fluctuating charge force field for proteins: II - Protein/solvent properties from molecular dynamics simulations using a nonadditive electrostatic model. J. Comput. Chem. 2004;25:1504–1514. doi: 10.1002/jcc.20077. [DOI] [PubMed] [Google Scholar]
- Perisic O, Fong S, Lynch DE, Bycroft M, Williams RL. Crystal structure of a calcium-phospholipid binding domain from cytosolic phospholipase A2. J. Biol. Chem. 1998;273:1596–1604. doi: 10.1074/jbc.273.3.1596. [DOI] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybylo M, Sýkora J, Humpolíckova J, Benda A, Zan A, et al. Lipid diffusion in giant unilamellar vesicles is more than 2 times faster than in supported phospholipid bilayers under identical conditions. Langmuir. 2006;22:9096–9099. doi: 10.1021/la061934p. [DOI] [PubMed] [Google Scholar]
- Rahdar M, Inoue T, Meyer T, Zhang J, Vazquez F, et al. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc. Natl. Acad. Sci. USA. 2009;106:480–485. doi: 10.1073/pnas.0811212106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern RE, Redfern DA, Furgason ML, Munson M, Ross AH, et al. PTEN phosphatase selectively binds phosphoinositides and undergoes structural changes. Biochemistry. 2008;47:2162–2171. doi: 10.1021/bi702114w. [DOI] [PubMed] [Google Scholar]
- Redfern RE, Daou M, Li L, Munson M, Gericke A, et al. A mutant form of PTEN linked to autism. Protein Sci. 2010;19:1948–1956. doi: 10.1002/pro.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AH, Gericke A. Phosphorylation keeps PTEN phosphatase closed for business. Proc. Natl. Acad. Sci. USA. 2009;106:1297–1298. doi: 10.1073/pnas.0812473106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalke M, Lösche M. Structural models of lipid surface monolayers from x-ray and neutron reflectivity measurements. Adv. Colloid Interf. Sci. 2000;88:243–274. doi: 10.1016/s0001-8686(00)00047-6. [DOI] [PubMed] [Google Scholar]
- Schalke M, Krüger P, Weygand M, Lösche M. Submolecular organization of DMPA in surface monolayers: Beyond the two-layer model. Biochim. Biophys. Acta. 2000;1464:113–126. doi: 10.1016/s0005-2736(99)00249-7. [DOI] [PubMed] [Google Scholar]
- Shekhar P, Nanda H, Lösche M, Heinrich F. Continuous dist ribution model for the investigation of complex molecular architectures near interfaces with scattering techniques. J. Appl. Phys. 2011;110:102216-1–12. doi: 10.1063/1.3661986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S, Moldovan R, Fitzpatrick J, Vanderah DJ, Deserno M, et al. In-plane homogeneity and lipid dynamics in tethered Bilayer Lipid Membranes (tBLMs) Soft Matter. 2010;6:1263–1274. doi: 10.1039/B919988H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S, Shekhar P, Heinrich F, Daou M-C, Gericke A, et al. Membrane association of the PTEN tumor suppressor: Molecular details of the protein-membrane complex from SPR binding studies and neutron reflection. PLoS ONE. 2012;7:e32591. doi: 10.1371/journal.pone.0032591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L, Parsons R. PTEN: Life as a tumor suppressor. Exp. Cell Res. 2001;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- Stiles BL. Phosphatase and tensin homologue deleted on chromosome 10: Extending its PTENtacles. Int. J. Biochem. Cell Biol. 2009;41:757–761. doi: 10.1016/j.biocel.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valincius G, McGillivray DJ, Febo-Ayala W, Vanderah DJ, Kasianowicz JJ, et al. Enzyme activity to augment the characterization of tethered bilayer membranes. J. Phys. Chem. B. 2006;110:10213–10216. doi: 10.1021/jp0616516. [DOI] [PubMed] [Google Scholar]
- Valincius G, Heinrich F, Budvytyte R, Vanderah DJ, McGillivray DJ, et al. Soluble amyloid β-oligomers affect dielectric membrane properties by bilayer insertion and domain formation: Implications for cell toxicity. Biophys. J. 2008;95:4845–4861. doi: 10.1529/biophysj.108.130997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Devreotes P. Regulation of PTEN function as a PIP3 gatekeeper through membrane interaction. Cell Cycle. 2006;5:1523–1527. doi: 10.4161/cc.5.14.3005. [DOI] [PubMed] [Google Scholar]
- Vazquez F, Ramaswamy S, Nakamura N, Sellers W. Phosphorylation of the PTEN tail regulates protein stability and function. Mol. Cell. Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Matsuoka S, Sellers WR, Yanagida T, Ueda M, et al. Tumor suppressor PTEN acts through dynamic interaction with the plasma membrane. Proc. Natl. Acad. Sci. USA. 2006;103:3633–3638. doi: 10.1073/pnas.0510570103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vockenroth IK, Ohm C, Robertson JWF, McGillivray DJ, Lösche M, et al. Stable insulating tethered bilayer membranes. Biointerphases. 2008;3:FA68–FA73. doi: 10.1116/1.2912097. [DOI] [PubMed] [Google Scholar]
- White SH, Wimley WC. Membrane protein folding and stability: Physical Principles. Annu. Rev. Biomol. Struct. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- White SH, Ladokhin AS, Jayasinghe S, Hristova K. How membranes shape protein structure. J. Biol. Chem. 2001;276:32395–32398. doi: 10.1074/jbc.R100008200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.