Abstract
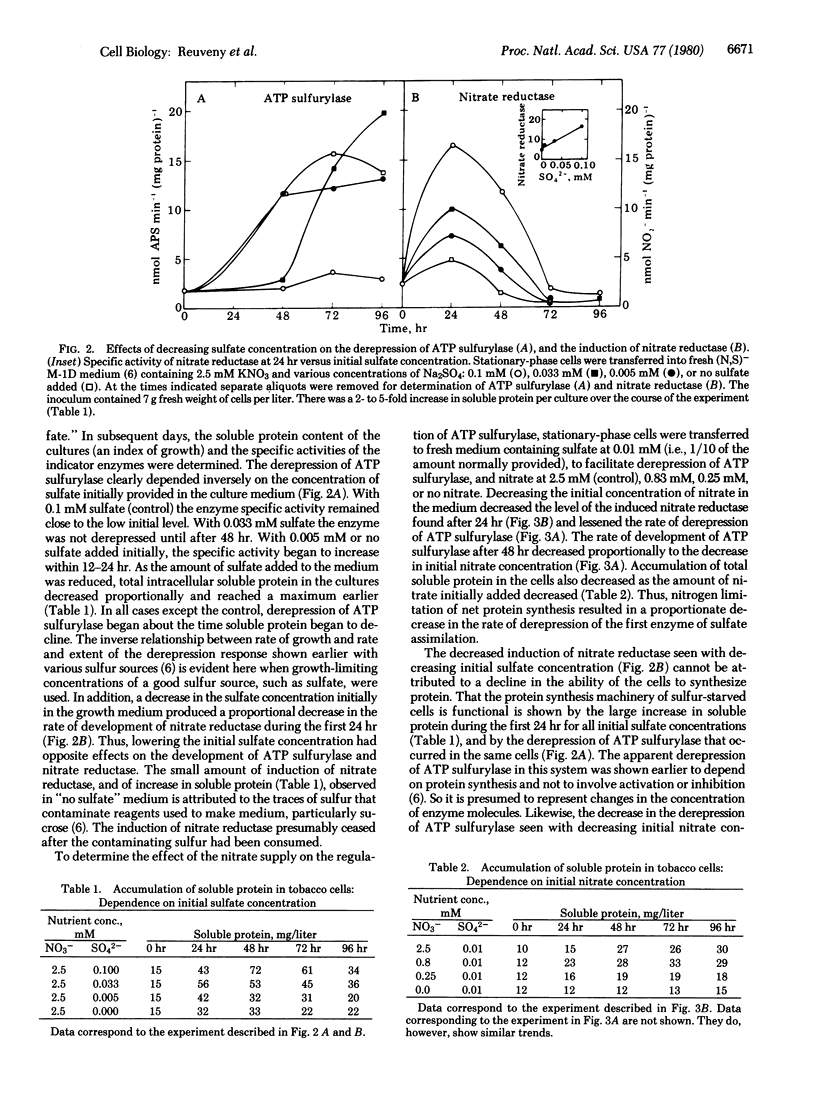
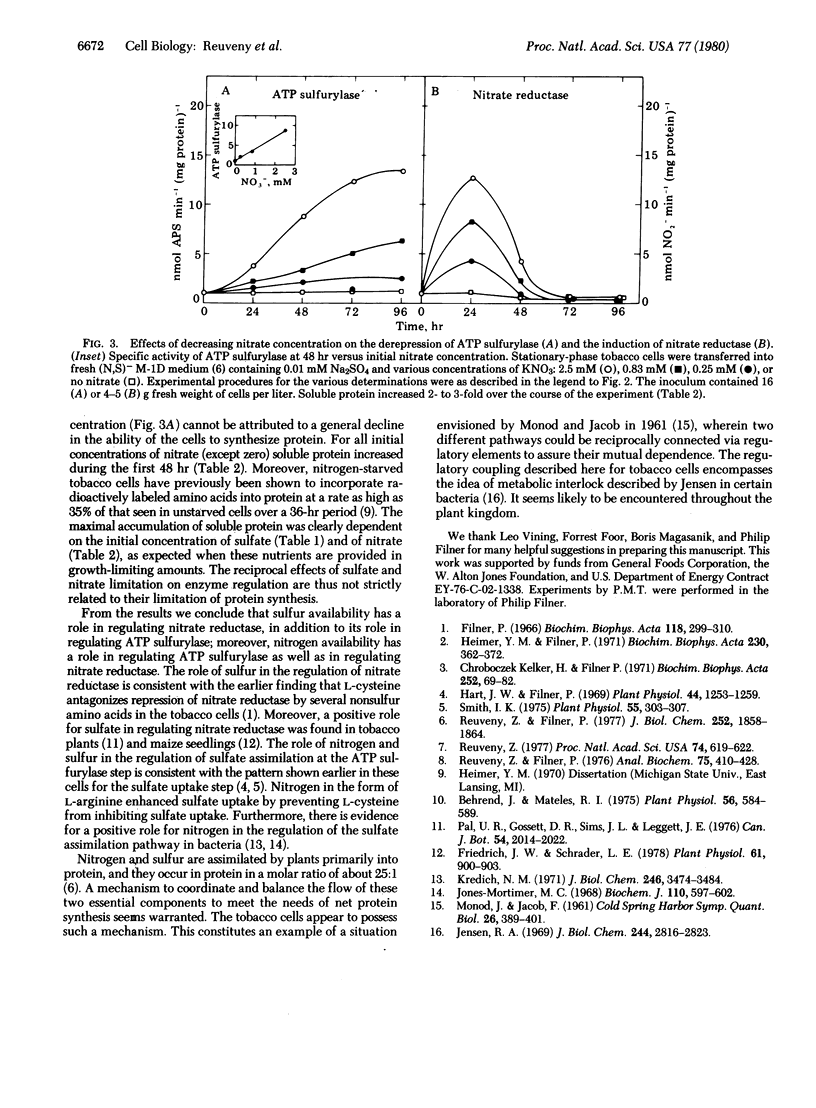
When cultured tobacco cells are provided growth-limiting concentrations of sulfur as sulfate, the rate of development of nitrate reductase (NADH:nitrate oxidoreductase, EC 1.6.6.1) is proportional to the initial sulfate concentration. When the cells are provided growth-limiting concentrations of nitrogen as nitrate, the rate of derepression of ATP sulfurylase (ATP:sulfate adenylyltransferase, EC 2.7.7.4) is proportional to the initial nitrate concentration. These results are taken to be indicative of a reciprocal regulatory coupling between the nitrate and sulfate assimilation pathways.
Keywords: nitrate reductase (NADH), sulfate adenylyltransferase, ATP sulfurylase, positive feedback regulation, nitrogen and sulfur metabolism
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrend J., Mateles R. I. Nitrogen metabolism in plant cell suspension cultures: I. Effect of amino acids on growth. Plant Physiol. 1975 Nov;56(5):584–589. doi: 10.1104/pp.56.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P. Regulation of nitrate reductase in cultured tobacco cells. Biochim Biophys Acta. 1966 May 5;118(2):299–310. doi: 10.1016/s0926-6593(66)80038-3. [DOI] [PubMed] [Google Scholar]
- Friedrich J. W., Schrader L. E. Sulfur deprivation and nitrogen metabolism in maize seedlings. Plant Physiol. 1978 Jun;61(6):900–903. doi: 10.1104/pp.61.6.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J. W., Filner P. Regulation of sulfate uptake by amino acids in cultured tobacco cells. Plant Physiol. 1969 Sep;44(9):1253–1259. doi: 10.1104/pp.44.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway in cultured tobacco cells. 3. The nitrate uptake system. Biochim Biophys Acta. 1971 Feb 23;230(2):362–372. doi: 10.1016/0304-4165(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Jensen R. A. Metabolic interlock. Regulatory interactions exerted between biochemical pathways. J Biol Chem. 1969 Jun 10;244(11):2816–2823. [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. The nature of the pleiotropic cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):597–602. doi: 10.1042/bj1100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelker H. C., Filner P. Regulation of nitrite reductase and its relationship to the regulation of nitrate reductase in cultured tobacco cells. Biochim Biophys Acta. 1971 Oct;252(1):69–82. doi: 10.1016/0304-4165(71)90093-6. [DOI] [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- MONOD J., JACOB F. Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb Symp Quant Biol. 1961;26:389–401. doi: 10.1101/sqb.1961.026.01.048. [DOI] [PubMed] [Google Scholar]
- Reuveny Z. Derepression of ATP sulfurylase by the sulfate analogs molybdate and selenate in cultured tobacco cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):619–622. doi: 10.1073/pnas.74.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny Z., Filner P. A new assay for ATP sulfurylase based on differential solubility of the sodium salts of adenosine 5'-phosphosulfate and sulfate. Anal Biochem. 1976 Oct;75(2):410–428. doi: 10.1016/0003-2697(76)90095-6. [DOI] [PubMed] [Google Scholar]
- Reuveny Z., Filner P. Regulation of adenosine triphosphate sulfurylase in cultured tobacco cells. Effects of sulfur and nitrogen sources on the formation and decay of the enzyme. J Biol Chem. 1977 Mar 25;252(6):1858–1864. [PubMed] [Google Scholar]
- Smith I. K. Sulfate transport in cultured tobacco cells. Plant Physiol. 1975 Feb;55(2):303–307. doi: 10.1104/pp.55.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]


