Abstract
Background and Aims
Differences in dormancy and germination requirements have been documented in heteromorphic seeds of many species, but it is unknown how this difference contributes to maintenance and regeneration of populations. The primary aim of this study was to compare the seed bank dynamics, including dormancy cycling, of the two seed morphs (black and brown) of the cold desert halophyte Suaeda corniculata and, if differences were found, to determine their influence on regeneration of the species.
Method
Seeds of the two seed morphs were buried, exhumed and tested monthly for 24 months over a range of temperatures and salinities, and germination recovery and viability were determined after exposure to salinity and water stress. Seedling emergence and dynamics of the soil seed bank were also investigated for the two morphs.
Key Results
Black seeds had an annual dormancy/non-dormancy cycle, while brown seeds, which were non-dormant at maturity, remained non-dormant. Black seeds also exhibited an annual cycle in sensitivity of germination to salinity. Seedlings derived from black seeds emerged in July and August and those from brown seeds in May. Seedlings were recruited from 2·6 % of the black seeds and from 2·8 % of the brown seeds in the soil, and only 0·5 % and 0·4 % of the total number of black and brown seeds in the soil, respectively, gave rise to seedlings that survived to produce seeds. Salinity and water stress induced dormancy in black seeds and decreased viability of brown seeds. Brown seeds formed only a transient soil seed bank and black seeds a persistent seed bank.
Conclusions
The presence of a dormancy cycle in black but not in brown seeds of S. corniculata and differences in germination requirements of the two morphs cause them to differ in their germination dynamics. The study contributes to our limited knowledge of dormancy cycling and seed bank formation in species producing heteromorphic seeds.
Keywords: Dormancy, halophyte seeds, salinity, seed germination, seedling recruitment, seed bank dynamics, Suaeda corniculata
INTRODUCTION
The production of two or more distinct types of seeds by a single plant is known as seed heteromorphism (Venable, 1985; Mandák, 1997; Imbert, 2002). In addition to morphological differences, heteromorphic seeds also differ in dispersal capacity (Flint and Palmblad, 1978; Venable and Levin, 1985; Imbert, 1999) and germination behaviour (Baskin and Baskin, 1976; Venable and Levin, 1985; Wang et al., 2008). Imbert (2002) compiled a worldwide list of 218 heteromorphic species, the majority of which belong to the Asteraceae and Chenopodiaceae (now included in Amaranthaceae). Studies on seed heteromorphism have focused on differences in germination responses (El-Keblawy, 2003; Carter and Ungar, 2003; Yao et al., 2010), seedling growth (Khan and Ungar, 1984; Katembe et al., 1998; Song et al., 2008) and plasticity in production of heteromorphic seeds, i.e. the proportion of heteromorphic seeds may change when the plants are grown in different environments (Imbert and Ronce, 2001; El-Keblawy, 2003). However, there are only a few comprehensive studies on the contribution of heteromorphic seeds to population regeneration (Venable and Levin, 1985).
Seed banks play an important role in ensuring persistence of plant populations (Hutchings and Booth, 1996; Leck and Simpson, 1987; Anderson et al., 2012) and contribute to future genetic variability of species (Tanksley and McCouch, 1997; McCue and Holtsford, 1998). Seeds in seed banks may go through annual dormancy/non-dormancy cycles (Baskin and Baskin, 1985; Baskin et al., 1987; Baskin et al., 1993), which regulate seed germination and seedling emergence and thus determine the optimal timing for plant establishment (Baskin et al., 1993; Baskin and Baskin, 1998). However, little information is available on seed banks (Carter and Ungar, 2003; Joley et al., 2003) and even less on dormancy cycles of the different morphs (Carter and Ungar, 2003). The only other study we are aware of on dormancy cycling in heteromorphic species was done on the amphicarphic weedy species Emex australis (Panetta and Randall, 1993). However, these investigators apparently used only aerial diaspores in their study. They did not mention that Emex produces aerial and subterranean achenes that are dimorphic (Evenari et al., 1977; Weiss, 1980).
Suaeda corniculata subsp. mongolica (hereafter Suaeda corniculata) is a leaf succulent halophyte belonging to the Chenopodiaceae (now placed in the Amaranthaceae) (Lomonosova et al., 2008), a family in which seed heteromorphism is common (Mandák and Pyšek, 2001; Imbert, 2002; Atia et al., 2011). Suaeda corniculata is an annual herb widely distributed in steppes and semi-desert zones of central southern Siberia, Mongolia and northern China (Lomonosova et al., 2008). It produces two distinct seed morphs: black seeds and brown seeds (Lomonosova et al., 2008).
The purpose of this study was to determine if there are differences in seed dormancy cycles of the two seed morphs buried in the soil under natural environmental conditions and if morphs vary in their ability to persist in the soil, i.e. to form a persistent seed bank. If so, how do the differences influence persistence of soil seed banks and regeneration of the population? To answer these questions, we tested germination of each of the two seed morphs and investigated the dynamics of their soil seed banks as well as seedling emergence. In some species of Suaeda, brown seeds are less dormant than black seeds (Song et al., 2008; Wang et al., 2008), and we hypothesized that this is the case for S. corniculata. Consequently, brown seeds in the soil are ready to germinate whenever environmental conditions are favourable for germination, while black seeds can attain high germination only when they come out of dormancy. In which case, brown seeds may be depleted from the soil seed banks faster than black ones, and more seedlings may be derived from brown than from black seeds. These aspects of the hypothesis were tested in this study. In addition, salinity and drought stress are the two most important environmental stresses facing S. corniculata, which grows in saline arid areas. Thus, germination tests were conducted on the two seed morphs after exposure to salinity and water stress to determine effects of soil salinity and drought stress on dormancy and viability of seeds in soil.
MATERIALS AND METHODS
Habitat and seed source
Mature seeds of Suaeda corniculata subsp. mongolica were collected on 20 November 2009 and 15 November 2010 from several hundred plants growing in a saline steppe on the Ordos Plateau in Inner Mongolia, northern China (38°14′N, 107°29′E; 1311 m a.s.l.). This area has a typical continental semi-arid climate with mean annual precipitation of 250–490 mm, primarily from June to August. Mean annual temperature is about 6·0–9·0 °C. Brown seeds and black seeds were hand separated after harvest, and they were tested separately in all experiments. Seeds collected in 2009 were dried at room temperature (10–18 °C, 17–32 % relative humidity) for 1 week before initiation of germination tests for fresh seeds and burial of seeds in soil. After air-drying at room temperature and hand separation, seeds collected in 2010 were dry stored at approx. –20 °C until use, except those tested for dormancy breaking and sown in the field (see below).
Black:brown seed ratio and seed mass
Fifteen individual plants were collected in natural habitats in November 2009, and all seeds were obtained from each of the 15 plants and mixed together. Five replicates of about 1000 seeds arbitrarily chosen from the mixed seed lot were divided into black or brown seeds based on colour of the testa, and the black:brown seed ratio was determined. Also, black and brown seeds were hand separated from the mixed seed lot, and the thousand-seed mass of each type was determined by weighing five replicates of 1000 seeds of each morph.
Germination tests of fresh seeds
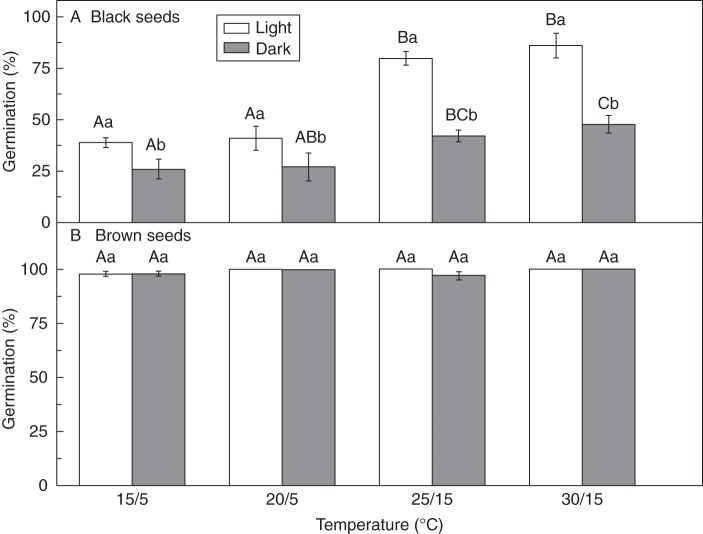
Fresh seeds of both morphs were tested for germination on filter paper moistened with distilled water at 15/5, 20/5, 25/15 and 30/15 °C (12/12 h) in the light (12 h photoperiod, approx. 100 µmol m−2 s−1, cool white fluorescent light) and in continuous darkness. The higher temperature coincided with a 12 h light period and the lower temperature with a 12 h dark period. The alternating temperature regimes represent the approximate mean daily maximum and minimum air temperatures for each month during the growing season on the Ordos Plateau: April and October, 15/5 °C; May and September, 20/5 °C; June and August 25/15 °C; and July, 30/15 °C. Four replicates of 25 seeds each were used in each test condition. Final germination percentages were determined after 20 d. Emergence of the radicle was the criterion for this and all other germination experiments. After all germination tests, non-germinated seeds were examined under a dissecting microscope to determine whether the embryo was firm and white, indicating that they were viable, or soft and grey, indicating that they were non-viable. Tetrazolium tests confirmed that the firm, white embryos were viable and the soft, grey ones non-viable. Only viable seeds were used in calculating germination percentages unless otherwise stated. Viability (%V) for each morph was calculated as [(NG = NV)/NT] × 100, where NG is the number of seeds that germinated, NV the number of non-germinated but viable seeds and NT the total number of seeds in each dish (i.e. 25) incubated at 30/15 °C in the light.
Germination of each seed morph was also tested at 0·2, 0·4, 0·6, 0·8, 1·2 and 1·6 m NaCl at 30/15 °C, using four replicates of 25 fresh seeds each. Final germination percentages were determined after 20 d. Non-germinated black seeds were tested for viability using the methods described above, and only viable seeds were used in calculating germination percentages. However, salinity decreased the viability of brown seeds, so germination percentages may be overestimated when only viable seeds were taken into account. Since viability of brown seeds was 100 % in distilled water, germination percentages (%G) of brown seeds in each Petri dish at each of the salinities was calculated as (NG/25) × 100, where NG was the number of seeds that germinated.
Dormancy breaking of black seeds
Since fresh mature black seeds were dormant (see the Results), cold stratification, gibberellic acid (GA3) and testa scarification were used to break their dormancy. Mature black seeds that were freshly collected in November 2010 were used in this experiment.
Cold stratification
Freshly collected black seeds were arranged evenly on two layers of filter paper in a metal box (20 cm length × 10 cm width × 10 cm depth) containing wet sand (10–13 % water content). The metal box was covered with a lid and kept in darkness in a refrigerator at 5 °C. After 0 (CK), 5, 10, 15 and 20 d cold stratification, four replicates of 25 seeds each were arbitrarily chosen to test germination at 15/5, 20/5, 25/15 and 30/15 °C (12/12 h) in the light (12 h photoperiod, approx. 100 µmol m−2 s−1, cool white fluorescent light). The higher temperature coincided with the 12 h light period and the lower temperature with the 12 h dark period. Final germination percentages were determined after 20 d.
GA3 treatments
Four replicates of 25 freshly collected black seeds each were tested for germination in 0 (CK), 0·1 and 1 mmol L−1 GA3 solutions at 15/5 °C (12/12 h) in the light (12 h photoperiod, approx. 100 µmol m−2 s−1, cool white fluorescent light). The higher temperature coincided with the 12 h light period and the lower temperature with the 12 h dark period. Final germination percentages were determined after 20 d.
Testa scarification
Seed coats of four replicates of 25 freshly collected seeds were scarified using a scalpel, being careful not to touch the embryos. Scarified seeds were tested for germination at 15/5 °C as described above.
Light requirement for germination of non-dormant black seeds
Fresh black seeds germinated to a higher percentage in light than in darkness (see below, Fig. 5). To determine if non-dormant black seeds also have a light requirement for germination, we tested germination of buried black seeds exhumed in May 2010, which were non-dormant (see the Results), in light (12 h photoperiod, approx. 100 µmol m−2 s−1, cool white fluorescent light), and in total darkness at 15/5, 20/5, 25/15, and 30/15 °C (12/12 h). Four replications of 25 seeds each were used. Final germination percentages were determined after 20 d.
Fig. 5.
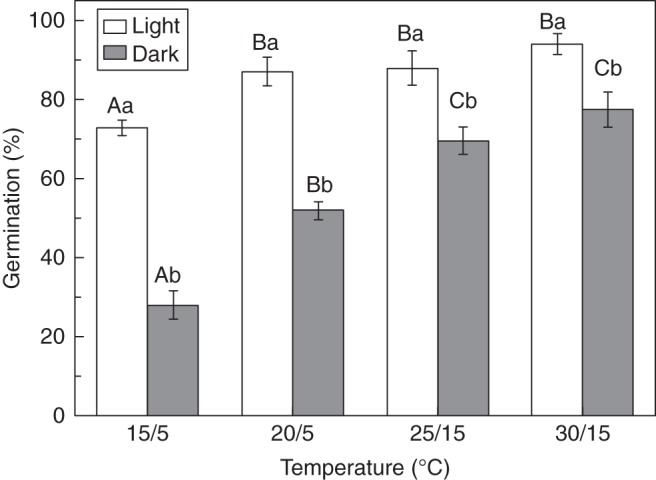
Germination percentages of black seeds of Suaeda corniculata exhumed in May 2010 and incubated at various temperatures in the light (12 h photoperiod) and in continuous darkness. Bars with the same uppercase letters are not significantly different (P > 0·05) across all temperature regimes. The same lowercase letters indicate no significant difference (P > 0·05) between germination percentages (means ± s.e.) in the light and continuous darkness with the same thermoperiod.
Dormancy cycling of the two seed morphs during burial
Following initial germination tests of fresh seeds in 2009, about 1000 seeds each of the black and brown morphs were placed in 24 nylon bags. Each of the 48 bags was buried at a depth of 2 cm in 20 cm diameter × 50 cm tall plastic pots with drainage holes. The pots were filled with soil collected from the habitat from which the seeds were collected and then buried on nearly flat ground at the Ordos Sandland Ecological Research Station of the Chinese Academy of Sciences on 1 December 2009. Mean daily maximum and minimum monthly air temperatures for each month during the study were obtained from data collected at the Research Station.
Seeds of the two morphs were exhumed and tested for germination on the first day of each month for 24 months, beginning in May 2010. Germination tests were conducted at 15/5, 20/5, 25/15 and 30/15 °C in light (12 h photoperiod) as described above. Four replications of 25 seeds each were used. Final germination percentages were determined after 20 d. Following the germination tests, non-germinated seeds were tested for viability as described above.
Effect of salinity on germination of buried seeds
Each time buried seeds were exhumed, they were incubated at four salinities and in distilled water. Since almost all brown seeds had germinated before the first exhumation in May 2010, germination tests were conducted only on black seeds. The buried black seeds were tested for germination in 0·2, 0·4, 0·6 and 0·8 m NaCl. Seeds were placed in Petri dishes on two layers of filter paper moistened with 3 mL of test solution, and the dishes were wrapped with plastic film. Four replicates of 25 seeds each were used. The seeds were incubated at 30/15 °C in light (see above). Final germination percentages were determined after 20 d. Following the germination tests, non-germinated seeds were tested for viability as described above.
A relative germination percentage (%GR) was used to evaluate the effect of salinity on germination of buried seeds: %GR = (GS/GW) × 100, where GS is the germination percentage at salinity S at 30/15 °C and GW is the germination percentage in distilled water.
Effect of salinity and water stress on dormancy and viability of the two seed morphs
Black and brown seeds that had been stored dry at –20 °C for 5 months were exposed to 1·0 m NaCl and isotonic polyethylene glycol (PEG)-8000 solutions for different periods of time in April 2011, after which they were tested for germination in distilled water and for viability. The osmotic potential of 1·0 m NaCl solution was determined by the Van't Hoff equation π = cRT, where c is osmolality in mol L−1, R the gas constant (8·31 J K−1 mol−1) and T temperature (K). The isotonic PEG-8000 solution was prepared according to Michel (1983).
For both black and brown seeds, 24 samples of 25 seeds each were placed in 5 cm Petri dishes; 3·5 mL NaCl solution was added to each of 12 of the dishes, and 3·5 mL of PEG-8000 solution was added to each of the other 12. The dishes were placed in an incubator at a constant temperature of 20 °C. This temperature was chosen to replicate the average temperature of early summer (before the beginning of the rainy season) when seeds were most likely to experience salinity and drought stress. A constant temperature rather than alternating temperatures was used to avoid changes in osmotic potential of PEG-8000 solutions. Four dishes were selected randomly after 20, 40 and 60 d pre-treatment in each of the NaCl and PEG-8000 solutions, and the seeds were transferred to Petri dishes and moistened with distilled water at 30/15 °C. Seeds that had germinated during the pre-treatment were counted and removed, and the remaining seeds were washed in running water twice before being transferred to distilled water. The seeds transferred to distilled water were incubated for 20 d before determination of final germination percentages, and non-germinated seeds were tested for viability as described above. Recovery germination percentage (%RG) was determined as [(a – b)/(c – b)] × 100 %, where a is the number of seeds germinated in solutions plus those that germinated after being transferred to distilled water, b is the number of seeds germinated during the pre-treatment with NaCl or PEG-8000 solution, and c is the total number of seeds in a dish (Pujol et al., 2000; Guma et al., 2010). To detect the effect of salinity and water stress on the viability of black and brown seeds, the percentage of viable seeds (%V) after exposure to stress was determined as [(a – b + d)/(c – b)] × 100 %, where a, b and c are the same as described above and d is the number of non-germinated viable seeds after 20 d incubation in distilled water.
The control consisted of 16 dishes of 25 seeds without pre-treatment with NaCl or PEG-8000 solution dry stored in the 20 °C incubator. Four dishes of seeds were incubated in distilled water at 30/15 °C after 0, 20, 40 and 60 d of dry storage at 20 °C. Final germination percentages and viability of non-germinated seeds were determined after 20 d.
Dynamics of soil seed banks of the two seed morphs
Soil seed banks of S. corniculata were investigated monthly from April to November in 2011 in the natural habitat where they were collected. Ten soil cores (5 cm diameter × 10 cm depth) were collected in a monospecific stand of S. corniculata. The soil cores were sub-divided into three layers: 0–2 cm, 2–5 cm and 5–10 cm. The soil samples were washed with water through a 0·5 mm mesh sieve and seeds were hand sorted from the residue (roots and vegetative parts). The number of viable black and brown seeds each was determined, as described above. Only viable seeds were used in calculating seed density in the soil seed bank.
Seedling emergence and survivorship of sown dimorphic seeds
Ten samples each of 100 black and brown seeds collected in 2010 were sown in ten (20 cm × 20 cm) plots in the natural habitat of S. corniculata in November 2010. Soil to a depth of 10 cm was removed from the plots, and then the plots were refilled with soil from the natural habitat that had been sieved by a 0·5 mm mesh sieve to remove seeds of S. corniculata. A nylon cloth (0·2 mm mesh) was used to isolate the soil in each plot from soil outside the plot and to prevent transport of seeds into and out of each plot. The edge of the cloth extended about 0·5 cm above the soil surface. To prevent escape of seeds from, or entry into, the plots, we also had a buffer zone around each of them. Soil in a 10 cm wide × 10 cm deep zone around each plot was replaced by the sieved soil mentioned above. In each plot, 100 seeds were planted evenly at a depth of about 1 cm. All newly emerged seedlings were labelled and recorded each month from April to September 2011, To avoid missing any seedlings that emerged but soon died, investigation of newly emerged seedlings was conducted twice each month (on the 15th and the last day), and the number of emerging seedlings was summed each month. The number of seedlings that survived until the end of the experiment was counted (see below).
The experiment was terminated on 30 September 2011 when the plants had turned red, indicating the end of the growing season. On this date, the number of living seedlings that had emerged in each month was recorded, and the soil in the plots was retrieved to determine the number of remaining viable seeds. The number of viable seeds remaining in the soil was determined by the same method used to determine the number of seeds in soil seed banks. The percentage of seedling emergence (%PE) was determined as (NES/NT) × 100, where NES is the number of seedlings that emerged during the experiment and NT the total number of seeds sown at each plot (100 seeds). Survival percentage (%SP) of seedlings was determined as (NLS/NES) × 100, where NLS is the number of living seedlings on September 30 and NES the same as described above.
All plants that survived until the end of the experiment produced some seeds. Success of reproduction (%PSR) was determined as (%PE × %SP), where %PE is the percentage of seedling emergence and %SP the survival percentage of seedlings as described above. During the experiment, soil water content (SWC), soil salinity, precipitation and temperature at the study site were monitored. Eight soil cores 0–5 cm deep were collected randomly within the study population of S. corniculata on the 15th day of each month from April to September 2011. The moisture content of the soil samples was determined by oven-drying at 105 °C for 48 h. The salinity of soil samples was analysed by the residue drying quality method (Bao, 2000). Monthly mean temperature and monthly mean precipitation data were obtained from a national weather station 10 km away from the field site.
Statistical analyses
Means of germination percentages of fresh seeds and germination percentages and percentage of viable seeds after various periods of pre-treatments of salinity and drought stress were compared by analyses of variances (ANOVAs). Fisher's l.s.d. test was performed for multiple comparisons to determine significant (P < 0·05) differences between means. An independent sample t-test was performed to determine significant differences between germination percentages of fresh seeds in the light and in continuous darkness and between germination percentages and percentages of viable seeds after the same period of exposure to NaCl and PEG-8000 solutions. Variables were arcsine square-root transformed to meet assumptions of ANOVA for normality and homogeneity of variance when necessary. Statistical analyses were performed using SPSS Version 13·0 for windows (SPSS, Chicago, IL, USA).
RESULTS
Black:brown seed ratio and seed mass
The ratio of black to brown seeds collected from 15 individuals was 4:1, thus, there were four times more black than brown seeds produced. Black and brown seeds had a thousand-seed mass of 240·35 ± 3·86 mg (mean ± s.e., n = 5) and 315·78 ± 1·75 mg, respectively.
Germination of fresh seeds
The viability of fresh black and brown seeds was 96·0 ± 2·8 and 100 %, respectively. In distilled water, fresh black seeds germinated to significantly higher percentages at high (25/15 and 30/15 °C) than at low (15/5 and 20/5 °C) temperatures in both the light and continuous darkness (Fig. 1A). Light promoted germination of fresh black seeds at all temperature regimes (Fig. 1A). Brown seeds germinated to >97 % at all four temperature regimes in both light and continuous darkness (Fig. 1B). Salinity had detrimental effects on germination of both black and brown seeds, but black seeds were more sensitive to all salinities tested than brown seeds (Fig. 2). Germination of black seeds was <12 % at 0·4 m NaCl, whereas germination of brown seeds did not decrease to <12 % unless they were incubated at 1·6 m NaCl.
Fig. 1.
Germination percentages of fresh black (A) and brown (B) seeds of Suaeda corniculata at various temperatures in the light (12 h photoperiod) and in continuous darkness. Bars with the same uppercase letters are not significantly different (P > 0·05) across all temperature regimes. The same lowercase letters indicate no significant difference (P > 0·05) between germination percentages (means ± s.e.) in light and continuous darkness with the same thermoperiod.
Fig. 2.
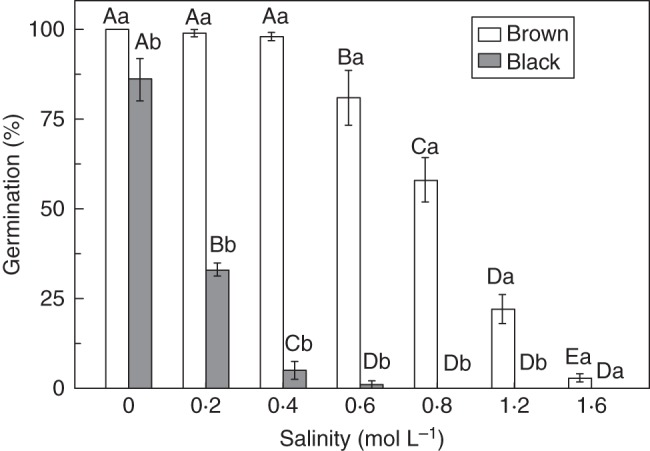
Germination percentages of fresh black and brown seeds of Suaeda corniculata at various salinities at 30/15 °C in a 12 h photoperiod. Bars with the same uppercase letters indicate no significant difference (P > 0·05) between germination percentages (means ± s.e.) of the same seed morphs across all salinities. The same lowercase letters indicate no significant difference (P > 0·05) between germination percentages (means ± s.e.) of the two seed morphs at the same salinity.
Dormancy breaking of black seeds
Cold stratification gradually increased germination percentage of black seeds at 15/5 °C (Fig. 3). Black seeds germinated to >70 % at 15/5 °C after 15 d cold stratification. Throughout the cold stratification period, black seeds maintained high germination percentages at 20/5, 25/15 and 30/15 °C (Fig. 3). Treatments with GA3 and testa scarification also significantly increased the germination percentage of black seeds at 15/5 °C (Fig. 4).
Fig. 3.
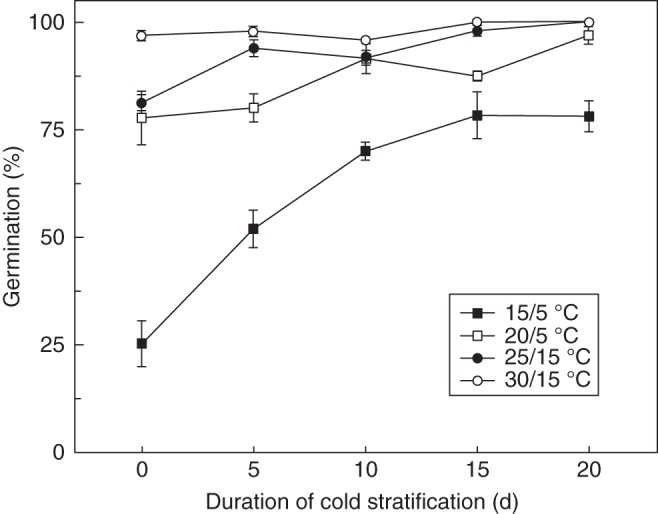
Germination percentages of black seeds of Suaeda corniculata incubated at various alternating temperatures in a 12 h photoperiod after 0, 5, 15 and 20 d of cold stratification at 5 °C in continuous darkness.
Fig. 4.
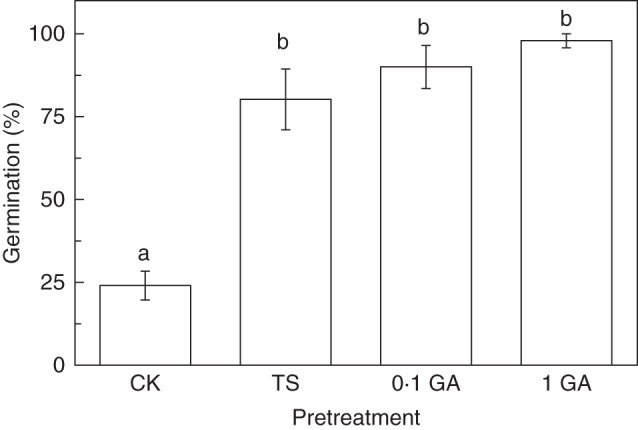
Germination percentages of fresh black seeds of Suaeda corniculata in 0·1 (0·1 GA) and 1 mmol L−1 GA3 (1 GA) and after testa scarification (TS) at 15/5 °C (12 h/12 h) in a 12 h photoperiod. CK is the untreated control. Values are means ± s.e. Bars with the same letters indicate no significant difference (P > 0·05) between germination percentages.
Light requirement for germination of non-dormant black seeds
Buried black seeds exhumed in May 2010 germinated to >75 % at all temperatures tested in the light (Fig. 5). However, darkness prohibited seed germination significantly at all temperatures (P < 0·05). Germination of black seeds exhumed in May 2010 had decreased to <30 % at 15/5 °C in darkness (Fig. 5).
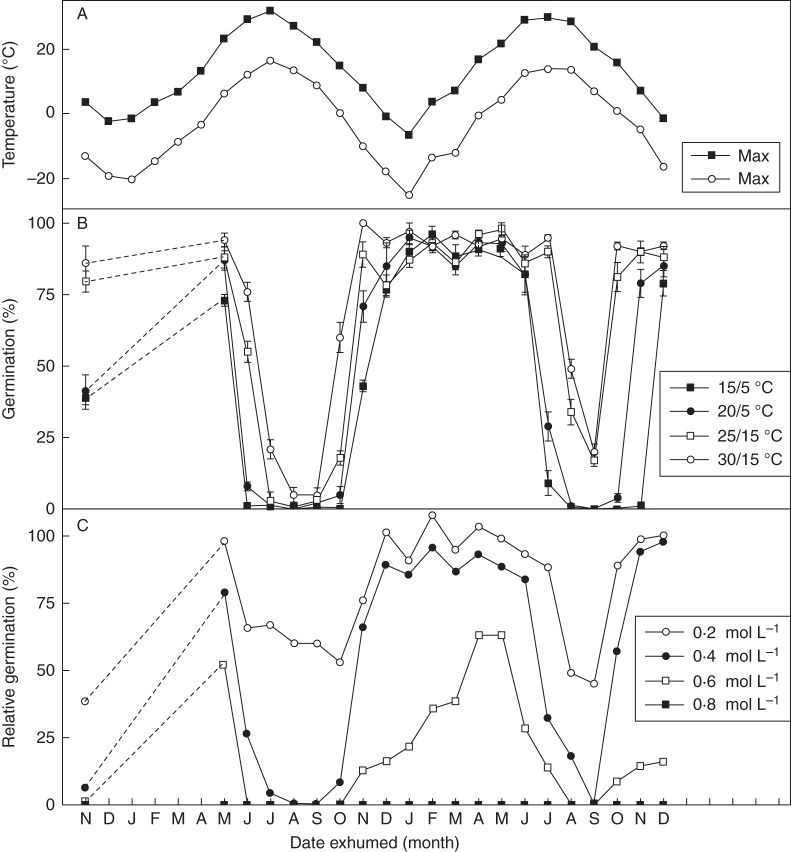
Dormancy cycling of the two seed morphs during burial
Black seeds exhumed in May 2010 germinated to a higher percentage at 15/5 and 20/5 °C compared with fresh black seeds, and, regardless of temperature, seeds germinated to ≥70 % (Fig. 6B). However, germination of exhumed black seeds decreased dramatically in summer, and only 5 % of them germinated at the most favourable temperature regime (30/15 °C) in August and September 2010. Seeds exhumed in October to December 2010 exhibited an increase in germination percentage, and those exhumed in December germinated to high percentages at all temperature regimes. Seeds exhumed monthly from December to May had high germination percentages, but in summer germination decreased, as it did in 2010 (Fig. 6B).
Fig. 6.
(A) Mean monthly maximum and minimum air temperatures at the seed burial site. (B) Germination percentages (means ± s.e.) of black seeds of Suaeda corniculata incubated at various temperature regimes for a 12 h photoperiod following 0–24 months of burial in soil in the field. (C) Relative germination percentages of black seeds of S. corniculata incubated at 30/15 °C in a 12 h photoperiod at salinities of 0·2, 0·4, 0·6 and 0·8 mol L−1 NaCl solutions following 0–24 months of burial in soil.
When the seeds were first exhumed in May 2010, scarcely any brown seeds were viable, as most of them had germinated during burial.
Effect of salinity on germination of buried seeds
Dormancy cycling varied quantitatively with the salinity at which germination was tested (Fig. 6C), but the pattern was similar to that of seeds incubated in distilled water at 30/15 °C. When the black seeds were first exhumed in May 2010, they had higher relative germination percentages at salinities of 0·2, 0·4 and 0·6 m NaCl than fresh seeds (Fig. 6C). Relative germination percentages at salinities of 0·2, 0·4 and 0·6 m NaCl were high in spring, autumn and winter, but low in summer (Fig. 6C). Throughout the experiment, only one seed germinated at 0·8 m NaCl (in January 2011).
Effect of salinity and water stress on dormancy and viability of the two seed morphs
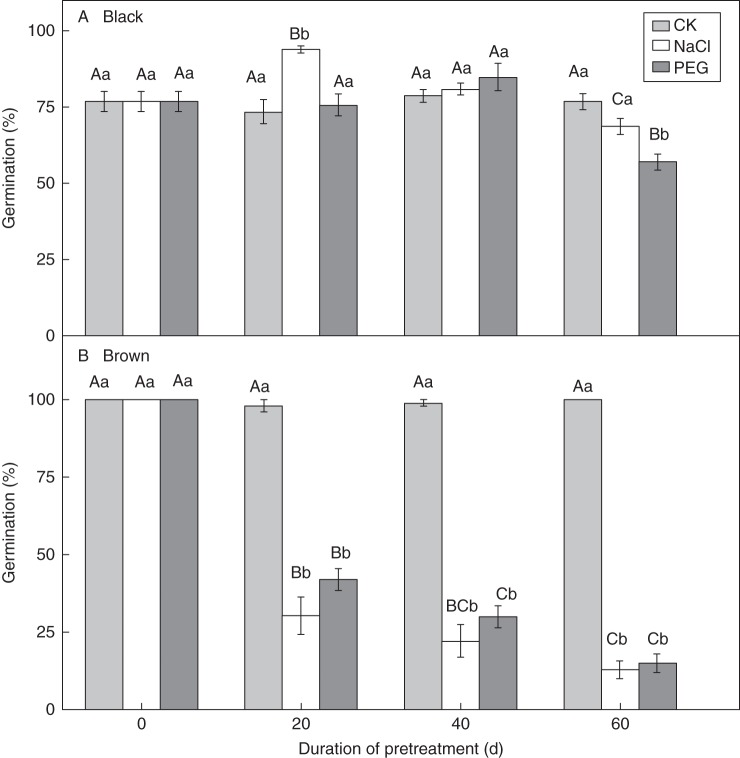
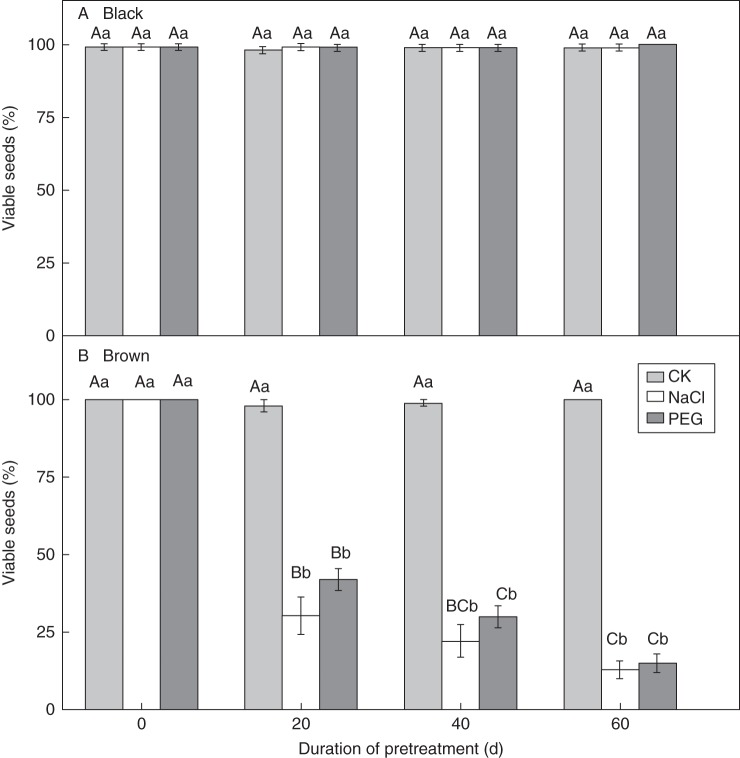
Exposure of black seeds to a salinity of 1 m NaCl for 20 d increased seed germination, but germination decreased after exposure for 40 or 60 d (Fig. 7A). Exposure to the isotonic PEG-8000 solution for 20 and 40 d did not affect germination of black seeds, but 60 d of exposure decreased germination significantly (P < 0·05, Fig. 7A). Exposure to a salinity of 1 m NaCl and to isotonic PEG solutions did not decrease the viability of black seeds within 60 d (Fig. 8A).
Fig. 7.
Germination percentages (means ± s.e.) of black (A) and brown (B) seeds of Suaeda corniculata in a 12 h photoperiod at 30/15 °C after 0, 20, 40 and 60 d of exposure to 1 mol L−1 NaCl or isotonic PEG-8000 solutions for a 12 h photoperiod at 20 °C. CK seeds were dry stored at 20 °C for 0, 20, 40 and 60 d. Bars with the same uppercase letters indicate no significant difference (P > 0·05) between germination percentages of seeds after various periods of exposure to NaCl, PEG-8000 solution or dry storage. The same lowercase letters indicate no significant difference (P > 0·05) between germination percentages of seeds pre-treated with NaCl or PEG-8000 solution for the same periods.
Fig. 8.
Percentages (means ± s.e.) of viable black (A) and brown (B) seeds of Suaeda corniculata in a 12 h photoperiod at 30/15 °C after 0, 20, 40 and 60 d of exposure to 1 mol L−1 NaCl or isotonic PEG-8000 solutions for a 12 h photoperiod at 20 °C. CK seeds were dry stored at 20 °C for 0, 20, 40 and 60 d. Bars with the same uppercase letters indicate no significant difference (P > 0·05) between percentages of viable seeds (%PV) after various periods of exposure to NaCl, PEG-8000 solution or dry storage. The same lowercase letters indicate no significant difference (P > 0·05) between percentages of viable seeds pre-treated by NaCl or PEG-8000 solution for the same periods.
The viability of brown seeds that were not pre-treated in 1 m NaCl or in isotonic PEG-8000 solution was 100 % (Fig. 8B), and they had 100 % germination in distilled water (Fig. 7B). Viability of brown seeds exposed to both 1 m NaCl and isotonic PEG solution for 20 d decreased with time (Fig. 8B). There were 12·8 ± 2·8 % and 15·0 ± 3·0 % brown seeds that were viable after 60 d pre-treatment in 1 m NaCl and isotonic PEG-8000 solution, respectively. Thus, about 85 and 87 % of the brown seeds died following exposure to salinity and water stress for 60 d. No viable non-germinated brown seeds were found after 20 d germination tests in distilled water after they had been transferred from NaCl or PEG-8000 solution. Thus, after being pre-treated in 1 m NaCl and isotonic PEG-8000 solution, the recovery germination percentage (%RG) of brown seeds changed in the same way as did the percentage of viable seeds (%V).
Dynamics of soil seed banks for dimorphic seeds
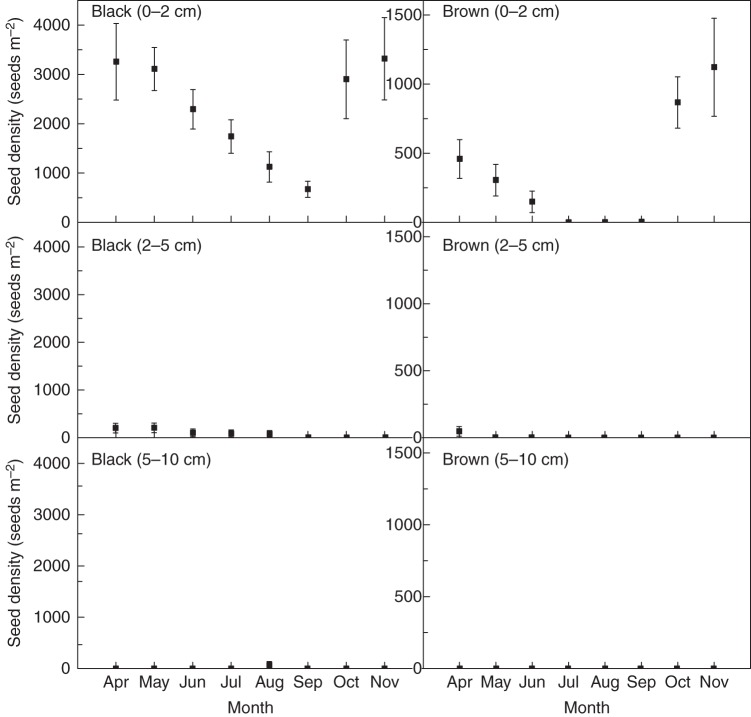
Both black and brown seeds were most abundant in the 0–2 cm level of soil, and seed density decreased with an increase in soil depth (Fig. 9). No seeds, except for one black seed (found in a soil core obtained in August 2011), were found at the 5–10 cm soil depth.
Fig. 9.
Densities of black (left) and brown (right) seeds at different soil depth in their natural habitat from April to November 2011. Values are means ± s.e. Only one black seed (in August) and no brown seeds were present at the 5–10 cm soil depth.
There were 3464 ± 803 (mean ± s.e.) black seeds m−2 at the 0–10 cm soil depth in April 2011 (Fig. 9) and the number declined month by month, reaching 662 ± 153 seeds m−2 in September. Seed density began to increase in October 2011, and it reached 3311 ± 829 seeds m−2 in November (Fig. 9).
The number of brown seeds in the soil showed similar temporal changes to those of black seeds (Fig. 9). The density of brown seeds was 509 ± 170 seeds m−2 in April 2011, which declined to 153 ± 78 seeds m−2 in June and to 0 in July, August and September. As a result of input of brown seeds into the soil in autumn (mostly October) 2010, the density reached 1120 ± 354 seeds m−2 in November 2011 (Fig. 9).
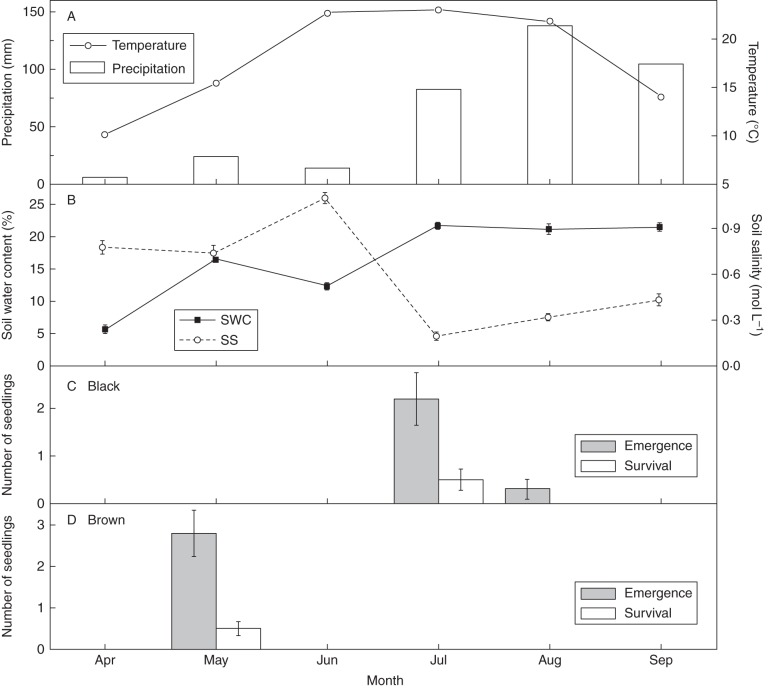
Seedling emergence and survivorship of sown seed morphs
Seedlings from black seeds emerged in July and August when the primary precipitation event of the year occurred in 2011 (Fig. 10). Only 2·6 ± 0·7 % of the black seeds recruited seedlings (%PE), of which approx. 85 % (2·2/2·6) emerged in July (Fig. 10). None of the seedlings that emerged in August survived until the end of the growing season (30 September). About 19 % (0·5/2·6) of the seedlings that emerged survived (%SP) and produced seeds. Thus, 0·5 % of the seeds sown produced plants that reproduced (%PSR); however, at the end of the experiment 35·1 ± 3·4 % of the black seeds were still alive in the soil.
Fig. 10.
(A) Mean air temperature and precipitation, (B) soil water content (SWC) and salinity (SS), and seedling emergence and survival from (C) black and (D) brown seeds of Suaeda corniculata.
Seedlings derived from sown brown seeds appeared in the field plots in May (Fig. 10). Seedling emergence percentage (%PE) was 2·8 ± 0·6 %, and about 14 % (0·4/2·8) of the seedlings survived to reproduce successfully (%SP). No brown seeds persisted in soil at the end of the experiment. Thus, altogether, only 0·4 ± 0·2 % of the brown seeds sown produced a plant that reproduced (%PSR).
DISCUSSION
Black seeds of S. corniculata have a dormancy mechanism at maturity that prevents germination at low temperatures. Germination of black seeds can be improved by testa scarification and GA3, which are a characteristic of seeds with physiological dormancy (Baskin and Baskin, 1998). Also, a very short period of cold stratification (15 d) increased germination of black seeds remarkably. Taken together, the effectiveness of these three treatments on dormancy break indicates that black seeds have non-deep physiological dormancy (Baskin and Baskin, 1998). Artificial cold stratification failed to increase germination of black seeds to >75 % at low temperatures. However, exposure to low winter temperatures in the field resulted in >95 % germination of buried black seeds at all temperature regimes tested. This difference may be due to differences in conditions between artificial and natural cold stratification. Wetson et al. (2008) found that differences in temperature and salinity during dormancy affected subsequent germination of S. maritima seeds.
Black seeds of S. corniculata have less mass than brown ones and need light to germinate, while the brown seeds do not require light. A light requirement for germination generally decreases with an increase in seed mass in many species in temperate areas (Milberg et al., 2000; Schutz et al., 2002), and it increases the ability of a species to form a persistent soil seed bank (Baskin and Baskin, 1989; Pons, 1991). Black seeds of S. corniculata maintain a persistent soil seed bank, while brown seeds form only a transient soil seed bank. Thus, black seeds persist for a longer period in the soil than brown seeds. The light requirement for germination in black seeds may also act as a depth-sensing cue.
Fresh black seeds of S. corniculata can germinate to high percentages at high temperatures and to low percentages at low temperatures, thus the seeds are conditionally dormant. Seeds are non-dormant in late winter–spring of the next year and they can germinate to high percentages at both high and low temperatures (Fig. 6). By July or August, most of the seeds had entered (2010) or re-entered (2011) dormancy via conditional dormancy (CD). A small percentage of the seeds, especially in the second year of burial (2011), remained conditionally dormant and retained the ability to germinate at 25/15 and 30/15 °C in August and September. Thus, it generally can be stated that when black seeds are first produced they are in a state of CD, and they become non-dormant by the following late winter–spring. Further, those that do not germinate in the first spring subsequently cycle between dormancy (D) and non-dormancy (ND): CD → ND ↔ D. This pattern of annual dormancy changes fits that of summer annuals shown in Baskin and Baskin (1998; panel b of fig. 4·4).
In addition to dormancy cycles, black seeds of S. corniculata also exhibit an annual cycle of sensitivity of germination to salinity. The cycle of sensitivity to salinity corresponds closely to dormancy cycles of seeds in the soil seed bank. Inhibitory effects of salinity on germination decrease as black seeds come out of dormancy and increase as non-dormant seeds re-enter dormancy. Baskin and Baskin (1998) concluded that the temperature range for seed germination and sensitivity of seeds to light, substrate moisture and hormones is correlated with the continuum of dormancy states. In our study, sensitivity of seed germination to salinity for black seeds of S. corniculata is also shown to be a correlate with the dormancy continuum. Thus, the increase in salt tolerance as seeds come out of dormancy and the decrease as seeds enter dormancy adds a new correlate to those formulated by Baskin and Baskin (1998) for seeds as they cycle back and forth through a dormancy continuum.
In contrast, brown seeds of S. corniculata do not form a persistent soil seed bank, and of course they cannot have dormancy cycles. Brown seeds were non-dormant at maturity, and they germinated to high percentages across the range of high to low temperatures and in continuous darkness. Thus, it is not surprising that the buried brown seeds had germinated in the spring when they were exhumed. Not unexpectedly, low winter temperature did not induce non-dormant brown seeds of this summer annual into dormancy (Baskin and Baskin, 1998). Carter and Ungar (2003) also found different dormancy characteristics in dimorphic seeds of the two summer annual halophytes Atriplex prostrate and Salicornia europaea (Amaranthaceae). Small seeds of both A. prostrate and S. europaea had dormancy cycling, whereas large seeds were non-dormant (S. europaea) or conditionally dormant (A. prostrate) at maturity and were non-dormant in the following spring (Carter and Ungar, 2003).
The different performance of black and brown seeds after exposure to salinity and drought stress may explain to some extent the mechanism of the difference in their dynamics of dormancy. Black seeds were induced into dormancy during exposure to salinity and water stress, while brown seeds remained non-dormant. It can be inferred that non-dormant black seeds that have not germinated in a germination season may become dormant and persist in the soil seed bank when they experience environmental stress. The viability of brown seeds is largely reduced by salinity and drought stress, and very few seeds maintain viability after exposure to salinity and drought stress. However, the seeds that remain viable germinate readily after removal of environmental stress. Brown seeds can therefore form only a transient soil seed bank. The differences in responses of dimorphic seeds of S. corniculata to salinity are similar to those of S. splendens (Redondo-Gómez et al., 2008), which also produces brown and black seeds. Black seeds of S. splendens maintain viability for a longer period of time under high salinities than brown seeds (Redondo-Gómez et al., 2008). However, it is unclear whether dimorphic seeds of S. splendens differ in persistence in the soil seed bank.
Dormancy cycles of seeds have been shown in many species growing in humid temperate regimes (Baskin and Baskin, 1980, 1985, 1989), and they usually have been considered to be driven primarily by seasonal temperature changes (Vanlerberghe and Van Assche, 1986; Van Assche and Vanlerberghe, 1989; Bouwmeester and Karssen, 1992). In our study, a 60 d exposure to 1·0 m salinity or to an isotonic PEG solution induced black seeds of S. corniculata into dormancy, which suggests that soil salinity and drought may also play a role in regulating dormancy cycles of black seeds of S. corniculata in their cold desert habitat. In this study, seeds were exposed to soil salinity (1·0 m) in the field for only 1 month (June) in summer, and soil salinity was decreased by precipitation in July when seedlings appeared in the plots. Thus, it seems unlikely that soil salinity induced dormancy in black seeds of S. corniculata. However, it should be noted that soil salinity in May is also very high, and the temperature may be around 20 °C. Also, in July there is high variability in time when substantial precipitation will occur. Thus, black seeds of S. corniculata could experience high salinity in the soil in May, June and July, and this period would be long enough for high salinity to promote dormancy induction in black seeds of S. corniculata. Although soil salinity may play a role in regulating dormancy induction in black seeds, it only induces a small proportion of them into dormancy (Fig. 7). However, a high percentage of the buried black seeds are induced into dormancy in summer (Fig. 6), leading us to conclude that high temperatures in summer may also be involved in regulating dormancy cycling of black seeds of S. corniculata.
Black seeds of S. corniculata have a ‘cautious’ strategy of germination (Gutterman, 1993). They maintain viability after a period of exposure to salinity stress and can commence germination once this stress is removed, which is a typical response of halophyte seeds to salinity stress (Ungar, 1962; Khan and Ungar, 1997; Hanslin and Eggen, 2005; Song et al., 2005). A long exposure to salinity and drought stress induced some black seeds into dormancy, which allows the species to withstand unfavourable periods of environmental stress. Brown seeds can germinate at high salinity of 1 m NaCl, which makes them distinct from seeds of glycophytes (non-salt-tolerant plants). However, most of them cannot maintain viability during salinity stress, which makes them different from other halophyte seeds. Brown seeds of S. corniculata show an ‘opportunistic’ strategy of germination (Gutterman, 1993), and they are ready to germinate whenever there are favourable conditions for germination, which gives the population a competitive advantage via early germination (Ross and Harper, 1972).
The ecological literature is sometimes confusing on the importance of seed dormancy and the maintenance of persistent soil seed banks. Cavieres and Arroyo (2001) state that formation of a persistent seed bank depends upon seed dormancy. However, Thompson et al. (2003) did not find a close relationship between seed dormancy and persistence in the soil by comparing the longevity index and dormancy states of 599 species. In our study, retention of viability of seeds of S. corniculata played a big role in maintenance of a seed bank. Exposure to high salinity and drought stress led to a dramatic decrease in viability of brown seeds. Furthermore, since brown seeds germinate to high percentages in darkness, even if they could remain viable the seed bank would be depleted by in situ germination. The black seeds maintain viability and enter dormancy after exposure to high salinity and drought stress, and they have a light requirement for germination, which delays depletion of the soil seed bank. Differences in the ability to maintain viability and in dormancy characteristics of the two seed morphs of S. corniculata lead to difference in their persistence in soil seed banks.
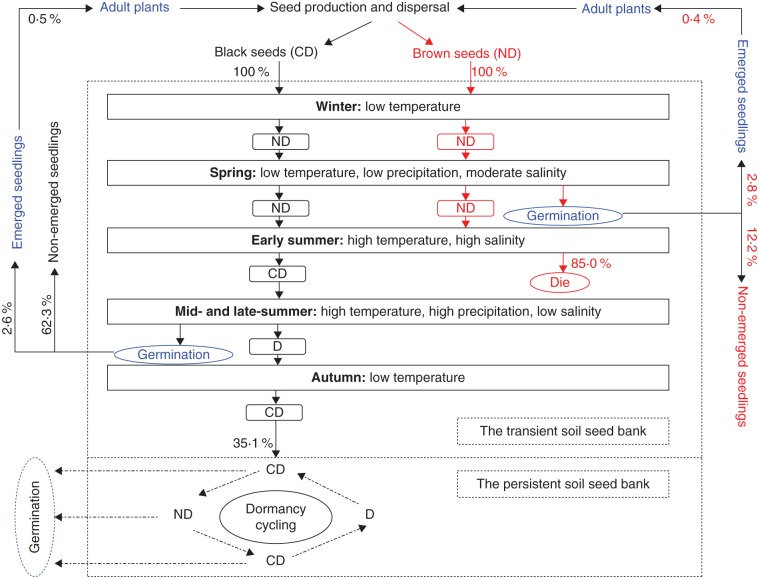
A conceptual model of the seed dynamics of black and brown seeds of S. corniculata is shown in Fig. 11. Black seeds are conditionally dormant when they mature in autumn, and they become non-dormant after exposure to cold temperature during early winter and maintain ND in late winter and spring. Black seeds of S. corniculata are different from type C seeds of Salsola affinis (Amaranthaceae), which came out of dormancy in the field (Junggar Basin, NW China) in late winter or early spring (Wei et al., 2007). Although daily minimum temperature in early winter (November) is below 0 °C, daily maximum temperature (8·6 °C) in the natural habitat during this time is in the temperature range for cold stratification for seeds of many summer annuals in temperate regions (Baskin and Baskin, 1998). A 15 d cold stratification at 5 °C can break dormancy of black seeds. Thus, dormancy release of black seeds can occur in early winter. High temperature and high salinity in the soil may induce dormancy in black seeds in summer, and the seeds become conditionally dormant after they are exposed to high salinity. High precipitation in summer decreases soil salinities, which lead to 2·6 % germination of black seeds. However, only 0·5 % of the black seeds had the probability to recruit seedlings that survived to produce seeds the first year. At the end of the growing season, 35·1 % of black seeds entered the persistent soil seed bank, and they had an annual dormancy cycle of D ↔ ND.
Fig. 11.
Conceptual model of changes in dormancy status of black (in black) and brown (in red) seeds of Suaeda corniculata and soil seed bank dynamics and seedling regeneration (in blue) of the population. The percentages indicate the proportions of seeds in a transient soil seed bank that recruit seedlings and enter the persistent soil seed bank. Abbreviations: CD, conditional dormancy; D, dormancy; ND, non-dormancy. The dashed-dotted lines represent possible fates of black seeds in the persistent soil seed bank.
Brown seeds of S. corniculata perform differently from black seeds (Fig. 11). Brown seeds are non-dormant when they are shed from the mother plant and enter the transient soil seed bank in autumn. They are non-dormant through winter and in the spring of the following year, and their germination can be triggered by the relatively small amount of precipitation in spring. Thus, brown seeds can result in establishment of the population in earlier summer than black seeds. About 2·8 % of brown seeds in the transient soil seed bank recruited seedlings, but only seedlings derived from 0·4 % of seeds produced seeds. Apparently, high soil salinity in early summer leads to a dramatic decrease in viability of brown seeds, and no brown seeds were found in the soil in July, August or September. Thus, it can be inferred that all brown seeds were non-viable in the field by July.
Differences in performance of the two seed morphs of S. corniculata in the soil seed bank increase fitness of the species in unpredictable saline environments. Brown seeds can germinate under a wider range of temperatures and salinities than black seeds. Seed germination and seedling emergence is triggered by small precipitation events in early spring. Early germination gives plants a selective advantage, since they are less likely to be attacked by predators and pathogens than late germinating individuals (Seiwa, 1998). Early germination also endows early germinating individuals with a competitive advantage to capture resources (Ross and Harper, 1972), and thus they are also likely to grow much larger and thus to produce more seeds than those that germinate late. Germination of black seeds is regulated by an annual dormancy cycle of D ↔ ND, and non-dormant black seeds have stricter germination requirements than brown seeds. They cannot germinate in natural saline habitats until soil salinity is decreased by precipitation. Thus, seedlings derived from black seeds of S. corniculata emerge mainly in the summer rainy season. Although seedlings of late-germinating black seeds have a shorter growing season than those of early-germinating brown seeds, young seedlings are exposed to more favourable moisture conditions for growth than those from brown seeds. However, if seedlings/plants from brown seeds can survive until the summer rainy season they would already be established and could respond rapidly to improve moisture conditions for growth.
ACKNOWLEDGEMENTS
This work was funded by the Key Basic Research and Development Plan of China (2010CB951304) and the National Natural Science Foundation of PR China (30872074, 30970461 and 31170383).
LITERATURE CITED
- Anderson TM, Schütz M, Risch AC. Seed germination cues and the importance of the soil seed bank across an environmental gradient in the Serengeti. Oikos. 2012;121:306–312. [Google Scholar]
- Atia A, Rabhi M, Debez A, Barhoumi Z, Abdelly C, Smaoui A. Factors controlling germination and dormancy processes in dimorphic fruits of Atriplex inflata (Chenopodiaceae) Plant Ecology and Evolution. 2011;144:307–312. [Google Scholar]
- Bao SD. Soil chemistry and agriculture analysis. (In Chinese.) Beijing: China Agriculture Press; 2000. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Baskin CC, Chesson PL, Baskin JM. Annual seed dormancy cycles in two desert winter annuals. Journal of Ecology. 1993;81:551–556. [Google Scholar]
- Baskin JM, Baskin CC. Germination dimorphism in Heterotheca subaxillaris var. Bulletin of the Torrey Botanical Club. 1976;103:201–206. subaxillaris. [Google Scholar]
- Baskin JM, Baskin CC. Ecophysiology of secondary dormancy in seeds of Ambrosia artemisiifolia. Ecology. 1980;61:475–480. [Google Scholar]
- Baskin JM, Baskin CC. The annual dormancy cycle in buried weed seeds: a continuum. BioScience. 1985;35:492–498. [Google Scholar]
- Baskin JM, Baskin CC. Physiology of dormancy and germination in relation to seed bank ecology. In: Leck MA, Parker VT, Simpson RL, editors. Ecology of soil seed banks. San Diego, CA: Academic Press; 1989. [Google Scholar]
- Baskin JM, Baskin CC, McCormick JF. Seasonal changes in germination responses of buried seeds of Portulaca smallii. Bulletin of the Torrey Botanical Club. 1987;114:169–172. [Google Scholar]
- Bouwmeester HJ, Karssen CM. The dual role of temperature in the regulation of the seasonal changes in dormancy and germination of seeds of Polygonum persicaria L. Oecologia. 1992;90:88–94. doi: 10.1007/BF00317813. [DOI] [PubMed] [Google Scholar]
- Carter CT, Ungar IA. Germination response of dimorphic seeds of two halophyte species to environmentally controlled and natural conditions. Canadian Journal of Botany. 2003;81:918–926. [Google Scholar]
- Cavieres LA, Arroyo MTK. Persistent soil seed banks in Phacelia secunda (Hydrophyllaceae): experimental detection of variation along an altitudinal gradient in the Andes of central Chile (33 °S) Journal of Ecology. 2001;89:31–39. [Google Scholar]
- El-Keblawy A. Effects of achene dimorphism on dormancy and progeny traits in the two ephemerals Hedypnois cretica and Crepis aspera (Asteraceae) Canadian Journal of Botany. 2003;81:550–559. [Google Scholar]
- Evenari M, Kadouri A, Gutterman Y. Eco-physiological investigations on the amphicarpy of Emex spinosa (L.) Campd. Flora. 1977;166:223–238. [Google Scholar]
- Flint SD, Palmblad IG. Germination dimorphism and developmental flexibility in the ruderal weed Heterotheca grandiflora. Oecologia. 1978;36:33–43. doi: 10.1007/BF00344569. [DOI] [PubMed] [Google Scholar]
- Guma IR, Padron-Mederos MA, Santos-Guerra A, Reyes-Betancort JA. Effect of temperature and salinity on germination of Salsola vermiculata L. (Chenopodiaceae) from Canary Islands. Journal of Arid Environments. 2010;74:708–711. [Google Scholar]
- Gutterman Y. Seed germination in desert plants. Berlin: Springer-Verlag; 1993. [Google Scholar]
- Hanslin HM, Eggen T. Salinity tolerance during germination of seashore halophytes and salt-tolerant grass cultivars. Seed Science Research. 2005;15:43–50. [Google Scholar]
- Hutchings MJ, Booth KD. Studies on the feasibility of re-creating chalk grassland vegetation on ex-arable land. I. The potential roles of the seed bank and the seed rain. Journal of Applied Ecology. 1996;33:1171–1181. [Google Scholar]
- Imbert E. The effects of achene dimorphism on the dispersal in time and space in Crepis sancta (Asteraceae) Canadian Journal of Botany. 1999;77:508–513. [Google Scholar]
- Imbert E. Ecological consequences and ontogeny of seed heteromorphism. Perspectives in Plant Ecology, Evolution and Systematics. 2002;5:13–36. [Google Scholar]
- Imbert E, Ronce O. Phenotypic plasticity for dispersal ability in the seed heteromorphic Crepis sancta (Asteraceae) Oikos. 2001;93:126–134. [Google Scholar]
- Joley DB, Maddox DM, Schoenig SE, Mackey BE. Parameters affecting germinability and seed bank dynamics in dimorphic achenes of Centaurea solstitialis in California. Canadian Journal of Botany. 2003;81:993–1007. [Google Scholar]
- Katembe WJ, Ungar IA, Mitchell JP. Effect of salinity on germination and seedling growth of two Atriplex species (Chenopodiaceae) Annals of Botany. 1998;82:167–175. [Google Scholar]
- Khan MA, Ungar IA. The effect of salinity and temperature on the germination of polymorphic seeds and growth of Atriplex triangularis Willd. American Journal of Botany. 1984;71:481–489. [Google Scholar]
- Khan MA, Ungar IA. Effects of thermoperiod on recovery of seed germination of halophytes from saline conditions. American Journal of Botany. 1997;84:279–283. [PubMed] [Google Scholar]
- Leck MA, Simpson RL. Seed bank of a freshwater tidal wetland: turnover and relationship to vegetation change. American Journal of Botany. 1987;74:360–370. [Google Scholar]
- Lomonosova M, Brandt R, Freitag H. Suaeda corniculata (Chenopodiaceae) and related new taxa from Eurasia. Willdenowia. 2008;38:81–109. [Google Scholar]
- Mandák B. Seed heteromorphism and the life cycle of plants: a literature review. Preslia. 1997;69:129–159. [Google Scholar]
- Mandák B, Pyšek P. Fruit dispersal and seed banks in Atriplex sagittata: the role of heterocarpy. Journal of Ecology. 2001;89:159–165. [Google Scholar]
- McCue KA, Holtsford TP. Seed bank influences on genetic diversity in the rare annual Clarkia springvillensis (Onagraceae) American Journal of Botany. 1998;85:30–36. [PubMed] [Google Scholar]
- Michel BE. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiology. 1983;72:66–70. doi: 10.1104/pp.72.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberg P, Andersson L, Thompson K. Large-seeded species are less dependent on light for germination than small-seeded ones. Seed Science Research. 2000;10:99–104. [Google Scholar]
- Panetta FD, Randall RP. Variation between Emex australis populations in seed dormancy/non-dormancy cycles. Australian Journal of Ecology. 1993;18:275–280. [Google Scholar]
- Pons TL. Induction of dark dormancy in seeds: its importance for the seed bank in the soil. Functional Ecology. 1991;5:669–675. [Google Scholar]
- Pujol JA, Calvo JF, Ramirez-Diaz L. Recovery of germination from different osmotic conditions by four halophytes from southeastern Spain. Annals of Botany. 2000;85:279–286. [Google Scholar]
- Redondo-Gómez S, Mateos-Naranjo E, Cambrolle J, Luque T, Figueroa ME, Davy AJ. Carry-over of differential salt tolerance in plants grown from dimorphic seeds of Suaeda splendens. Annals of Botany. 2008;102:103–112. doi: 10.1093/aob/mcn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MA, Harper JL. Occupation of biological space during seedling establishment. Journal of Ecology. 1972;60:77–88. [Google Scholar]
- Schutz W, Milberg P, Lamont BB. Seed dormancy, after-ripening and light requirements of four annual Asteraceae in south-western Australia. Annals of Botany. 2002;90:707–714. doi: 10.1093/aob/mcf250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiwa K. Advantages of early germination for growth and survival of seedlings of Acer mono under different overstorey phenologies in deciduous broad-leaved forests. Journal of Ecology. 1998;86:219–228. [Google Scholar]
- Song J, Fan H, Zhao YY, Jia YH, Du XH, Wang BS. Effect of salinity on germination, seedling emergence, seedling growth and ion accumulation of a euhalophyte Suaeda salsa in an intertidal zone and on saline inland. Aquatic Botany. 2008;88:331–337. [Google Scholar]
- Song J, Feng G, Tian CY, Zhang FS. Strategies for adaptation of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum to a saline environment during seed-germination stage. Annals of Botany. 2005;96:399–405. doi: 10.1093/aob/mci196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksley SD, McCouch SR. Seed banks and molecular maps: unlocking genetic potential from the wild. Science. 1997;277:1063–1066. doi: 10.1126/science.277.5329.1063. [DOI] [PubMed] [Google Scholar]
- Thompson K, Ceriani RM, Bakker JP, Bekker RM. Are seed dormancy and persistence in soil related? Seed Science Research. 2003;13:97–100. [Google Scholar]
- Ungar IA. Influence of salinity on seed germination in succulent halophytes. Ecology. 1962;43:763–764. [Google Scholar]
- Van Assche JA, Vanlerberghe KA. The role of temperature on the dormancy cycle of seeds of Rumex obtusifolius L. Functional Ecology. 1989;3:107–115. [Google Scholar]
- Vanlerberghe KA, Van Assche JA. Dormancy phases in seeds of Verbascum thapsus L. Oecologia. 1986;68:479–480. doi: 10.1007/BF01036759. [DOI] [PubMed] [Google Scholar]
- Venable DL. The evolutionary ecology of seed heteromorphism. American Naturalist. 1985;126:577–595. [Google Scholar]
- Venable DL, Levin DA. Ecology of achene dimorphism in Heterotheca latifolia: I. Achene structure, germination and dispersal. Journal of Ecology. 1985;73:133–145. [Google Scholar]
- Wang L, Huang Z, Baskin CC, Baskin JM, Dong M. Germination of dimorphic seeds of the desert annual halophyte Suaeda aralocaspica (Chenopodiaceae), a C4 plant without Kranz anatomy. Annals of Botany. 2008;102:757–769. doi: 10.1093/aob/mcn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Dong M, Huang Z. Seed polymorphism, dormancy and germination of Salsola affinis (Chenopodiaceae), a dominant desert annual inhabiting the Junggar Basin of Xinjiang, China. Australian Journal of Botany. 2007;55:464–470. [Google Scholar]
- Weiss PW. Germination, reproduction and interference in the amphicarpic annual Emex spinosa (L.) Campd. Oecologia, 1980;45:244–251. doi: 10.1007/BF00346465. [DOI] [PubMed] [Google Scholar]
- Wetson AM, Cassaniti C, Flowers TJ. Do conditions during dormancy influence germination of Suaeda maritima? Annals of Botany. 2008;101:1319–1327. doi: 10.1093/aob/mcn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao SX, Lan HY, Zhang FC. Variation of seed heteromorphism in Chenopodium album and the effect of salinity stress on the descendants. Annals of Botany. 2010;105:1015–1025. doi: 10.1093/aob/mcq060. [DOI] [PMC free article] [PubMed] [Google Scholar]