Abstract
Background and Aims
The importance of thermal thresholds for predicting seed dormancy release and germination timing under the present climate conditions and simulated climate change scenarios was investigated. In particular, Vitis vinifera subsp. sylvestris was investigated in four Sardinian populations over the full altitudinal range of the species (from approx. 100 to 800 m a.s.l).
Methods
Dried and fresh seeds from each population were incubated in the light at a range of temperatures (10–25 and 25/10 °C), without any pre-treatment and after a warm (3 months at 25 °C) or a cold (3 months at 5 °C) stratification. A thermal time approach was then applied to the germination results for dried seeds and the seed responses were modelled according to the present climate conditions and two simulated scenarios of the Intergovernmental Panel on Climate Change (IPCC): B1 (+1·8 °C) and A2 (+3·4 °C).
Key Results
Cold stratification released physiological dormancy, while very few seeds germinated without treatments or after warm stratification. Fresh, cold-stratified seeds germinated significantly better (>80 %) at temperatures ≥20 °C than at lower temperatures. A base temperature for germination (Tb) of 9·0–11·3 °C and a thermal time requirement for 50 % of germination (θ50) ranging from 33·6 °Cd to 68·6 °Cd were identified for non-dormant cold-stratified seeds, depending on the populations. This complex combination of thermal requirements for dormancy release and germination allowed prediction of field emergence from March to May under the present climatic conditions for the investigated populations.
Conclusions
The thermal thresholds for seed germination identified in this study (Tb and θ50) explained the differences in seed germination detected among populations. Under the two simulated IPCC scenarios, an altitude-related risk from climate warming is identified, with lowland populations being more threatened due to a compromised seed dormancy release and a narrowed seed germination window.
Keywords: Base temperature, climate change, cold stratification, crop wild relative, IPCC scenarios, physiological dormancy, thermal time, Vitaceae, Vitis vinifera subsp. sylvestris
INTRODUCTION
The Intergovernmental Panel on Climate Change (IPCC) has predicted temperature increases of approx. 2–4 °C by 2090–2099 according to different emission scenarios. Predictions have been grouped into four families of scenarios (A1, A2, B1 and B2) that explore alternative development pathways, covering a wide range of demographic, economic and technological driving forces and resulting greenhouse gas emissions (IPCC, 2007). In particular, large increases in temperature have been predicted and reported for the Mediterranean mountain ranges (Peñuelas and Boada, 2003; Nogués-Bravo et al., 2008).
Temperature is the main environmental factor governing seed germination in moist soil, determining both the fraction of seeds in a population that germinate and the rate at which they emerge (Heydecker, 1977). In non-dormant seeds, the germination response to accumulated temperature has been modelled by a thermal time (θ) approach (e.g. Garcìa-Huidobro et al., 1982; Covell et al., 1986; Ellis et al., 1986, 1987; Pritchard and Manger, 1990; Pritchard et al., 1996; Hardegree, 2006). In this model, seeds accumulate units of thermal time (°Cd) to germinate for a percentile g of the population. When seeds are subjected to temperatures (T) above a base temperature for germination (Tb), at which the germination rate is zero, and below an optimum temperature (To), above which germination rate starts to decrease (i.e. the sub-optimal temperature range), germination rate increases linearly with temperature (Garcìa-Huidobro et al., 1982). Thus, in this temperature range, germination occurs in the time tg when the thermal time accumulated has reached the critical value (θg) for a percentile g of the population. This response can be described as θg = (T – Tb)tg. Intraspecific variation in Tb among populations of the same species may be due to different environmental conditions during seed development (Daws et al., 2004).
Recently, Walck et al. (2011), in their review of climate change and plant reproduction by seed (i.e. release from dormancy and germination), highlighted that the ecophysiology of seed germination phenology is similar to that of budburst in some species, in which buds require chilling to release winter dormancy and a critical thermal time in spring for budburst. This germination behaviour, according to the seed dormancy classification system of Baskin and Baskin (2004), falls in the physiological dormancy class. Walck et al. (2011) argued that in species with this kind of dormancy, seedling emergence will be delayed with global warming, if the current length of the stratification period approximates its minimum requirement, as shortened winters will not adequately overcome dormancy. On the other hand, emergence will be earlier if the current length of stratification greatly exceeds the minimum required, as premature spring warm-up accelerates germination (Walck et al., 2011). However, despite the importance of the characterization of thermal time requirements for seed conservation purposes (Mira et al., 2011) and of the seed germination niche for identifying plants at risk from global warming (see Cochrane et al., 2011), very little attention has been given to how thermal thresholds [i.e. base temperature (Tb) and critical thermal time for germination (θg)] can be used to estimate the impact of predicted climate change scenarios on seed germination phenology.
Freshly extracted seeds of Vitis ssp. are usually physiologically dormant and may require considerable periods of cold stratification before they will germinate (e.g. Singh, 1961; Ellis et al., 1983, 1985; Conner, 2008; Wang et al., 2011). Subsequent efficient seed germination occurs at 20–30 °C and suggests that emergence will occur naturally in the spring after winter chilling (Baskin and Baskin, 1998). Wang et al. (2009) modelled the effect of stratification on dormancy release in seeds of Beichun grape (a cross-breed of V. vinifera × V. amurensis) and found that dormancy was consistently released with prolonged stratification time at temperatures <15 °C (with an optimum at approx. 10 °C), while stratification at 25 °C induced secondary dormancy. Alternatively, warm and dry after-ripening can break dormancy in grape seeds (Ellis et al., 1985). For example, in V. amurensis seeds, dormancy was released by 90 d at low seed moisture and 25 °C (Wang et al., 2011). However, to our knowledge, no information is available on seed dormancy release and germination for the wild grapevine (Vitis vinifera subsp. sylvestris; hereafter Vitis sylvestris) across an altitudinal range.
Therefore, the aims of this work were to (1) characterize the thermal thresholds for seed dormancy and germination of the rare crop wild relative Vitis sylvestris, (2) model its phenology of seed germination and (3) predict the impact of different IPCC scenarios of increasing temperatures on its seed germination phenology.
MATERIALS AND METHODS
Study species
The genus Vitis consists of approx. 65 inter-fertile species growing almost exclusively in the northern hemisphere (This et al., 2006). Vitis sylvestris is one of the most important taxa of this genus due to its contribution of genetic variability to modern cultivars of the domesticated grapevine V. vinifera subsp. vinifera (Levadoux, 1956; Cunha et al., 2007). V. sylvestris reached the Mediterranean probably from the Caucasian area (Grassi et al., 2006) and is now distributed from North Africa to Central Europe (Arnold et al., 1998). It grows from 0 to 1000 m a.s.l., along riverbanks, on screes (colluvial sites) of hilly humid slopes and occasionally on coastal sheers and beaches (Ocete et al., 2008), in separate micro- or meta-populations often comprising only a few individuals (Terral et al., 2010).
Study area and seed lot details
Sardinia is the second largest island in the Mediterranean Sea. Its isolation and high geological diversity have created a wide range of habitats, especially on its mountain massifs, where there are conditions of ecological insularity (Médail and Quézel, 1997). During the Quaternary glaciations, the island constituted a climatic refuge area for V. sylvestris (Grassi et al., 2008), which is still present in numerous, well-preserved, large populations (Lovicu et al., 2009). Sardinian populations range from 60 to 800 m a.s.l. on different substrates (mainly Palaeozoic), except for limestones. The isolation of these populations in natural areas, located far both from urban areas and from vineyards, indicates the low probability that intraspecific hybridization with V. vinifera cultivars has occurred (Zecca et al., 2010) and highlights the possibility that subtle variations in ecological niche requirements might make some populations more at risk from climate change.
Berries of V. sylvestris were collected in two consecutive seasons (2009–2010) from six different localities belonging to four regions at the time of natural dispersal (Tables 1 and 2). Immediately after collection, seeds were separated from the fleshy fruits by squashing through sieves with a tepid water wash, then spread in a thin layer to dry. Seeds collected in 2009 were stored in a dry room at 15 % relative humidity and 15 °C for 15 months. At the end of the drying period the moisture content was calculated gravimetrically after 17 h at 103 °C (ISTA, 2006), based on three replicates of 50 seeds each (Table 2). Seeds collected in 2010 were not dried and the mass and moisture content were calculated immediately after cleaning (Table 2).
Table 1.
Seed lot details of the investigated populations of Vitis sylvestris
| Population code | Region | Locality | Geographical coordinates (Datum WGS84) | Substrate (Carmignani et al., 2001) | Altitude (m a.s.l.) | Habitat |
|---|---|---|---|---|---|---|
| SU1 | Sulcis | Gutturu Mannu – Assemini (CA) | 39°10′N, 08°55′E | Granites | 86–114 | Riparian woods with Alnus glutinosa |
| SU2 | Sulcis | Medau truba manna – Siliqua (CA) | 39°12′N, 08°46′E | Metamorphic rocks | 119–183 | Riparian woods with Alnus glutinosa |
| IG1/2 | Iglesiente | Rio Antas – Fluminimaggiore (CI) | 39°24′N, 08°28′E | Metamorphic rocks | 130–270 | Riparian woods with Alnus glutinosa – Mantle shrubs with Rubus ulmifolius |
| SA1 | Sarcidano | Cuccuru – Laconi (OR) | 39°52′N, 09°05′E | Granites | 715–718 | Mantle shrubs with Rubus ulmifolius – Deciduous woods with Quercus sp. pl. |
| SA2 | Sarcidano | Mitz'eurgia carbone – Laconi (OR) | 39°52′N, 09°05′E | Granites/travertines | 622–756 | Mantle shrubs with Rubus ulmifolius – Deciduous woods with Quercus sp. pl. |
| BA1/2 | Barbagia di Belvì | Errist'aulu – Aritzo (NU) | 39°55′N, 09°15′E | Granites | 710–780 | Deciduous woods with Quercus sp. pl. |
Table 2.
Variation in mass and moisture contents for Vitis sylvestris seed lots; data are the mean of three replicates of 50 seeds each and values with the same letter are not significantly different (P > 0·05) by one-way ANOVA followed by post-hoc Fisher LSD test
| Population code | Collection date (day/month/year) | Fresh seed mass (mg; mean ± s.d.) | Moisture content (%; mean ± s.d.) | Dry seed mass (mg; mean ± s.d.) |
|---|---|---|---|---|
| Dry seed lots | ||||
| SU1 | 20/09/09 | – | 5·42 ± 0·05a | 26·05 ± 0·44a |
| IG1 | 03/10/09 | – | 5·52 ± 0·04ab | 25·83 ± 0·22a |
| SA1 | 09/10/09 | – | 5·66 ± 0·09c | 24·46 ± 0·21b |
| BA1 | 25/10/09 | – | 5·60 ± 0·04bc | 24·67 ± 0·16b |
| P < 0·01 | P < 0·001 | |||
| Fresh seed lots | ||||
| SU2 | 09/10/10 | 32·35 ± 0·40a | 19·70 ± 0·09a | 25·98 ± 0·35a |
| IG2 | 18/09/10 | 32·68 ± 1·55a | 17·48 ± 0·37b | 18·99 ± 0·89b |
| SA2 | 28/10/10 | 35·43 ± 1·18b | 28·80 ± 0·44c | 25·23 ± 0·82a |
| BA2 | 23/10/10 | 36·95 ± 0·35b | 22·31 ± 0·26d | 28·71 ± 0·18c |
| P < 0·001 | P < 0·001 | P < 0·001 | ||
Germination tests
Three replicates of 20 seeds each, from both 2009 (i.e. dried) and 2010 (i.e. fresh) seedlots, were sown on the surface of 1 % agar water (with the ventral face of the seeds turned towards the substrate) in 90-mm-diameter plastic Petri dishes and incubated in the light (12 h light/12 h dark) at a range of germination temperatures (10, 15, 20, 25 and 25/10 °C). Further replicates were given a warm (3 months at 25 °C) or a cold (3 months at 5 °C) stratification, before these temperature treatments. In the alternating temperature regime, the 12-h light period coincided with the warm period. Germination was defined as visible radicle emergence to ≥1 mm and germinated seeds were scored three times a week. At the end of the germination tests, when no additional germination had occurred for 2 weeks, a cut-test was carried out to determine the viability of the remaining seeds. Soft, mouldy seeds were considered to be non-viable.
Data analysis
Thermal time analysis was carried out for cold-stratified seeds collected in 2009 (see Table 2) as this treatment yielded the highest proportion of non-dormant seeds and reflected the alternating dry/warm and cold/wet season under a Mediterranean climate. Estimates of time (tg, days) taken for cumulative germination to reach different percentiles (g) for successive increments of 10 % germination were interpolated from the germination progress curves (Covell et al., 1986). Germination rate (1/tg) was plotted as a function of temperature (constant 10–25 °C) and regressed using a linear model, to estimate the base temperature (Tb) for each g percentile, according to Garcìa-Huidobro et al. (1982):
| (1) |
An average (± 1 s.d.) of the x-intercept among percentiles was calculated for the suboptimal temperature range to establish the Tb for each population (Ellis et al., 1986; Pritchard and Manger, 1990). Linear regression equations were then recalculated for each percentile, but constrained to pass through Tb (Hardegree, 2006). A comparison of regressions was then made between this model and one in which the base temperatures were allowed to vary for all the percentiles and the best estimate was considered to be that which resulted in the smallest residual variance (Covell et al., 1986). Thermal time (θ, °Cd) estimates for each population were then calculated separately as the inverse of the sub-optimal regression equations (Covell et al., 1986; see eqn 1). Germination percentages were transformed to probits using tabular data from Finney (1971).
Linear regression was used to express probit(g) as a function of both θ and logθ for the sub-optimal temperature range for each seed lot and the best model evaluated on the basis of the r2 values (Hardegree, 2006). Linear distributed θ showed the highest r2 values, and thus the following equation was used to describe the form of cumulative germination response of seeds (Covell et al., 1986):
| (2) |
where K is an intercept constant when thermal time (θg) is zero and σ is the standard deviation of the response to θg (i.e. the reciprocal of the slope), and represents the sensitivity of the population to θg (Covell et al., 1986). Thus, the flatter the slope of the fitted line, the greater the variation in response to thermal time between individual seeds (Daws et al., 2004). On a plot of probit(g) against θg, the median thermal time required for seed germination of the population (g = 50 %; θ50) corresponds to the thermal time when probit(g) = 5 (Finney, 1971).
Environmental heat sum was calculated starting from March (i.e. after the winter cold stratification period), for each population (see Table 1) as follows:
| (3) |
where Tb is the base temperature for germination, EnvTm is the average monthly temperature of the month (m) and tm is the number of days of the month (m), until reaching the median thermal time value (θ50).
Climatic data (historical series of monthly averages of temperatures and rainfall from nearby climatic stations) were obtained for each population site (weather stations of Desulo, Fluminimaggiore, Laconi and Capoterra, for Barbagia, Iglesiente, Sarcidano and Sulcis, respectively), from Regione Autonoma della Sardegna (http://www.regione.sardegna.it/j/v/25?s=131338&v=2&c=5650&t=1). IPCC scenarios of temperature increase by 2090–2099 relative to the period 1980–1999 were then simulated adjusting the mean monthly temperatures according to the best estimate temperature change (+1·8 and +3·4 °C for B1 and A2 scenarios, respectively; IPCC, 2007). The threshold of a mean winter temperature of 15 °C, above which cold stratification does not occur with a significant rate, was applied, according to the findings of Wang et al. (2009).
Linear regressions were fitted using SigmaPlot 2002 for Windows version 8·0 (SPSS, Chicago, IL, USA). One-way ANOVA, followed by a post-hoc Fisher least significant difference test (LSD), was carried out to test for differences in seed traits (mass and moisture content) among populations, final germination percentages and base temperature values. For these analyses R v. 2·12·0 (R Development Core Team, 2010) was used.
RESULTS
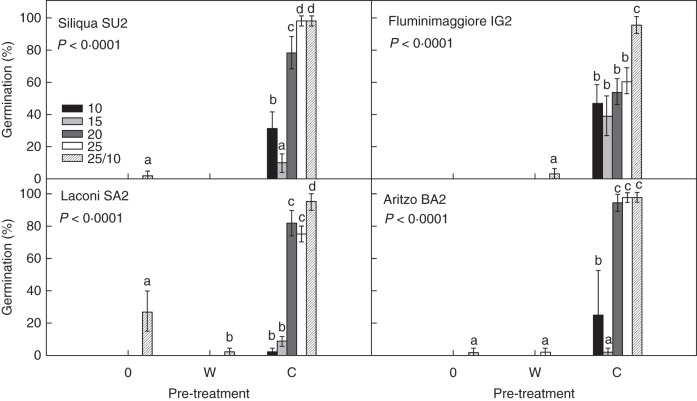
Freshly collected seeds of V. sylvestris germinated to high percentages only after a cold stratification period (C), while just a few seeds germinated in the control (0) or after warm stratification (W) for all the investigated seed lots (Fig. 1). After 3 months of cold stratification, seeds from all lots germinated significantly better (P < 0·05) at constant temperatures ≥20 °C (>80 %), than at 10 and 15 °C (<40 %) except for IG2, seeds of which germinated with percentages ranging from approx. 40 % (15 °C) to 60 % (25 °C), without significant differences among temperatures (Fig. 1). Fresh, cold-stratified seeds reached their maximum germination when incubated in the alternating temperature regime (25/10 °C), with final percentages of 98 ± 3 % for BA2 and SU2 seed lots and 95 ± 5 % for IG2 and SA2 seed lots. This condition was the only temperature regime that allowed some seed germination (approx. 25 %) for the SA2 seed lot without any pre-treatment (Fig. 1).
Fig. 1.
Final germination percentages (mean ± s.d.) of fresh seeds for the four investigated populations at different temperatures for each pre-treatment: 0 = control, W = warm stratification (25 °C for 3 months) and C = cold stratification (5 °C for 3 months). Data are the mean of three replicates. For each seed lot one-way ANOVA, followed by post-hoc Fisher's LSD test, was carried out; bars with the same letters are not different at P < 0·05.
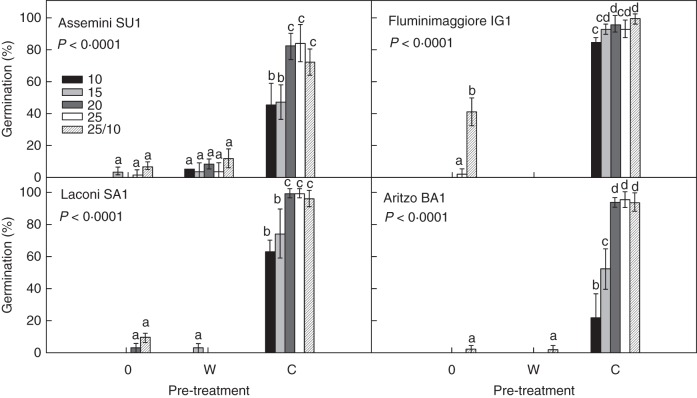
Dried seeds showed a similar trend, with seeds germinating at high percentages only after cold pre-treatment, particularly when moved to constant temperatures ≥20 °C and at 25/10 °C in all seed lots (Fig. 2). However, dried seeds germinated >40 % also at temperatures lower than 20 °C (i.e. at 10 and 15 °C), except for BA1 with approx. 20 % germination at 10 °C (Fig. 2). Few dried seeds germinated also without any pre-treatment and after warm stratification. In particular, seeds from IG1 germinated to approx. 40 % at 25/10 °C without any pre-treatment, while SU1 seeds reached approx. 10 % germination at almost all temperatures in the control and after warm stratification pre-treatment (Fig. 1). The great majority (approx. 90 %) of non-germinated seeds from the tests on both freshly used and pre-dried seeds appeared viable at the end of the experiments when checked by the cut-test.
Fig. 2.
Final germination percentages (mean ± s.d.) of dry seeds for the four investigated populations at different temperatures for each pre-treatment: 0 = control, W = warm stratification (25 °C for 3 months) and C = cold stratification (5 °C for 3 months). Data are the mean of three replicates. For each seed lot one-way ANOVA, followed by post-hoc Fisher's LSD test, was carried out; bars with the same letters are not different at P < 0·05.
Freshly collected seeds varied significantly (P < 0·001) among seed lots in their fresh (from 32·3 to 36·9 mg, P < 0·001) and dry (from 18·9 to 28·7 mg, P < 0·001) mass and in their moisture content (from 17·5 to 28·8 %, P < 0·001; Table 2). Moisture content of dried seeds ranged from 5·4 to 5·7 % (P < 0·01) and dry mass from 24·5 to 26·0 mg (P < 0·001; Table 2). The post-hoc test highlighted that seeds from the two low-altitude populations (SU1 and IG1) showed significant differences (P < 0·05) in their fresh mass and moisture content compared with those from high altitude (SA1 and BA1), low-altitude seeds being smaller and with lower moisture contents (Table 2). At constant temperatures ≥20 °C and at 25/10 °C, final germination percentages of cold-stratified fresh seeds were indifferent to their initial seed moisture content (linear regression P > 0·05; data not shown). However, at low temperatures (10 and 15 °C) these values were negatively related to the seed moisture content of each seed lot (P < 0·001; r2 = 0·41; data not shown).
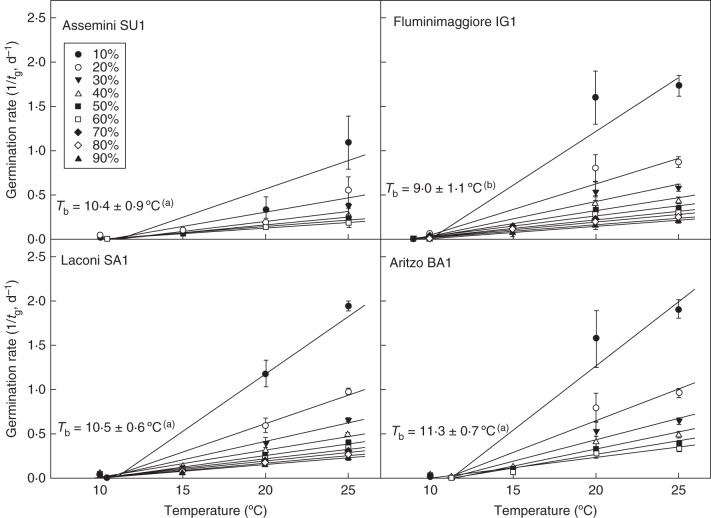
It was possible to estimate the Tb of each percentile (g) within all the four populations from the linear regressions of the relationship between constant temperatures and germination rate of cold-stratified seeds collected in 2009 (1/tg; Fig. 3). The Tb of each population, calculated as the average value of the x-intercepts of the percentiles for which regression lines had a P < 0·05, were 10·4 ± 0·9, 9·0 ± 1·1, 10·5 ± 0·6 and 11·3 ± 0·7 °C for SU1, IG1, SA1 and BA1 populations, respectively. These values were statistically different (P < 0·0001) by one-way ANOVA and the post-hoc Fisher's LSD test highlighted that this difference was determined by the Tb value of IG1, as it was the only value which differed at P < 0·05 from the others (Fig. 3). For the different populations, linear regressions were re-calculated for each percentile, constraining them to pass through the mean Tb (Fig. 3). This model led to no differences in residual sum of squares compared with when Tb was allowed to vary for each percentile and showed highest values of r2 for all of the linear regression equations (r2 > 0·89 for BA1, r2 > 0·85 for IG1, r2 > 0·92 for SA1 and r2 > 0·77 for SU1).
Fig. 3.
Base temperatures for germination (Tb) for the four populations, calculated after cold stratification (3 months at 5 °C) and incubation at constant temperatures (10–25 °C). Within each population, the linear regressions for the different percentiles were constrained to the common value of Tb. For percentiles for which regression lines had a P > 0·05, Tb values were not calculated. Statistical differences among populations were analysed by one-way ANOVA followed by post-hoc Fisher's LSD test; values with the same letters are not significantly different at P < 0·05.
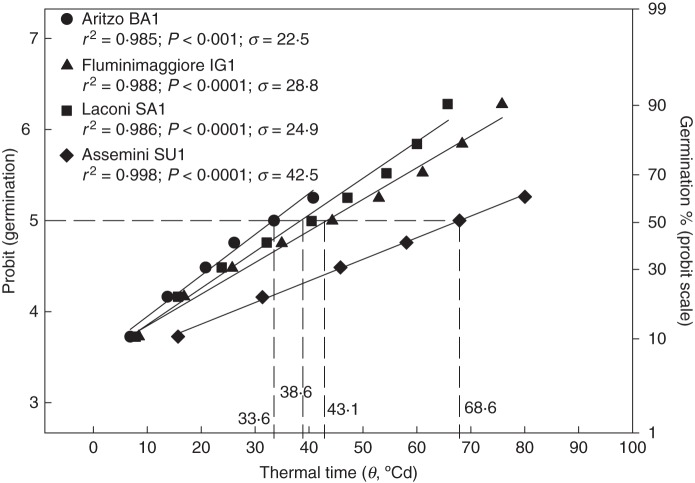
Figure 4 shows the estimate of the relationship between thermal time (θ), on a linear scale, and germination, expressed in probits, for the four investigated V. sylvestris populations, calculated according to eqn (2) (Fig. 4). All the regression lines accounted for approx. 99 % of the variation of the responses of germination to θ. Probit germination of seeds showed similar thermal time requirements for BA1, IG1 and SA1 populations. In these populations, thermal units needed to reach 50 % germination (θ50) were 33·6, 38·6 and 43·1 °Cd, respectively, whereas population SU1 needed approx. 76 °Cd (Fig. 4). In addition, seeds of SU1 had a greater σ value (42·5 °Cd) than the other seed lots (ranging from 22·5 to 28·8 °Cd; Fig. 4). The differences detected among populations in the θ50 values were negatively related to altitude, by an exponential non-linear regression, with a rapid decrease up to approx. 300 m a.s.l. (Fig. 5).
Fig. 4.
Germination in probits of dried seeds as a function of thermal time requirement (θ, °Cd), according to the equation: probit(g) = K + θg/σ. Thermal times were calculated assuming base temperatures for germination of 11·3, 9·0, 10·5 and 10·4 °C for BA1, IG1, SA1 and SU1 seed lots, respectively. Thermal time to reach 50 % of germination [θ50, corresponding to probit(g) = 5] are showed for each population. The germination percentages on a probit scale are also reported for reference on the right.
Fig. 5.
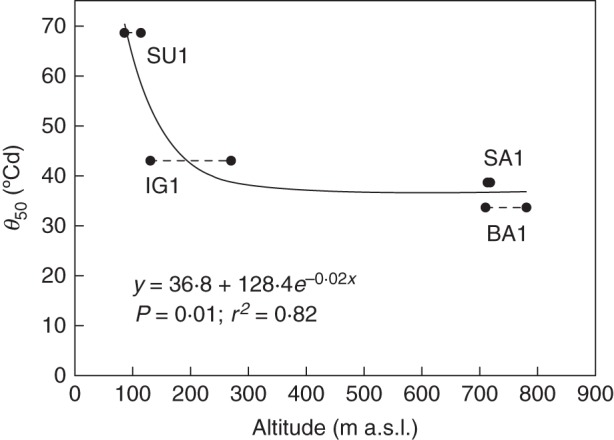
Dependency of thermal time to reach 50 % of germination (θ50, °Cd) with altitude (m a.s.l.). Points correspond to the minimum and maximum altitude of BA1, IG1, SA1 and SU1 populations.
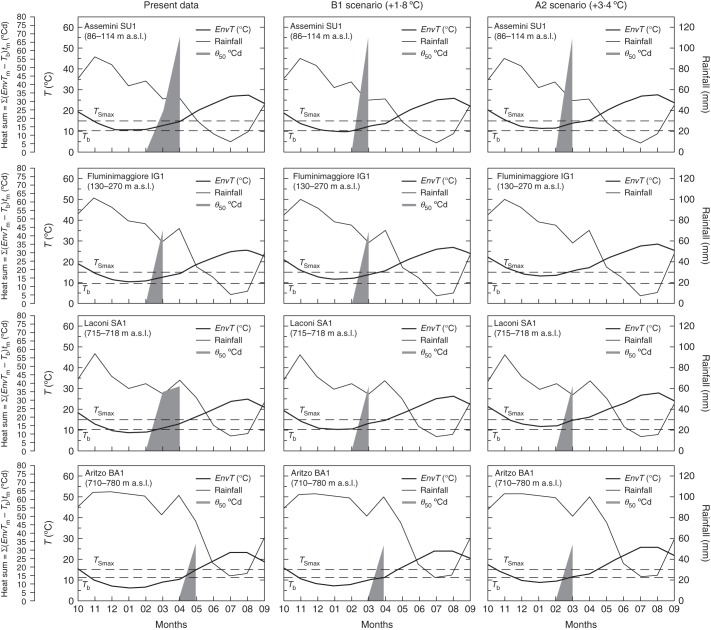
Available climatic data allowed calculation of the heat sum (Σ°Cd) that non-dormant seeds accumulate in each investigated population after winter, when mean temperatures (Tm) rise above the base temperatures for germination (Tb, Fig. 6). Although all four populations showed a bi-seasonal Mediterranean trend, there were relevant differences in their mean monthly temperatures (Tm), according to their altitude (see Table 1). These varied from minimum values of 5·9, 10·2, 8·7 and 10·1 °C for BA1, IG1, SA1 and SU1, respectively (January; Fig. 6), to maximum values of 22·9–27·4 °C (August; Fig. 6). Due to these differences, seeds from BA1 should start to accumulate thermal units (°Cd) in April, when environmental temperatures (EnvTm) exceed the base temperatures for germination (Tb). In contrast, the higher mean temperatures (and for IG1 also a lower Tb) should allow seeds to accumulate thermal units as early as February for IG1, SU1 and SA1 (Fig. 6). Thus, the differences in mean monthly temperatures together with those in Tb and θ50 values lead to potentially large differences across populations on their timing for germination. Non-dormant seeds belonging to BA1 needed 33·6 °Cd to reach 50 % germination (see Fig. 4). According to the climatic data, seeds would reach this value in May (Fig. 6), when EnvTm is 14·9 °C. Similarly, seeds from SA1 are expected to reach 38·6 °Cd in April, when EnvTm is 13·2 °C (Fig. 6). Seeds from IG1 are predicted to reach 50 % germination (θ50 of 43·1 °Cd) in March when EnvTm is 10·5 °C, whereas seeds from SU1 would achieve this level of germination in April (EnvTm = 12·6 °C), with a θ50 value of 68·6 °Cd (Fig. 6).
Fig. 6.
Environmental heat sum for non-dormant seeds calculated for each population for the present data and under two different IPCC scenarios (B1, +1·8 °Cd and A2, +3·4 °Cd ) as the sum of mean monthly temperature – base temperature for germination (Tm – Tb) per the number of days (tm), until reaching the median thermal times for germination (θ50, °Cd). Base temperatures for germination (Tb) are assumed to be 11·3, 9·0, 10·5 and 10·4 °C for BA1, IG1, SA1 and SU1 seed lots, respectively (dashed lines). Maximum stratification temperature for dormancy release (TSmax) set to 15 °C, according to Wang et al. (2009). Annual trends of temperature and rainfall are also reported for each population, starting from the month of natural dispersal (10 = October).
The increase in temperature of 1·8 °C predicted by the B1 scenario should lead to mean winter temperatures ranging from approx. 8 °C (BA1) to 12 °C (IG1) and therefore lower than the maximum temperature for dormancy release (15 °C; Fig. 6). In addition, an increased heat sum would accelerate germination of non-dormant seeds, starting in March for SU1, IG1 and SA1, with EnvTm of 10·8, 10·9 and 12·5 °C, respectively, and in April for BA1 (EnvTm = 8·3 °C; Fig. 6).
An increase in mean temperature of 3·4 °C (A2 scenario) would compromise seed dormancy release for IG1 where during the winter the mean temperature would be 14·1 °C and therefore close to the maximum stratification temperature for dormancy release (TSmax) of 15 °C (reported in Wang et al., 2009; Fig. 6). In all the other populations mean temperature during winter stratification will range from 9·9 °C (BA1) to 12·5 °C (SA1) allowing seed dormancy to be released (Fig. 6). Germination of seeds for all these populations would be in March (EnvTm of 12·1, 15·0 and 14·6 °C for BA1, SA1 and SU1, respectively; Fig. 6) as in the B1 scenario for SU1 and SA1 and 1 month earlier for BA1 (Fig. 6).
DISCUSSION
Freshly harvested seeds of V. sylvestris from all the investigated populations did not germinate, or reached very low germination percentages, when incubated without any pre-treatment. Thus, they are dormant, as already described for other species of this genus (e.g. Singh, 1961; Ellis et al., 1983, 1985; Conner, 2008; Wang et al., 2011). Warm stratification prior to the germination test did not enhance germination, whereas cold stratification completely released dormancy. Drying had a positive effect on germination of V. sylvestris, widening the temperature range at which seeds reached high germination percentages after cold stratification. Seeds of V. vinifera are reported to be orthodox (Royal Botanic Gardens Kew, 2008) and V. sylvestris seeds are clearly highly desiccation-tolerant, considering the high germination percentages achieved at low moisture content (<6 %). Drying before or after stratification markedly promoted the effect of 5 °C stratification on seed dormancy release of different varieties of V. amurensis (Wang et al., 2011), suggesting that a combination of dry after-ripening and moist cold stratification may be a common pattern of dormancy breaking for species of this genus, probably mimicking the natural conditions of moist and cold seasons (i.e. winter) followed by warm and dry ones (i.e. summer).
The base temperature for germination (Tb) in non-dormant (i.e. cold-stratified) seeds of V. sylvestris ranged from approx. 9 to 11 °C, depending on the provenance. To our knowledge this is the first report of Tb for a member of the Vitaceae. The analysis carried out in this study confirmed that Tb is a constant within a population (Covell et al., 1986; Ellis et al., 1987; Pritchard and Manger, 1990), while this value may slightly or significantly change among populations of the same species (i.e. Ellis et al., 1987; Daws et al., 2004). In addition, treatments for dormancy release can modify the Tb for seeds belonging to the same population (Pritchard et al., 1999). Considering that no seeds of V. sylvestris germinated without treatment at the tested constant temperatures, a Tb ≥ 25 °C may be supposed for dormant seeds of the investigated populations. However, this remains to be confirmed by incubating seeds without stratification at higher temperatures (i.e. up to 40 °C). The detected Tb values are consistent with the current circum-Mediterranean distribution (Arnold et al., 1998) and the altitudinal range (0–1000 m a.s.l.; Ocete et al., 2008) of this species. Such an environment and thermal characteristics of the seed populations would probably prevent seedling establishment in northern and higher-elevation regions, where spring temperatures are too low to facilitate seed development and germination. All the conditions tested in this study were in the sub-optimal range and, therefore, the optimal temperature for germination of non-dormant seeds of V. sylvestris may be assumed to be ≥25 °C.
Seed lot provenance clearly influences the sensitivity of the seed germination response to thermal time (Daws et al., 2004). Populations of V. sylvestris varied in their thermal time estimates (θ50). Sensitivity to the accumulated thermal units (°Cd) increased with altitude, leading to reduced θ50 values in the highest populations, i.e. faster germination in a potentially shorter growing season. Daws et al. (2004) correlated differences in thermal time requirements to the environmental parameters (heat sum) of the sites where seeds developed. These authors detected a latitudinal gradient (from Greece to England) for seeds of A. hippocastanum, with Tb and σ values increasing and decreasing, respectively, as latitude increased (Daws et al., 2004). Consistent with these findings, at the small geographical scale of the present study, where all the populations are located at 39 °N, altitude acted as a driver for thermal requirement sensitivity.
Typically under Mediterranean climate, germination is limited to winter, which maximizes the length of the growing season before the onset of summer drought (Thanos et al., 1991; Doussi and Thanos, 2002). In contrast, in temperate and alpine regions spring germination prevails mainly due to a requirement of cold stratification over winter (Baskin and Baskin, 1998). The dormancy breaking and thermal time requirements identified in this study together with the available climatic data allowed a spring germination to be predicted for seeds of V. sylvestris. In the laboratory experiments, winter conditions were simulated by 90 d of cold stratification at 5 °C. Climatic data available for the investigated populations showed a mean temperature from December to February (winter) of 9·0, 10·7, 9·1 and 6·5 °C for SU1, IG1, SA1 and BA1, respectively. Although these values were higher than the tested stratification temperature (except for BA1), they were close to 10 °C (the optimum temperature for dormancy release of Beichun grape seeds; Wang et al., 2009) and lower than 15 °C, the threshold temperature below which stratification consistently released dormancy of Beichun grape seeds (Wang et al., 2009). Therefore, seeds from IG1, SA1 and BA1 may germinate from March, April and May, respectively, and the seedling growth period, i.e. from seed germination to the onset of the summer drought, increases as the altitude decreases (1 month for BA1, 2 months for SA1 and IG1). This is due to θ50 values decreasing with altitude and, for seeds from IG1, also to their lower Tb. An early spring germination for seeds of the IG1 population was confirmed by the positive effect detected for the alternating temperature, which promotes field emergence prior to canopy closure (Daws et al., 2002). In contrast, seeds from the lowest population (SU1), due to their high Tb and θ50, cannot start germinating before April, i.e. only 1 month before the start of the summer drought. In this population, growing in a region characterized by more than 4 months of droughts, plants were found exclusively within the riparian Alnus glutinosa woods, where watercourses do not dry in summer.
The different environmental conditions detected for the investigated populations, also during seed maturation, resulted in significantly different moisture levels in freshly collected seeds among populations and may lead to phenological shifts (see Reader, 1984; Blionis et al., 2001). Changes in phenological phases of V. vinifera have been correlated with warming-induced earlier maturity of grapevines (Webb et al., 2011). According to the findings of our study, higher temperatures would not be detrimental per se for seed germination in wild grapevine. Moreover, it appears that the requirement of cold stratification for dormancy release detected for seeds of this species will ensure germination occurs in the spring. However, in the Mediterranean region, a declining trend of precipitation was observed from 1900 to 2005, increasing the area affected by drought (IPCC, 2007) and projections for Mediterranean mountains predict lower levels of precipitation mainly during spring (Nogués-Bravo et al., 2008). Therefore, the seedling growing season could be shortened by a reduction in soil moisture and water availability. Rising temperatures may reduce the rate of dormancy loss in seeds of species that need cold stratification before germination occurs (Steadman and Pritchard, 2004). However, an increase in temperatures of 1·8 °C (B1 scenario; IPCC, 2007) would still adequately overcome dormancy for all the investigated populations (winter temperatures <15 °C; Wang et al., 2009), whereas with +3·4 °C (A2 scenario; IPCC, 2007) the higher winter temperature would not allow seed dormancy to be overcome in the IG1 population. The different responses detected along the altitudinal gradient highlighted that low-altitude populations are, potentially, at greater risk from climate change, due to both temperatures too high for seed dormancy release at efficient rates and a narrowed favourable phenological window for germination. A common opinion is that the species most vulnerable to a warming climate are those already restricted by physical limits of their range such as higher altitudes and upper mountain locations (e.g. Walther et al., 2002; Parolo and Rossi, 2008). However, the present distribution and altitudinal range of V. sylvestris should allow populations to shift both to northern regions and to higher altitudes, where conditions, based on our findings, will still be favourable for seed dormancy release and germination.
In conclusion, the thermal thresholds for seed germination identified in this study (Tb and θ50) explain the differences in seed germination detected among populations of this rare crop wild relative and are consistent with its present geographical and altitudinal ranges (Arnold et al., 1998; Ocete et al., 2008). In addition, this study allowed the impact of climate warming on seed germination of the modelled species to be assessed according to two different IPCC scenarios (B1 and A2; IPCC, 2007) and highlighted the importance of thermal time requirements for predicting, in a quantitative way, plant ecological responses to climate change and the potential for natural regeneration.
ACKNOWLEDGEMENTS
We thank the ‘Agenzia per l'Agricoltura Sperimentale della Sardegna (AGRIS)’ for technical support. In particular, M. Farci, G. Lovicu, F. Piras and R. Zurru helped with seed collecting and A. Aru, G. Piano and M. Sedda with seed cleaning. The CCB is supported by the ‘Provincia di Cagliari – Assessorato Tutela Ambiente’. The Royal Botanic Gardens, Kew, receives grant in-aid from Defra, UK.
LITERATURE CITED
- Arnold C, Gillet F, Gobat JM. Situation de la vigne sauvage Vitis vinifera subsp. sylvestris en Europe. Vitis. 1998;37:159–170. [Google Scholar]
- Baskin CC, Baskin JM. Seeds. Ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Blionis GJ, Halley JM, Vokou D. Flowering phenology of Campanula on Mt Olympos, Greece. Ecography. 2001;24:696–706. [Google Scholar]
- Carmignani L, Oggiano G, Barca S, et al. Note illustrative della Carta Geologica della Sardegna in scala 1:200·000 – Memorie descrittive della Carta Geologica d'Italia. Rome: Servizio Geologico d'Italia; 2001. [Google Scholar]
- Cochrane A, Daws MI, Hay FR. Seed-based approach for identifying flora at risk from climate warming. Austral Ecology. 2011;36:923–935. [Google Scholar]
- Conner PJ. Effects of stratification, germination, temperature, pre-treatment with gibberellic acid and hydrogen peroxide on germination of ‘Fry’ Muscardine (Vitis rotundifolia) seed. HortScience. 2008;43:853–856. [Google Scholar]
- Covell S, Ellis RH, Roberts EH, Summerfield RJ. The influence of temperature on seed germination rate in grain legumes. I. A comparaison of chickpea, lentil, soyabean, and cowpea at constant temperatures. Journal of Experimental Botany. 1986;37:705–715. [Google Scholar]
- Cunha J, Baleiras-Couto M, Cunha JP, et al. Characterization of Portuguese populations of Vitis vinifera L. ssp. sylvestris (Gmelin) Hegi. Genetic Resources and Crop Evolution. 2007;54:981–988. [Google Scholar]
- Daws MI, Burslem DFRP, Crabtree LM, Kirkman P, Mullins CE, Dalling JW. Differences in seed germination responses may promote coexistence of four sympatric Piper species. Functional Ecology. 2002;16:258–267. [Google Scholar]
- Daws MI, Lydall E, Chmielarz P, et al. Developmental heat sum influences recalcitrant seed traits in Aesculus hippocastanum across Europe. New Phytologist. 2004;162:157–166. [Google Scholar]
- Doussi MA, Thanos CA. Ecophysiology of seed germination in Mediterranean geophytes. 1. Muscari spp. Seed Science Research. 2002;12:193–201. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. A note on the development of a practical procedure for promoting the germination of dormant seed of grape (Vitis spp.) Vitis. 1983;22:211–219. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. Handbook of seed technology for Genebanks no. 3. Vol. II. Compendium of specific germination information and test recommendations. Reading, UK: University of Reading; 1985. [Google Scholar]
- Ellis RH, Covell S, Roberts EH, Summerfield RJ. The influence of temperature on seed germination rate in grain legumes. II. Intraspecific variation in chickpea (Cicer arietinum L.) at constant temperatures. Journal of Experimental Botany. 1986;37:1503–1515. [Google Scholar]
- Ellis RH, Simon G, Covell S. The influence of temperature on seed germination rate in grain legumes. III. A comparison of five faba bean genotypes at constant temperatures using a new screening method. Journal of Experimental Botany. 1987;38:1033–1043. [Google Scholar]
- Finney DJ. Probit analysis. 3rd edn. Cambridge: Cambridge University Press; 1971. [Google Scholar]
- Garcìa-Huidobro J, Monteith JL, Squire GR. Time, temperature and germination of Pearl Millet (Pennisetum typhoides S. & H.) I. Constant temperature. Journal of Experimental Botany. 1982;33:288–296. [Google Scholar]
- Grassi F, Labra M, Imazio S, et al. Phylogeographical structure and conservation genetics of wild grapevine. Conservation Genetics. 2006;7:837–845. [Google Scholar]
- Grassi F, De Mattia F, Zecca G, Sala F, Labra M. Historical isolation and Quaternary range expansion of divergent lineages in wild grapevine. Biological Journal of the Linnean Society. 2008;95:611–619. [Google Scholar]
- Hardegree SP. Predicting germination response to temperature. I. Cardinal-temperature models and subpopulation-specific regression. Annals of Botany. 2006;97:1115–1125. doi: 10.1093/aob/mcl071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydecker W. Stress and seed germination: an agronomic view. In: Khan AA, editor. The physiology and biochemistry of seed dormancy and germination. Oxford: Oxford Biochemical Press; 1977. pp. 237–277. [Google Scholar]
- IPCC. Climate change 2007: synthesis report in Core Writing Team. In: Pachauri RK, Reiginger A, editors. Contribution of Working Groups I, II, III to the 4th Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: IPCC; 2007. [Google Scholar]
- ISTA. International rules for seed testing. Edition 2006. Bassersdorf, Switzerland: The International Seed Testing Association (ISTA); 2006. [Google Scholar]
- Levadoux L. Les populations sauvages et cultivées de Vitis vinifera. Annales Amélioration Plantes Cultivées. 1956;6:50–110. [Google Scholar]
- Lovicu G, Farci M, Orrú M, Ocete ME, López MA, Ocete R. Presencia aislada de filoxera y yesca sobre vid silvestre. Vitis vinifera L. subespecie sylvestris (Gmelin) Hegi, en Cerdeña. XXXI Jornadas de viticultura y enología Tierra de Barros. Cultural Santa Ana, Centro Universitario Almendralejo, dal 4 al 8 de mayo de 2009, 2009:105–112. [Google Scholar]
- Médail F, Quézel P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Annals of the Missouri Botanical Garden. 1997;84:112–127. [Google Scholar]
- Mira S, González-Benito ME, Ibars MI, Estrelles E. Dormancy release and seed ageing in the endangered species Silene diclinis. Biodiversity and Conservation. 2011;20:345–358. [Google Scholar]
- Nogués-Bravo D, Araújo MB, Lasanta T, López Moreno JI. Climate change in Mediterranean mountains during the 21st century. Ambio. 2008;37:280–285. doi: 10.1579/0044-7447(2008)37[280:ccimmd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ocete R, López MA, Gallardo A, Arnold C. Comparative analysis of wild and cultivated grapevine (Vitis vinifera) in the Basque Region of Spain and France. Agriculture Ecosystems and Environment. 2008;123:95–98. [Google Scholar]
- Parolo G, Rossi G. Upward migration of vascular plants following a climate warming trend in the Alps. Basic and Applied Ecology. 2008;9:100–107. [Google Scholar]
- Peñuelas J, Boada M. A global change-induced biome shift in the Montseny mountains (NE Spain) Global Change Biology. 2003;9:131–140. [Google Scholar]
- Pritchard HW, Manger KR. Quantal response of fruit and seed germination rate in Quercus robur L. and Castanea sativa Mill. to constant temperatures and photon dose. Journal of Experimental Botany. 1990;41:1549–1557. [Google Scholar]
- Pritchard HW, Tompsett PB, Manger KR. Development of a thermal time model for the quantification of dormancy loss in Aesculus hippocastanum seeds. Seed Science Research. 1996;6:127–135. [Google Scholar]
- Pritchard HW, Steadman KJ, Nash JV, Jones C. Kinetics of dormancy release and the high temperature germination response in Aesculus hippocastanum seeds. Journal of Experimental Botany. 1999;50:1507–1514. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2010. http://www.R-project.org . [Google Scholar]
- Reader RJ. Comparison of the annual flowering schedules for Scottish heathland and Mediterranean-type shrublands. Oikos. 1984;43:1–8. [Google Scholar]
- Royal Botanic Gardens Kew. Seed Information Database (SID). Version 7·1. 2008 http://data.kew.org/sid/ (accessed 9 February 2012) [Google Scholar]
- Singh SN. Germination of grape (Vitis vinifera L.) hybrid seeds by chilling. Current Science. 1961;30:62. [Google Scholar]
- Steadman KJ, Pritchard HW. Germination of Aesculus hippocastanum seeds following cold-induced dormancy loss can be described in relation to a temperature-dependent reduction in base temperature (Tb) and thermal time. New Phytologist. 2004;161:415–425. doi: 10.1046/j.1469-8137.2003.00940.x. [DOI] [PubMed] [Google Scholar]
- Terral J, Tabard E, Bouby L, et al. Evolution and history of grapevine (Vitis vinifera) under domestication: new morphometric perspectives to understand seed domestication syndrome and reveal origins of ancient European cultivars. Annals of Botany. 2010;105:443–455. doi: 10.1093/aob/mcp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos CA, Georghiou K, Douma DJ, Marangaki CJ. Photoinhibition of seed germination in Mediterranean maritime plants. Annals of Botany. 1991;68:469–475. [Google Scholar]
- This P, Lacombe T, Thomas MR. Historical origins and genetic diversity of wine grapes. Trends in Genetics. 2006;22:511–519. doi: 10.1016/j.tig.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. Climate change and plant regeneration from seed. Global Change Biology. 2011;17:2145–2161. [Google Scholar]
- Walther G, Post E, Convey P, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Wang WQ, Song SQ, Li SH, Gan YY, Wu JH, Cheng HY. Quantitative description of the effect of stratification on dormancy release of grape seeds in response to various temperatures and water contents. Journal of Experimental Botany. 2009;60:3397–3406. doi: 10.1093/jxb/erp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WQ, Song SQ, Li SH, Gan YY, Wu JH, Cheng HY. Seed dormancy and germination in Vitis amaurensis and its variation. Seed Science Research. 2011;21:255–265. [Google Scholar]
- Webb LB, Whetton PH, Barlow EWR. Observed trends in winegrape maturity in Australia. Global Change Biology. 2011;17:2707–2719. [Google Scholar]
- Zecca G, De Mattia F, Lovicu G, Labra M, Sala F, Grassi F. Wild grapevine: sylvestris, hybrids or cultivars that escaped from vineyards? Molecular evidence in Sardinia. Plant Biology. 2010;12:558–562. doi: 10.1111/j.1438-8677.2009.00226.x. [DOI] [PubMed] [Google Scholar]