Abstract
The biological activity of reducing-end-modified oligogalacturonides was quantified in four tobacco (Nicotiana tabacum) tissue culture bioassays. The derivatives used were oligogalacturonides with the C-1 of their reducing end (a) covalently linked to a biotin hydrazide, (b) covalently linked to tyramine, (c) chemically reduced to a primary alcohol, or (d) enzymatically oxidized to a carboxylic acid. These derivatives were tested for their ability to (a) alter morphogenesis of N. tabacum cv Samsun thin cell-layer explants, (b) elicit extracellular alkalinization by suspension-cultured cv Samsun cells, (c) elicit extracellular alkalinization by suspension-cultured N. tabacum cv Xanthi cells, and (d) elicit H2O2 accumulation in the cv Xanthi cells. In all four bioassays, each of the derivatives had reduced biological activity compared with the corresponding underivatized oligogalacturonides, demonstrating that the reducing end is a key element for the recognition of oligogalacturonides in these systems. However, the degree of reduction in biological activity depends on the tissue culture system used and on the nature of the specific reducing-end modification. These results suggest that oligogalacturonides are perceived differently in each tissue culture system.
Carbohydrates that act as signal molecules in plants (oligosaccharins) have been isolated from plant and fungal cell wall polysaccharides, from the cell walls of bacterial symbionts of plants, and from fungal glycopeptides (for review, see Ryan and Farmer, 1991; Darvill et al., 1992; Côté and Hahn, 1994). We are interested in determining the mechanism by which one type of the plant cell wall-derived oligosaccharins, the oligogalacturonides, elicit biological responses in plants.
The biological effects elicited in plants by oligosaccharins are diverse (for review, see Ryan and Farmer, 1991; Darvill et al., 1992; Côté and Hahn, 1994) but can generally be placed in two groups: delayed responses and rapid responses. Delayed responses are usually observed hours or days after oligosaccharin treatment and are often directly involved in adaptation to environmental conditions, whereas rapid responses generally occur at the plant cell surface and are observed within minutes after addition of oligosaccharins. The delayed responses elicited by oligogalacturonides can be broadly divided into those in which defense responses are induced and those in which growth and development are modified. The defense-related responses, depending on the plant species, include phytoalexin accumulation (Hahn et al., 1981), lignification of cell walls (Robertsen, 1986), and proteinase inhibitor accumulation (Bishop et al., 1984). The responses involving growth and development include induction of ethylene in tomato fruit (Brecht and Huber, 1988), inhibition of auxin-induced pea stem elongation (Branca et al., 1988), and regulation of tobacco (Nicotiana tabacum) TCL explant morphogenesis (Eberhard et al., 1989). The rapid responses induced by oligogalacturonides include enhanced protein phosphorylation in tomato and potato membranes (Farmer et al., 1989), transmembrane ion flux accompanied by membrane depolarization in tobacco suspension cells (Mathieu et al., 1991) and carrot protoplasts (Messiaen et al., 1993), and H2O2 accumulation in suspension-cultured soybean cells (Apostol et al., 1989).
Rapid responses elicited by oligosaccharins may act as signal transduction events in the initiation of the delayed responses; however, this has not been directly demonstrated. Isolation and characterization of oligosaccharin receptors would facilitate the identification of the oligosaccharin-induced signal-transduction events. High-affinity binding sites for three oligosaccharins, the oligo-β-glucosides, the oligochitins, and a yeast N-glycan, have been identified using biologically active, radiolabeled derivatives (Cosio et al., 1990; Cheong and Hahn, 1991; Basse et al., 1993; Shibuya et al., 1993; Baureithel et al., 1994). The specificity of these binding sites has been characterized by measuring the ability of structural analogs having a range of biological activities to displace labeled derivatives in competitive binding studies. A direct correlation between the competitive binding ability of the analogs and their biological activity has been observed for each binding site, providing evidence that the sites identified are physiological receptors of the oligosaccharins. Characterizing the specificity of binding proteins in this manner requires homogeneous oligosaccharins, labeled oligosaccharin derivatives, and oligosaccharin analogs.
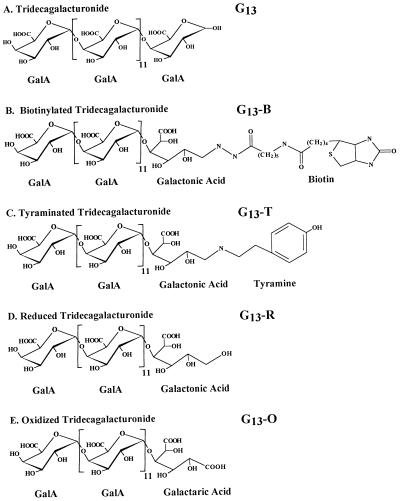
With the ultimate goal of identifying and characterizing oligogalacturonide receptors, we have quantified the biological activities of discernibly homogeneous reducing- end-derivatized oligogalacturonides in four tobacco tissue- culture bioassays. The oligogalacturonide derivatives assayed include G13-T, G13-B, G13-O, and G13-R (Fig. 1). Biotinylated and 125I-labeled tyraminated oligogalacturonides could be used to detect low-abundance oligogalacturonide-binding proteins. Unlabeled oligogalacturonides, including reduced and oxidized oligogalacturonides, could be used to characterize the specificity of oligogalacturonide-binding proteins.
Figure 1.
Structures of G13 and the four reducing-end derivatives of G13 used in this study.
MATERIALS AND METHODS
Purified orange fruit uronic acid oxidase was a gift from Russell Pressey (U.S. Department of Agriculture, Athens, GA). All reagents were obtained from Sigma.
Preparation of Homogeneous Oligogalacturonides and Their Reducing-End Derivatives
In this paper the term oligogalacturonide refers to native or unmodified, as well as reducing-end-derivatized oligogalacturonides, such as G13-T, G13-B, G13-R, and G13-O. Oligogalacturonides, generated by partial endopolygalacturonase digestion of polygalacturonic acid, were purified to homogeneity by a three-step protocol (Spiro et al., 1993) consisting of size-selective precipitation with ethanol, followed by Q-Sepharose anion-exchange chromatography, and then semipreparative HPAEC-PAD. All oligogalacturonides used in this study were homogeneous and chemically stable at −20°C, as determined by analytical HPAEC-PAD and by determination of their Mr by electrospray MS.
Oligogalacturonides derivatized with tyramine or biotin-x-hydrazide (6-[biotinoylamino] caproic acid hydrazide) at C-1 of the reducing-end galacturonic acid were synthesized and purified to homogeneity as described previously (Spiro et al., 1996; Ridley et al., 1997).
G13-R was generated by treating the G13 (5 mg in 1 mL of 1 m NH4OH) for 16 h at 4°C with NaBH4 (5 mg). The reaction was terminated by the addition of 5 volumes of 10% acetic acid in methanol, which destroyed any remaining NaBH4. This solution was kept for 2 h at −20°C and the resulting precipitate was collected by centrifugation. The supernatant was discarded. The pellet was washed twice with methanol (5 mL) to remove residual salts. The final pellet was dried under a stream of air at 25°C. The residue was dissolved in water (0.5 mL) and stored at −20°C.
G13-O was generated by treating G13 (5 mg) in 10 mm Tris-HCl, pH 8.5 (1 mL), with 3 units of uronic acid oxidase (Pressey, 1993) for 23 h at 37°C. This solution was sterilized by centrifugation through a 0.2-μm nylon filter prior to incubation.
Neither the chemical reduction nor the enzymatic oxidation of G13 proceeded to completion; therefore, G13-R and G13-O were individually purified to homogeneity by semipreparative HPAEC-PAD (Spiro et al., 1993). Electrospray MS analysis confirmed that the expected product had been synthesized.
TCL Bioassay
Tobacco (Nicotiana tabacum cv Samsun) plants were grown as described by Mohnen et al. (1990). The TCL explant bioassay was performed as described by Eberhard et al. (1989). Briefly, thin strips of tissue, approximately 1 mm wide and composed of 6 to 10 cell layers, were cut from surface-sterilized tobacco floral branches. TCL explants, approximately 10 mm long, were cut from the tissue strips. The explants were placed in individual wells of 12-well culture dishes (ICN) containing 2 mL of Linsmaier and Skoog (1965) medium, pH 5.8, supplemented with 167 mm Glc, 3 mm IAA, and 0.3 mm kinetin. The oligogalacturonides to be assayed were sterilized by filtration through 0.2-μm nylon membrane syringe filters (Nalgene, Rochester, NY). The TCL explants were incubated at 24°C under continuous, cool-white fluorescent light. The overall TCL explant morphology and the number of flowers formed on each TCL explant after 23 or 24 d of culture were determined by examination with a dissecting microscope.
Maintenance of Suspension-Cultured Tobacco Cells
Suspension-cultured N. tabacum cv Samsun cells, initiated from pith parenchyma callus, were grown in Linsmaier and Skoog (1965) medium, pH 6.0, supplemented with 4.5 μm 2,4-D and 3% Suc. The cells were cultured under continuous light at 26°C on a rotary shaker at 110 rpm. The PCV obtained after centrifugation (1000g for 5 min) was used as a measure of growth. An aliquot of the cell suspension giving an initial PCV of 2.7% was transferred to 100 mL of fresh medium every 7 d. The PCV after 7 d of culture was between 26 and 33%. The culture of the N. tabacum cv Samsun cells and the alkalinization bioassays using these cells were carried out at the Complex Carbohydrate Research Center (Athens, GA).
Suspension-cultured N. tabacum cv Xanthi cells were grown in Gamborg B-5 medium, pH 5.7, supplemented with 1 μm 2,4-D, 40 nm kinetin, and 2% Suc. The cells were grown under continuous light on a rotary shaker at 160 rpm and were subcultured every 7 d by transferring 10 mL of the culture into 100 mL of fresh medium. The PCV after 7 d of culture was between 18 and 22%. The culture of the N. tabacum cv Xanthi cells and the alkalinization and H2O2 accumulation bioassays using these cells were carried out at the Centre National de la Recherche Scientifique laboratories (Gif-sur-Yvette, France).
Analysis of Oligogalacturonide-Induced Extracellular Alkalinization by Suspension-Cultured Tobacco Cells
Aliquots of 7-d-old exponentially growing N. tabacum cv Samsun suspension-cultured cells (4 mL) in 25-mL Erlenmeyer flasks were equilibrated for 2 h on a rotary shaker (110 rpm). The pH after this period was between 4.4 and 4.6. The pH of the oligogalacturonide solutions (50 μL in water) was adjusted to that of the equilibrated cells. The addition of oligogalacturonides to each successive flask was staggered by 60 s so that pH measurements could be made at fixed times after sample addition. The extracellular pH was measured immediately upon addition of the oligogalacturonides and subsequently at 14-min intervals through at least 56 min using a Micro-Combination pH electrode (Microelectrodes, Inc., Londonderry, NH) connected to an Accumet 950 pH meter (Fisher Scientific).
The analysis of oligogalacturonide-induced extracellular alkalinization by cv Xanthi cells was carried out in the same manner as for the cv Samsun cells with the following exceptions. Aliquots (3 mL) of the cell suspensions were placed in 12-mL glass vials, adjusted to 1 mm Mes-Tris (pH 5.2), and equilibrated for 2 h, at which time the pH was between 5.1 and 5.5. The pH was measured using a BRV4H glass electrode (Heito, Paris, France) connected to a PSD 11 pH meter (Heito).
Analysis of Oligogalacturonide-Induced Oxidative Burst
The accumulation of H2O2 in the growth medium of suspension-cultured cv Xanthi cells was determined in the same experiments as the measurement of extracellular alkalinization. Aliquots (10–40 μL) of the growth medium were removed at intervals and added to a series of cuvettes containing 8 μm scopoletin (7-OH-6-methoxycoumarin) and 5 μg of horseradish peroxidase (P 6782, Sigma) in 2 mL of 50 mm Mes-Tris, pH 5.2. The amount of H2O2 present in the culture medium was determined by the decrease in fluorescence of scopoletin and compared with the effect of a standard concentration series of H2O2 on scopoletin fluorescence (Root et al., 1975). Fluorescence measurements were made with a spectrofluorimeter (Photon Technology International, South Brunswick, NJ) using an excitation wavelength of 350 nm and an emission wavelength of 460 nm.
RESULTS
Reducing-End-Derivatized G13 at Submicromolar Concentrations Alter the Morphogenesis of N. tabacum cv Samsun TCL Explants
Oligogalacturonides induce flower formation, inhibit root formation, and reproducibly alter the overall morphology of tobacco TCL explants cultured on indolebutyric acid and kinetin-containing medium (Eberhard et al., 1989; Marfà et al., 1991; Bellincampi et al., 1993). Oligogalacturonides of DP 12 to 14, which have approximately equal activity, induce the alteration of TCL explant morphogenesis at submicromolar concentrations, whereas higher concentrations of shorter and longer oligomers are required to induce an equivalent response. The mechanism by which oligogalacturonides initiate these responses is unknown.
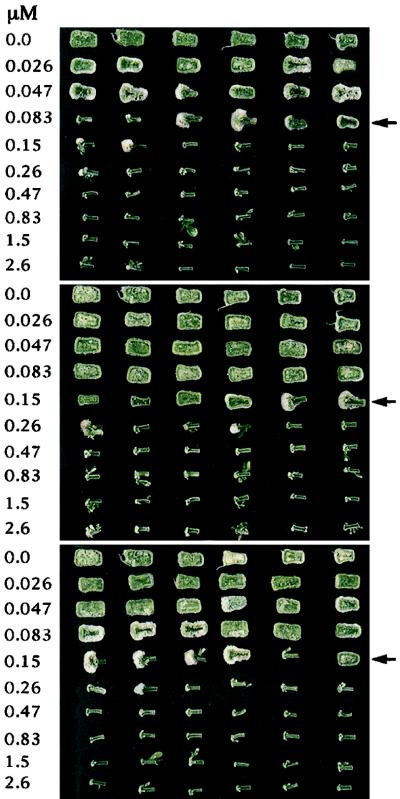
We determined the effect of derivatized and underivatized oligogalacturonides on TCL explants cultured in transition medium containing 3 μm IAA and 0.3 μm kinetin. A transition medium containing IAA rather than indolebutyric acid (Mohnen et al., 1990) was used, since the addition of oligogalacturonides to TCL explants cultured on IAA-containing transition medium causes a distinct concentration-dependent change in the overall morphology of the TCL explants. TCL explants, in the absence of added oligogalacturonides or at low concentrations of bioactive oligogalacturonides, grow more or less uniformly over their entire length, resulting in rectangular explants. In the presence of higher concentrations of bioactive oligogalacturonides, the TCL explants were significantly inhibited in their growth and had limited tissue enlargement only at one end, yielding mushroom-shaped explants (Fig. 2). In the absence of oligogalacturonides, the rectangular explants produced only roots, whereas they produced roots and occasionally flowers when exposed to low concentrations of bioactive oligogalacturonides. The mushroom-shaped explants that resulted from exposure to higher concentrations of bioactive oligogalacturonides frequently formed flowers but never roots.
Figure 2.
The TCL explants from a representative tobacco morphogenesis bioassay comparing the activities of G12 (top), G13-B (middle), and G13-T (bottom). The explants were incubated in growth medium containing the indicated oligogalacturonide concentrations, with six replicate TCL explants per concentration. The arrows indicate the concentrations at which oligogalacturonides induce a distinct change in the overall morphology of the TCL explants (the morphogenetic switch concentrations).
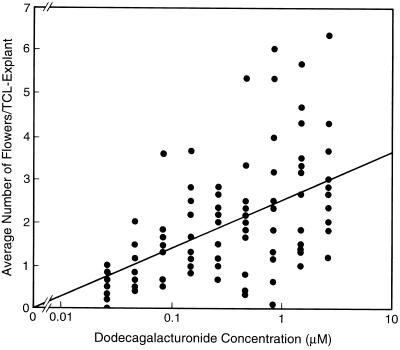
The ability of each oligogalacturonide to alter TCL-explant morphogenesis was tested at nine concentrations and compared with a similar concentration series of G12. Each oligogalacturonide was assayed in two independent experiments using six replicate TCL explants at each concentration. G12, G13-T, G13-B, and G13-R each induced a concentration-dependent increase in the number of flowers formed on TCL explants. The maximum of the average number of flowers induced by G12 (1.5 μm, 2.8 flowers/explant) was nearly identical to the maximum of the average number of flowers induced by the three reducing-end derivatives (2.6 μm, 3.1 flowers/explant). However, as shown in Figure 3 for G12, the number of flowers induced on TCL explants by a given oligogalacturonide concentration varied considerably, and thus the flowering data were not useful for a quantitative comparison of the biological activities of the various oligogalacturonides.
Figure 3.
Plot of the log of G12 concentration versus the average number of flowers induced per TCL explant. The data from 12 experiments are shown, and each data point represents the average number of flowers produced by the six replicate TCL explants used for each treatment in a given experiment. In the absence of G12, no flowers were formed. The line represents the dose-response curve for G12 as determined by least-squares analysis using log concentration.
However, the oligogalacturonide concentration at which the overall morphology of the TCL explants changes from rectangular to mushroom-shaped (the morphogenetic switch concentration) could be used to quantify the activities of the oligogalacturonides since it reproducibly fell within a less-than 4-fold concentration range (Fig. 2). The average morphogenetic switch concentration of G12 in 12 experiments was 0.058 ± 0.033 μm (Table I). Compared with G12, the morphogenesis switch concentration for G13-T was 2.5-fold higher; G13-B was 3.4-fold higher, and G13-R was 6.3-fold higher. The difference between the activity of G12 and each G13 derivative was statistically significant (Wilcoxon's Rank-Sum test, confidence level = 98.5%). There was no discernible difference in the activity of G12 when assayed in the presence of excess free biotin or free tyramine. These results indicate that derivatization of the reducing end of G13 results in a reduction in its ability to alter TCL explant morphogenesis.
Table I.
Comparison of the ability of reducing-end-derivatized and underivatized oligogalacturonides to alter TCL explant morphogenesis
| Oligogalacturonide | Morphogenetic Switch Concentration | Derivatized Oligogalacturonide | Morphogenetic Switch Concentration |
|---|---|---|---|
| μm | μm | ||
| G16 | 0.26 | G16-B | 0.65 |
| G15 | 0.15 | G13-B | 0.21 |
| G12 | 0.058 | G13-T | 0.15 |
| G10 | 0.83 | G13-R | 0.37 |
| G9 | 2.6 | G10-B | 1.7 |
| G7 | >2.6 | G8-B | >2.6 |
The concentration of each oligogalacturonide that induces a distinct change in the overall TCL explant morphology is reported as the morphogenetic switch concentration (as defined in the text). The values given for each oligogalacturonide are the averages of two independent bioassays, with the exception of the value for G12, which is the average of 12 independent experiments (sd = 0.033 μm).
We compared the morphogenetic switch concentration of various homogeneously sized biotinylated and underivatized oligogalacturonides to determine whether reducing-end derivatization alters the size requirement for activity. G16-B and G10-B were both reduced in activity compared with their corresponding underivatized oligogalacturonides, and the highest concentration of G8-B tested (2.6 μm) did not induce an alteration in the overall explant morphology (Table I). G13-B was significantly more active than longer or shorter biotinylated oligogalacturonides, which is consistent with the size requirements found using underivatized oligogalacturonides in this bioassay (Marfà et al., 1991; Bellincampi et al., 1993).
All Four Derivatives of G13 Were More Than 30-Fold Reduced in Their Ability to Induce Extracellular Alkalinization by Suspension-Cultured cv Samsun Cells
Transient membrane depolarization accompanied by rapid K+ ion efflux and extracellular alkalinization was induced when suspension-cultured tobacco or carrot cells were exposed to micromolar concentrations of oligogalacturonides having DPs of 10 to 15 (Mathieu et al., 1991; Messiaen and Van Cutsem, 1994). These rapid responses have been hypothesized to act as signaling events leading to delayed responses, such as the alteration of TCL-explant morphogenesis (Mathieu et al., 1991; Messiaen and Van Cutsem, 1994). We have characterized the ability of various sizes of reducing-end-derivatized and underivatized oligogalacturonides to induce extracellular alkalinization in cv Samsun suspension cultures, the same cultivar used for the tobacco morphogenesis bioassay.
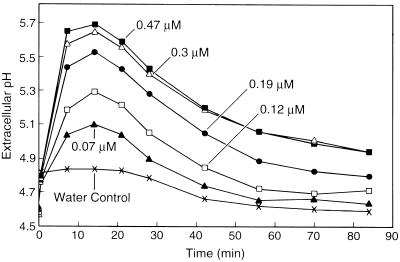
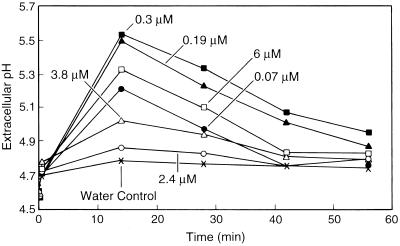
The ability of each oligogalacturonide to induce extracellular alkalinization by cv Samsun cells was assayed using seven concentrations of oligogalacturonides in at least two independent experiments. A standard concentration series of G12 (Fig. 4) was assayed in each experiment. Each bioactive oligogalacturonide elicited a rapid, concentration-dependent, and saturable extracellular alkalinization response that exhibited three characteristic phases: a period of rapid alkalinization beginning 30 s after oligogalacturonide addition, a plateau period during which the pH remained near its maximum level, and a period of gradual reacidification during which the pH of the medium returned to approximately its original value.
Figure 4.
Representative time courses of the extracellular alkalinization by suspension-cultured cv Samsun cells treated with the indicated concentrations of G12 at time 0.
The alkalinization response measured 14 min after oligogalacturonide addition was used to compare the activities of the derivatized and underivatized oligogalacturonides, since this time consistently fell within the period of maximum alkalinization. The change in pH was converted to the R to obtain a linear measure of the response. Rmax was always found to be essentially the same between replicates within the same experiment; however, Rmax varied significantly between experiments. The alkalinization response elicited by saturating concentrations of each bioactive oligogalacturonide within an experiment was essentially the same between replicates within the same experiment; however, Rmax varied significantly between experiments. To correct for variability of the responses between experiments, the response elicited by each sample was expressed as the fraction of the Rmax for that experiment (R/Rmax).
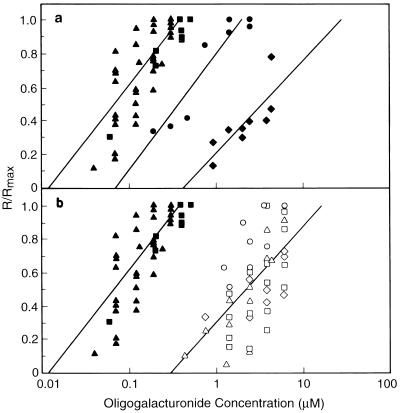
The R/Rmax values were used to generate dose-response curves (Fig. 5) and the EC50 were determined from these curves. G12 and G13 were the most active of the compounds tested (Fig. 5a) and the difference between their activities was not statistically significant (paired t test, level of confidence = 95%); therefore, the data from G12 and G13 were pooled to generate a single dose-response curve (EC50 = 0.068 μm). G15 was approximately 5-fold less active (EC50 = 0.36 μm) and G9 was 50-fold less active (EC50 = 3.4 μm). G3 was inactive over the entire range of concentrations tested (2–45 μm). These results establish that the size requirements for oligogalacturonides to elicit extracellular alkalinization by cv Samsun cells are similar to that required for alteration of TCL-explant morphogenesis (Marfà et al., 1991; Bellincampi et al., 1993).
Figure 5.
Effect of the DP (a) and reducing-end derivatization (b) of oligogalacturonides on their ability to induce extracellular alkalinization by suspension-cultured cv Samsun cells. The data were plotted as the alkalinization response of each sample divided by the alkalinization response induced by a saturating concentration of G12 in that experiment (R/Rmax). The R/Rmax values for the various oligogalacturonides are indicated as follows: G12, ▴; G13, ▪; G15, •; G9, ♦; G13-T, ⋄; G13-B, □; G13-O, ○; and G13-R, ▵. The data for G12 and G13 are shown on both plots to aid in comparison. The lines represent the linear portion of the dose-response curves as determined by least-squares analysis using log concentration. Separate dose-response curves were generated using combined data from G12 and G13 and combined data from the four reducing-end derivatives of G13 (see text).
Each of the reducing-end derivatives of G13 elicited a concentration-dependent and saturable extracellular alkalinization by cv Samsun cells with the same kinetics as G12 and G13 (Fig. 6, e.g. G13-T), although they were all approximately 30-fold less active (Fig. 5b). There was no statistical difference (paired t test, level of confidence = 95%) between the activities of the four G13 derivatives in this bioassay. When the data for all four derivatives were used to generate a single dose-response curve, an EC50 of 2.2 μm was obtained, which was approximately 32-fold higher than that of G12 and G13 (Fig. 5b). The reduction in the bioactivity of the reducing-end-derivatized oligogalacturonides was at least 6 times greater in this bioassay than for any of the G13 derivatives in the tobacco morphogenesis bioassay. G10-B has an EC50 of 5 μm, and G16-B did not induce a measurable response at 2.4 μm, the highest concentration tested. Thus, G13-B is more active than the shorter or longer biotinylated oligogalacturonides, which is in accord with the size requirement for the underivatized oligogalacturonides in this bioassay.
Figure 6.
Representative time courses for extracellular alkalinization by cv Samsun cells induced by the indicated concentrations of G12 (closed symbols) or G13-T (open symbols). More than a 30-fold higher concentration of G13-T, compared with G12, is required to induce an equivalent alkalinization response.
The Ability of the Reducing-End-Derivatized Oligogalacturonides to Induce Extracellular Alkalinization by cv Xanthi Cells Differs Depending on the Particular Reducing-End Modification
The size requirement for oligogalacturonides to elicit extracellular alkalinization by cv Samsun cells is similar to that described for cv Xanthi cells (Mathieu et al., 1991). However, the EC50 for G12 was approximately 15 times higher for the cv Xanthi cells than for the cv Samsun cells. Furthermore, with the cv Xanthi cells, the oligogalacturonide-induced alkalinization and subsequent reacidification occurred at about half the rate attained with cv Samsun cells. These differences in the alkalinization response between the two bioassays may have been due to differences in the cell culture conditions but were not attributable to the bioassay conditions, since the differences in sensitivity and kinetics remained when the two cell lines were assayed using the same conditions (data not shown).
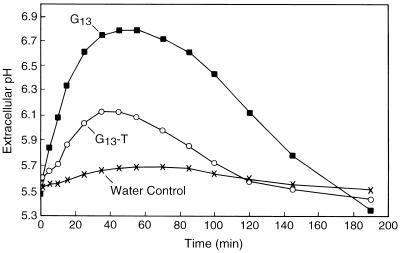
We measured the ability of G13-R and G13-T to elicit extracellular alkalinization by suspension-cultured cv Xanthi cells to compare the structural requirements for activity between the two alkalinization bioassays. Both G13 derivatives elicited a concentration-dependent alkalinization response, with kinetics similar to those elicited by underivatized G13 (Fig. 7, e.g. G13-T). G13-R induced a saturating response, whereas G13-T did not, even at the highest concentration tested (8.7 μm). The same method used to determine EC50s in the cv Samsun alkalinization bioassay was used for this bioassay. G13 was 1.6-fold more active than G13-R and 8.7-fold more active than G13-T, as determined by comparison of their EC50 values (Table II). Both of these differences are statistically significant (paired t test, level of confidence = 99.5%). Thus, the bioactivities of the two G13 derivatives tested in the cv Xanthi alkalinization bioassay depend on the specific reducing-end modification, whereas each of the G13 derivatives have approximately equal activity in the cv Samsun alkalinization bioassay.
Figure 7.
Representative time courses of extracellular alkalinization by cv Xanthi cells elicited by G13 and of G13-T. All treatments were at 8.7 μm.
Table II.
Comparison of the abilities of G13, G13-R, and G13-T to elicit extracellular alkalinization and H2O2 accumulation by suspension-cultured cv Xanthi cells
| Compound | EC50
|
|
|---|---|---|
| Extracellular alkalinization | H2O2 accumulation | |
| toCTR | μm | |
| G13 | 1.0 | 1.4 |
| G13-R | 1.6 | 1.9 |
| G13-T | 8.7a | >8.7 |
Each of the oligogalacturonides was tested at five concentrations and the data were used to calculate the EC50 values.
The EC50 is determined based on the maximum response obtained with G13.
The Structural Requirements of the Reducing-End-Derivatized Oligogalacturonides for Induction of H2O2 Accumulation and for Induction of Extracellular Alkalinization using cv Xanthi Cells Are Approximately the Same
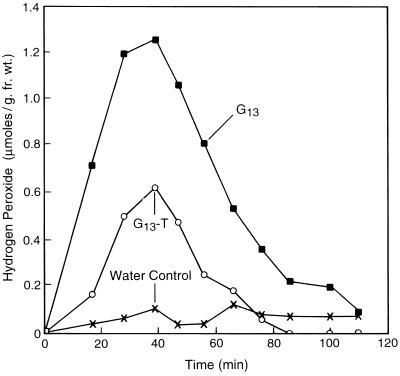
In the experiments with cv Xanthi described above, in which we measured extracellular alkalinization, we also measured the ability of G13, G13-R, and G13-T to induce the accumulation of extracellular H2O2. H2O2 accumulation is of interest since it is a rapid signaling event involved in the initiation of defense responses (Tenhaken et al., 1995). G13 and G13-R induced a rapid, concentration-dependent, and saturable accumulation of extracellular H2O2 by cv Xanthi cells. In contrast, G13-T induced a weak response (Fig. 8); only 20% of the maximum response induced by G13 was induced at the highest concentration of G13-T tested (8.7 μm). The kinetics of H2O2 accumulation and extracellular alkalinization were at first very similar. However, the H2O2 concentration returned to its original level in about half the time required for the alkalinization response to return to normal (compare Figs. 7 and 8). Approximately 1.5-fold higher concentrations were required for half-maximum induction of H2O2 accumulation than for half-maximum induction of extracellular alkalinization in the cv Xanthi suspension-cultured system. The H2O2 induction activity of both derivatives, relative to G13, was similar to their activity in the extracellular alkalinization bioassays (Table II). G13 was 1.3-fold more active than G13-R and in excess of 6-fold more active than G13-T in the H2O2 induction assay. These results indicate that, in the cv Xanthi tissue culture system, the structural requirements for activity of the reducing-end-derivatized oligogalacturonides are essentially the same for induction of H2O2 accumulation and extracellular alkalinization.
Figure 8.
Representative time courses of H2O2 accumulation in the growth medium of cv Xanthi cells elicited by the addition of G13 and G13-T. These measurements were carried out simultaneously using the same suspension cells as used for the measurement of extracellular alkalinization (Fig. 7). All treatments were at 8.7 μm.
DISCUSSION
Derivatization or modification of the reducing end of oligogalacturonides significantly reduced their biological activity in all four tobacco tissue culture bioassays studied. However, the degree of reduction in activity depends on the specific modification as well as the tissue-culture system used. Each of the three tissue-culture systems we investigated exhibits a unique capacity to respond to the different reducing-end-derivatized oligogalacturonides, suggesting that there is diversity in the way oligogalacturonides are perceived by these systems. For example, the biological activities of the reducing-end derivatives of G13 relative to the activities of the corresponding underivatized oligogalacturonides (relative activities) of each derivative tested in the cv Samsun tobacco morphogenesis bioassay (G13-T, 38%; G13-B, 29%; G13-R, 16%) are different from their relative activities in the cv Samsun extracellular alkalinization bioassay, where all four derivatives have approximately the same relative activity (3%). On the other hand, the relative activities of the derivatives tested in the cv Xanthi extracellular alkalinization bioassay (G13-R, 63%; G13-T, 12%) are similar to their relative activities in the cv Xanthi H2O2 accumulation bioassay (G13-R, 75%; G13-T, <17%). The similarity of the results with these two bioassays suggests that oligogalacturonide induction of extracellular alkalinization and H2O2 accumulation use signal- transduction pathways with common elements.
It is clear from our results that the reducing-end C-1 of the oligogalacturonides is a key recognition element in the bioassays tested. In a previous study, the reducing-end C-1 of di- and trigalacturonides was shown to be required for induction of proteinase inhibitors in tomato plants (Moloshok et al., 1992). Induction of tomato proteinase inhibitors likely involves a different receptor than the responses we have investigated here, since the proteinase inhibitors are induced by oligogalacturonides as small as DP 2 (Bishop et al., 1984). Derivatization of the reducing end of glucan or chitin oligosaccharins does not affect their biological activity regardless of the modification used (Sharp et al., 1984a, 1984b; Cheong et al., 1991; Shibuya et al., 1993; Baureithel et al., 1994). However, the nature of the modification of the reducing end of a chitosan-derived octasaccharide does effect its ability to induce phytoalexin accumulation in pea; the methyl glycoside derivative retains full activity and the methoxyphenyl glycoside derivative is inactive (Hadwiger et al., 1994). We have shown that the specific reducing-end modification differentially reduces the biological activity of oligogalacturonide derivatives in the tobacco morphogenesis bioassay and in both of the cv Xanthi suspension-culture bioassays, whereas the specific modification reduces the activity of the derivatives to an equal extent in the cv Samsun extracellular alkalinization bioassay.
The nonreducing end of oligogalacturonides can also play a role in the recognition of biologically active oligogalacturonides by plant cells. Pectate-lyase-catalyzed introduction of a 4,5-unsaturated bond in the nonreducing terminal residue of oligogalacturonides and lowered the most active size from DP 12 to 10 (Davis et al., 1986).
Radiolabeled reducing-end derivatives of an elicitor-active hepta-β-glucoside from Phytophthora sojae and of elicitor-active chitin-derived oligosaccharides have been used to identify high-affinity, specific binding sites in plant membrane fractions (Cosio et al., 1990; Cheong and Hahn, 1991; Shibuya et al., 1993; Baureithel et al., 1994). Each of these derivatives elicits biological responses (EC50 of ≤ 10 nm) that are indistinguishable from those elicited by their corresponding underivatized oligosaccharins, indicating that these derivatives bind to their physiological receptors with high affinity. Furthermore, the EC50 of these derivatives and a series of elicitor analogs are closely correlated with the concentration of each compound required for half-maximum binding to membrane proteins in vitro, suggesting that the binding proteins identified are the physiological receptors (Cheong et al., 1991).
We have characterized the bioactivities of G13-T and G13-B to assess their usefulness for the identification of oligogalacturonide receptors in the tobacco tissue-culture system. The G13 derivatives elicit responses that are qualitatively similar to those elicited by the underivatized oligogalacturonides, suggesting that in each system the derivatized oligogalacturonides bind to the physiologically relevant oligogalacturonide receptor. However, the EC50 of the labeled G13 derivatives are higher than those of the underivatized oligogalacturonides, suggesting that the derivatives have lower affinity for the receptors.
Suspension-cultured cells have been useful for identifying oligosaccharin-binding proteins because oligosaccharins elicit rapid, easily measured responses in suspension-cultured cells, and suspension-cultured cells provide a large amount of homogeneous tissue for the isolation of membranes (Cosio et al., 1990; Shibuya et al., 1993; Baureithel et al., 1994). However, the EC50s for the labeled G13 derivatives in our suspension-culture bioassays are 200- to 900-fold higher than the EC50 of the labeled oligosaccharin derivatives that have been used to identify binding proteins in other systems (Cosio et al., 1990; Cheong and Hahn, 1991; Shibuya et al., 1993; Baureithel et al., 1994). The high concentrations of G13-T and G13-B that would be required to identify binding sites in the suspension-culture systems could result in a high degree of nonspecific binding, which could be compounded by the polyanionic nature of the oligogalacturonides. This is likely to make it difficult to detect the low-abundance, specific, saturable binding that is characteristic of a receptor protein.
The labeled G13 derivatives have much lower EC50 in the tobacco morphogenesis bioassay (150–200 nm) than in the suspension culture bioassays (1.9 to >8.7 μm). The oligogalacturonide concentrations required in the tobacco morphogenesis bioassay are approximately 10-fold higher than the EC50 of the labeled oligosaccharin derivatives that have been successfully used to identify binding proteins (Cosio et al., 1990; Cheong and Hahn, 1991; Shibuya et al., 1993; Baureithel et al., 1994). Thus, the labeled G13 derivatives could be used to identify oligogalacturonide-binding proteins in membranes from TCL explants, although it would be challenging to obtain sufficient tissue from such small explants (approximately 10 mg each). The labeled derivatives could also be used to identify clones encoding oligogalacturonide-binding proteins from a cDNA expression library constructed with TCL-explant mRNA. Such an approach has been used to identify membrane-bound receptors (Marullo et al., 1989; Nakayama et al., 1992).
The differences in the way the three tissue culture systems respond to the reducing-end-derivatized oligogalacturonides could be due to different receptors. It is also possible that the reducing-end-derivatized oligogalacturonides are differentially degraded or sequestered in the different tissue culture systems. We have not directly tested this possibility; however, we have found that suspension-cultured tobacco cells are able to rapidly sequester a portion of reducing-end-reduced oligogalacturonides and to cleave the remaining reducing-end-labeled oligogalacturonide (Y. Mathieu, J. Guern, M.D. Spiro, M.A. O'Neill, K. Kates, A.G. Darvill and P. Albersheim, unpublished data). Alternatively, differences in pH, ionic strength, or ionic composition of the media used in the tissue-culture systems may affect the binding of oligogalacturonides to their receptors either directly or indirectly by altering the conformation of the oligogalacturonides.
Oligogalacturonides of DP ≥ 10 have been hypothesized to form a multimeric “egg-box” conformation through cooperative intermolecular binding of Ca2+ ions (Kohn, 1975, 1987; Powell et al., 1982). Monoclonal antibodies have been identified that are believed to recognize this Ca2+-requiring egg-box conformation. These antibodies can bind to oligogalacturonides of DP ≥ 9 only in the presence of millimolar Ca2+ (Liners et al., 1992). It is possible that oligogalacturonide receptors may specifically recognize the egg-box conformation in a manner similar to these antibodies. Indirect evidence has been presented that the lower limit of the size requirement for oligogalacturonides to elicit most responses is due to the inability of oligomers shorter than DP 9 to form the egg box. For example, induction of ion flux in carrot and tobacco cells and phytoalexin accumulation in carrot cells requires a minimum DP of 9 or 10 and the presence of millimolar Ca2+ (Mathieu et al., 1991; Messiaen et al., 1993; Messiaen and Van Cutsem, 1994). It is possible that the reduction in biological activity of reducing-end-derivatized oligogalacturonides reported in this paper is due to a shift in the equilibrium of the oligogalacturonides away from a physiologically active conformation, such as the egg box.
Abbreviations:
- DP
degree of polymerization
- EC50
oligogalacturonide concentration that includes a half-maximal response
- G12
dodecagalacturonide
- G13
tridecagalacturonide
- G13-B
biotinylated tridecagalacturonide
- G13-O
oxidized tridecagalacturonide (dodecagalacturonide-d-galactaric acid)
- G13-R
reduced tridecagalacturonide (dodecagalacturonide-l-galactonic acid)
- G13-T
tyraminated tridecagalacturonide
- HPAEC-PAD
high performance anion-exchange chromatography with pulsed amperometric detection
- PCV
packed cell volume
- R
change in free proton concentration
- Rmax
change in free proton concentration elicited by a saturating concentration of G12 or G13
- TCL
thin cell layer
Footnotes
This research was supported in part by the U.S. Department of Energy (DOE)-funded Center for Plant and Microbial Complex Carbohydrates (grant no. DE-FG05-93-ER20097) and by DOE grant no. DE-FG02-96ER20221 (to P.A.).
LITERATURE CITED
- Apostol I, Heinstein PF, Low PS. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Role in defense and signal transduction. Plant Physiol. 1989;90:109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basse CW, Fath A, Boller T. High affinity binding of a glycopeptide elicitor to tomato cells and microsomal membranes and displacement by specific glycan suppressors. J Biol Chem. 1993;268:14724–14731. [PubMed] [Google Scholar]
- Baureithel K, Felix G, Boller T. Specific, high affinity binding of chitin fragments to tomato cells and membranes. Competitive inhibition of binding by derivatives of chitin fragments and a nod factor of Rhizobium. J Biol Chem. 1994;269:17931–17938. [PubMed] [Google Scholar]
- Bellincampi D, Salvi G, De Lorenzo G, Cervone F, Marfà V, Eberhard S, Darvill A, Albersheim P. Oligogalacturonides inhibit the formation of roots on tobacco explants. Plant J. 1993;4:207–213. [Google Scholar]
- Bishop PD, Pearce G, Bryant JE, Ryan CA. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984;259:13172–13177. [PubMed] [Google Scholar]
- Branca C, De Lorenzo G, Cervone F. Competitive inhibition of the auxin induced elongation by α-d-oligogalacturonides in pea stem segments. Physiol Plant. 1988;72:499–504. [Google Scholar]
- Brecht JK, Huber DJ. Products released from enzymatically active cell wall stimulate ethylene production and ripening in preclimacteric tomato (Lycopersicon esculentum Mill.) fruit. Plant Physiol. 1988;88:1037–1041. doi: 10.1104/pp.88.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J-J, Birberg W, Fügedi P, Pilotti Å, Garegg PJ, Hong N, Ogawa T, Hahn MG. Structure-activity relationships of oligo-β-glucoside elicitors of phytoalexin accumulation in soybean. Plant Cell. 1991;3:127–136. doi: 10.1105/tpc.3.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J-J, Hahn MG. A specific, high-affinity binding site for the hepta-β-glucoside elicitor exists in soybean membranes. Plant Cell. 1991;3:137–147. doi: 10.1105/tpc.3.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio EG, Frey T, Verduyn R, Van Boom J, Ebel J. High-affinity binding of a synthetic heptaglucoside and fungal glucan phytoalexin elicitors to soybean membranes. FEBS Lett. 1990;271:223–226. doi: 10.1016/0014-5793(90)80411-b. [DOI] [PubMed] [Google Scholar]
- Côté F, Hahn MG. Oligosaccharins: structures and signal transduction. Plant Mol Biol. 1994;26:1375–1411. doi: 10.1007/BF00016481. [DOI] [PubMed] [Google Scholar]
- Bergmann ACC, Carlson RW, Cheong J-J, Eberhard S, Hahn MG, Darvill L, Augur V-M, Marfà V, Meyer B and others. Oligosaccharins-oligosaccharides that regulate growth, development and defense responses in plants. Glycobiology. 1992;2:181–198. doi: 10.1093/glycob/2.3.181. [DOI] [PubMed] [Google Scholar]
- Davis KR, Darvill AG, Albersheim P, Dell A. Host-pathogen interactions. XXX. Characterization of elicitors of phytoalexin accumulation in soybean released from soybean cell walls by endopolygalacturonic acid lyase. Z Naturforsch. 1986;41c:39–48. [Google Scholar]
- Eberhard S, Doubrava N, Marfà V, Mohnen D, Southwick A, Darvill A, Albersheim P. Pectic cell wall fragments regulate tobacco thin-cell-layer explant morphogenesis. Plant Cell. 1989;1:747–755. doi: 10.1105/tpc.1.8.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Moloshok TD, Saxton MJ, Ryan CA. In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci USA. 1989;86:1539–1542. doi: 10.1073/pnas.86.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MG, Darvill AG, Albersheim P. Host-pathogen interactions. XIX. The endogenous elicitor, a fragment of a plant cell wall polysaccharide that elicits phytoalexin accumulation in soybeans. Plant Physiol. 1981;68:1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger LA, Ogawa T, Kuyama H. Chitosan polymer sizes effective in inducing phytoalexin accumulation and fungal suppression are verified with synthetic oligomers. Mol Plant-Microbe Interact. 1994;7:531–553. doi: 10.1094/mpmi-7-0531. [DOI] [PubMed] [Google Scholar]
- Kohn R. Ion binding on polyuronates-alginate and pectin. Pure Appl Chem. 1975;42:371–397. [Google Scholar]
- Kohn R. Binding of divalent cations to oligomeric fragments of pectin. Carbohydr Res. 1987;160:343–353. [Google Scholar]
- Liners F, Thibault J-F, Van Cutsem P. Influence of the degree of polymerization of oligogalacturonates and of esterification pattern of pectin on their recognition by monoclonal antibodies. Physiol Plant. 1992;99:1099–1104. doi: 10.1104/pp.99.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsmaier EM, Skoog F. Organic growth factor requirement of tobacco tissue cultures. Physiol Plant. 1965;18:100–127. [Google Scholar]
- Marfà V, Gollin DJ, Eberhard S, Mohnen D, Darvill A, Albersheim P. Oligogalacturonides are able to induce flowers to form on tobacco explants. Plant J. 1991;1:217–225. [Google Scholar]
- Marullo S, Delavier-Klutchko C, Guillet JG, Charbit A, Strosberg AD, Emorine LJ. Expression of human β1 and β2 adrenergic receptors in E. coli as a new tool for ligand screening. Biotechnology. 1989;7:923–927. [Google Scholar]
- Mathieu Y, Kurkdijan A, Xia H, Guern J, Koller A, Spiro M, O'Neill M, Albersheim P, Darvill A. Membrane responses induced by oligogalacturonides in suspension-cultured tobacco cells. Plant J. 1991;1:333–343. doi: 10.1046/j.1365-313X.1991.t01-10-00999.x. [DOI] [PubMed] [Google Scholar]
- Messiaen J, Read ND, Van Cutsem P, Trewavas AJ. Cell wall oligogalacturonides increase cytosolic free calcium in carrot protoplasts. J Cell Sci. 1993;104:365–371. [Google Scholar]
- Messiaen J, Van Cutsem P. Pectic signal transduction in carrot cells: membrane, cytosolic and nuclear responses induced by oligogalacturonides. Plant Cell Physiol. 1994;35:677–689. [Google Scholar]
- Mohnen D, Eberhard S, Marfà V, Doubrava N, Toubart P, Gollin DJ, Gruber TA, Nuri W, Albersheim P, Darvill A. The control of root, vegetative shoot and flower morphogenesis in tobacco thin cell-layer explants (TCLs) Development. 1990;108:191–201. doi: 10.1242/dev.108.1.191. [DOI] [PubMed] [Google Scholar]
- Moloshok T, Pearce G, Ryan CA. Oligouronide signaling of proteinase inhibitor genes in plants: structure-activity relationships of di and trigalacturonic acids and their derivatives. Arch Biochem Biophys. 1992;294:731–734. doi: 10.1016/0003-9861(92)90748-l. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Yakota T, Arai K. Use of mammalian cell expression cloning systems to identify genes for cytokines, receptors, and regulatory proteins. Curr Opin Biotechnol. 1992;3:497–505. doi: 10.1016/0958-1669(92)90077-v. [DOI] [PubMed] [Google Scholar]
- Powell DA, Morris ER, Gidley MJ, Rees DA. Conformations and interactions of pectins. II. Influence of residue sequence on chain association in calcium pectate gels. J Mol Biol. 1982;155:517–531. doi: 10.1016/0022-2836(82)90485-5. [DOI] [PubMed] [Google Scholar]
- Pressey R. Uronic acid oxidase in orange fruit and other plant tissues. Phytochemistry. 1993;32:1375–1379. [Google Scholar]
- Ridley BL, Spiro MD, Glushka J, Albersheim P, Darvill AG, Mohnen D. A method for biotin labeling of biologically active oligogalacturonides using a chemically stable hydrazide linkage. Anal Biochem. 1997;249:10–19. doi: 10.1006/abio.1997.2165. [DOI] [PubMed] [Google Scholar]
- Robertsen B. Elicitors of the production of lignin-like compounds in cucumber hypocotyls. Physiol Mol Plant Pathol. 1986;28:137–148. [Google Scholar]
- Root RK, Metcalf J, Oshino N, Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975;55:945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA, Farmer EE. Oligosaccharide signals in plants: a current assessment. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:651–674. [Google Scholar]
- Sharp JK, McNeil M, Albersheim P. The primary structures of one elicitor-active and seven elicitor-inactive hexa(β-d-glucopyranosyl)-d-glucitols isolated from the mycelial walls of Phytophthora megasperma f. sp. glycinea. J Biol Chem. 1984a;259:11321–11336. [PubMed] [Google Scholar]
- Sharp JK, Valent B, Albersheim P. Purification and partial characterization of a β-glucan fragment that elicits phytoalexin accumulation in soybean. J Biol Chem. 1984b;259:11312–11320. [PubMed] [Google Scholar]
- Shibuya N, Kaku H, Kuchitsu K, Maliarik MJ. Identification of a novel high-affinity binding site for N-acetylchitooligosaccharide elicitor in the membrane fraction from suspension-cultured rice cells. FEBS Lett. 1993;329:75–78. doi: 10.1016/0014-5793(93)80197-3. [DOI] [PubMed] [Google Scholar]
- Spiro MD, Kates KA, Koller AL, O'Neill MA, Albersheim P, Darvill AG. Purification and characterization of biologically active (1→4)-linked α-d-oligogalacturonides after partial digestion of polygalacturonic acid with endopolygalacturonase. Carbohydr Res. 1993;247:9–20. [Google Scholar]
- Spiro MD, Ridley BL, Glushka J, Darvill AG, Albersheim P. Synthesis and characterization of tyramine-derivatized (1→4)-linked α-d-oligogalacturonides. Carbohydr Res. 1996;290:147–157. doi: 10.1016/0008-6215(96)00075-4. [DOI] [PubMed] [Google Scholar]
- Tenhaken R, Levine A, Brisson LF, Dixon RA, Lamb C. Function of the oxidative burst in hypersensitive disease resistance. Proc Natl Acad Sci USA. 1995;92:4158–4163. doi: 10.1073/pnas.92.10.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]