Abstract
Background and Aims
The pollination biology of very few Chloraeinae orchids has been studied to date, and most of these studies have focused on breeding systems and fruiting success. Chloraea membranacea Lindl. is one of the few non-Andean species in this group, and the aim of the present contribution is to elucidate the pollination biology, functional floral morphology and breeding system in native populations of this species from Argentina (Buenos Aires) and Brazil (Rio Grande do Sul State).
Methods
Floral features were examined using light microscopy, and scanning and transmission electron microscopy. The breeding system was studied by means of controlled pollinations applied to plants, either bagged in the field or cultivated in a glasshouse. Pollination observations were made on natural populations, and pollinator behaviour was recorded by means of photography and video.
Key Results
Both Argentinean and Brazilian plants were very consistent regarding all studied features. Flowers are nectarless but scented and anatomical analysis indicates that the dark, clavate projections on the adaxial labellar surface are osmophores (scent-producing glands). The plants are self-compatible but pollinator-dependent. The fruit-set obtained through cross-pollination and manual self-pollination was almost identical. The main pollinators are male and female Halictidae bees that withdraw the pollinarium when leaving the flower. Remarkably, the bees tend to visit more than one flower per inflorescence, thus promoting self-pollination (geitonogamy). Fruiting success in Brazilian plants reached 60·78 % in 2010 and 46 % in 2011. Some pollinarium-laden female bees were observed transferring pollen from the carried pollinarium to their hind legs. The use of pollen by pollinators is a rare record for Orchidaceae in general.
Conclusions
Chloraea membrancea is pollinated by deceit. Together, self-compatibility, pollinarium texture, pollinator abundance and behaviour may account for the observed high fruiting success. It is suggested that a reappraisal and re-analysis of important flower features in Chloraeinae orchids is necessary.
Keywords: Breeding system, Chloraea membrancea, Chloraeinae, Halictidae, Orchidaceae, orchids, pollination
INTRODUCTION
In its current delimitation, the orchid subtribe Chloraeinae Rchb. f. is, in essence, an Andean group that embraces approx. 70 species distributed in three genera: Bipinnula Comm. ex Juss., Chloraea Lindl. and Gavilea Poepp. (Correa and Sánchez, 2003; Chemisquy and Morrone, 2012; Cisternas et al., 2012a, b). The most recent monograph of Chloraea was prepared by Correa (1969) and recognizes 46 valid species. Correa (1969) stressed that only two species, C. bella Hauman – restricted to Uruguay and the Argentinean province of Entre Rios – and C. membranacea Lindl., occur outside the Andean range. Most of the remaining species inhabit Chilean–Argentinean Patagonia, and some species grow in north-western Argentina, Bolivia and Peru (Correa, 1969; Correa and Sánchez, 2003). Pollination biology has previously been investigated for only a handful of Patagonian Chloraea species, and these studies focused mainly on breeding systems and fruiting success, but unfortunately yielded little pollinator behaviour data (Clayton and Aizen, 1996; Lehnebach and Riveros, 2003; Humaña et al., 2008). They nevertheless agreed that all Chloraea spp. studied to date lack nectar and are self-compatible, yet are pollinator-dependent; i.e. a pollinator is required to set fruit and produce viable seed (Gumprecht, 1975; Clayton and Aizen, 1996; Lehnebach and Riveros, 2003; Humaña et al., 2008). Bees (Apidae and Colletidae), flies and Coleoptera have been observed pollinating these orchids, but such insect visits were rare and, consequently, few details of pollinator behaviour at the flowers are available (Clayton and Aizen, 1996; Lehnebach and Riveros, 2003; Humaña et al., 2008).
As all Chloraea spp. studied to date lack nectar (Lehnebach & Riveros, 2003; Humaña et al., 2008), it is likely that these flowers are pollinated by deceit, but due to the scarcity of pollination data this remains to be proved. Therefore, detailed analyses of floral features coupled with complete pollinator observations are necessary to fully ascertain the pollination biology of these orchids. The aim of the present contribution is to present a detailed study of the floral functional morphology, pollination biology and breeding system of C. membranacea, one of the few Chloraeinae orchids having a non-Andean distribution.
MATERIALS AND METHODS
Study sites
The pollination biology and breeding system of Chloraea membranacea was studied for natural populations occurring in Brazil (Flores da Cunha, Rio Grande do Sul: 29 °1′50″S, 51 °11′30″W, alt. approx. 756 m) and Argentina (Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, city of Buenos Aires: 34 °32′40″S, 58 °26′25″W, alt. approx. 6 m). Supplementary observations were also made on a cultivated plant grown at the city of Porto Alegre (State of Rio Grande do Sul: 30 °01′59″S, 51 °13′48″W, alt. approx. 10 m). For conservation purposes, we cite here only the coordinates of Brazilian municipalities and omit the exact location details of populations. These data, however, are available on request.
The Flores da Cunha locality occurs within the Atlantic Rain Forest biome/domain, and the vegetation consists of forests dominated by Araucaria angustifolia (Bertol.) Kuntze (Araucariaceae). Average annual rainfall is about 2400 mm and annual average temperature is approximately 14·5 °C (Moreno, 1961). The climate is characterized by a cool summer, a cold winter and the lack of a well-defined dry period (Nimer, 1989; Almeida, 2009). Both the Brazilian locality of Porto Alegre and Buenos Aires (Argentina) occur within the Pampa Biome. The Argentinean population occurs in the understorey of an elm (Ulmus sp.) forested park at the Campus of the Facultad de Ciencias Exactas y Naturales, near the Rio de la Plata river. Average annual rainfall is about 1306 mm and annual average temperature is approx. 18·05 °C (DCAO-FCEN-UBA, 2012). In Porto Alegre, the vegetation consists of a combination of grassy areas (‘campos’) surrounded by granitic outcrops and forests. Average annual rainfall is about 1321 mm and annual average temperature is approx. 19·3 °C (PMPA, 2012).
Species studied
Chloraea membranacea grows in north-eastern Argentina (from Buenos Aires Province, northwards to Santa Fé, Entre Rios and Corrientes), Uruguay and southern Brazil, reaching its northernmost limit at the State of Paraná (Ponta Grossa and Curitiba; Correa, 1969). Although common in Argentina and Uruguay, this plant is rare in Brazil, and all recent herbarium records (10 years or less) come from the State of Rio Grande do Sul. In the Brazilian State of Paraná, this species has not been collected for decades (the most recent voucher for Paraná State, as far as we can ascertain, was pressed in 1977; C. R. Buzatto, pers. observ.), and there are no known pressed vouchers from Santa Catarina State. Generally, flowering plants may reach a height of 40–70 cm. The lanceolate leaves are 10–15 cm long and 2–3 cm wide (see Supplementary Data Fig. S1A) and may be dry or absent during anthesis (Correa, 1969). The inflorescence may bear up to 20 greenish-white flowers. Morphologically, Hauman (1921) and Correa (1969) considered this species to be very similar to C. bella, the only other non-Andean species of Chloraea. Together, C. membranacea and C. bella constitute the so-called ‘eastern’ Chloraea species-group (Hauman, 1922; Correa, 1969), the only non-Andean species-group in the genus. Apart from its distribution, this ‘eastern’ group is characterized by distinctive column features: the stigmatic surface is long (two-thirds of the total length of the column) and dumb-bell-shaped, and the column wings are narrower and thicker towards the apex (Correa, 1969).
Recent phylogenetic studies (Chemisquy and Morrone, 2010, 2012; Cisternas et al., 2012a, b) cast doubt on the monophyly of the genera assigned to Chloraeinae as currently circumscribed. Indeed, the same analyses (see Chemisquy and Morrone, 2012; Cisternas et al., 2012a, b) place C. membranacea either as sister to, or in the same clade as, Chloraea virescens (Willd.) Lindl., the type species of the genus. In other words, even if Chloraea is redefined and some groups are segregated (as already proposed by Szlachetko & Margonska, 2001, and Szlachetko and Tukałło, 2008), it is likely that C. membranacea will remain within Chloraea. Plant vouchers were deposited at the ICN Herbarium of the Universidade Federal do Rio Grande do Sul (UFRGS) and also at the Museo Argentino de Ciencias Naturales – Bernardino Rivadavia Herbarium (BA).
Floral morphology and anatomy
Fresh flowers and their parts were photographed using a digital camera. They were then examined for secretory structures using a stereomicroscope and subsequently prepared for light microscopy (LM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM; see below).
Light microscopy and histochemistry
Following macroscopic observations, pieces of labellar tissue were tested for lipids and starch by treating each with a saturated solution of Sudan III in 70 % (v/v) ethanol and IKI, respectively, and examined microscopically (Jensen, 1962). Other labellar samples (approx. 1 mm3) were fixed in 2·5 % glutaraldehyde/4 % freshly de-polymerized paraformaldehyde in phosphate buffer (pH 7·2; 0·05 m) for 5 h at room temperature (initially under reduced pressure to facilitate penetration), carefully washed three times in phosphate buffer (30 min each) and post-fixed in 1 % (w/v) osmium tetroxide solution for 2 h at 0 °C. They were then dehydrated using a graded ethanol series, and infiltrated and embedded in Historesin (Leica). Sections were cut at 1 µm thickness on a Leica RM2155 microtome using a Jung carbide knife, stained with 0·25 % (w/v) toluidine blue O (TBO) in 0·25 % (w/v) aqueous sodium tetraborate solution, air-dried and mounted in synthetic Canada balsam.
Transmission electron microscopy
Pieces of labellar projections (approx. 1 mm3) were fixed as above and, following dehydration using a graded ethanol series, infiltrated and embedded in Spurr's resin. Following polymerization at 60 °C, sections were cut at 70 nm for TEM using a Sorvall Porter-Blum MT2-B ultramicrotome and Diatome diamond knife. Sections were then stained with uranyl acetate and lead citrate (Reynolds, 1963) and examined using a Zeiss EM 109T transmission electron microscope at an accelerating voltage of 80 kV.
Scanning electron microscopy
Labella and columns (n = 3, each) were dehydrated using a graded ethanol series, transferred to pure acetone, subjected to critical-point drying using liquid CO2 and sputter-coated with 15 nm gold (Davies and Stpiczyńska, 2009). The samples were examined using a Jeol JSM-6060 scanning electron microscope, at an accelerating voltage of 20 kV, located at the Centro de Microscopia Eletrônica of the Universidade Federal do Rio Grande do Sul (UFRGS).
Floral life span, pollination, fruiting success, pollination efficiency and breeding system
Floral life span was established in 2011 by monitoring 15 tagged, untouched flowers occurring on five bagged individuals (three flowers per inflorescence) from the Flores da Cunha population (see Table 1).
Table 1.
Summary of pollination observations in populations of Chloraea membranacea. Data of voucher specimens are detailed below each locality
| Locality/country | Year | Observation days | Observation period | Hours spent per locality |
|---|---|---|---|---|
| Flores da Cunha (RS, Brazil) M. Pedron s.n. (ICN) | 2010 | 2, 7, 8, 11, 13, 15, 19 and 20 November | 0800 to 1930 h | 57 |
| Flores da Cunha (RS, Brazil) M. Pedron s.n. (ICN) | 2011 | 31 October and 1–5 November and 7–11 November | 1030 to 1700 h | 33 |
| Porto Alegre (RS, Brazil) C.R. Buzatto 745 (ICN) | 2011 | 5 and 6 November | 1030 to 1700 h | 13 |
| UBA (CABA, Argentina) A. Sanguinetti s.n. (BA) | 2011 | 17, 22–25 October | 1100 to 1800 h | 15 |
| Total (h) | 118 |
Pollination biology of native Argentinean and Brazilian populations was studied in the field. The number of hours spent observing each population is indicated in Table 1. Preliminary observations carried out in 2009 clearly showed that the flowers were attractive to pollinators during daylight hours (1000 to 1800 h), a fact that was subsequently confirmed. Consequently, further observations were planned accordingly, and no crepuscular or nocturnal observations were made. Generally, the observation period extended from 0800 to 1830 h.
In Brazil (Flores da Cunha), the pollination biology of C. membranacea was studied for a period of two consecutive years (2010, 2011; Table 1). In 2010, observations were made on five plants that produced a total of 51 flowers. In 2011, 11 individuals that produced a total of 213 flowers were monitored. Fruiting success (fruits/flowers produced) was recorded at the Flores da Cunha locality for both years, as a measure of pollinator efficiency. A t-test was applied to analyse possible differences in fruiting success for each of the years observed. Between 17 and 19 November 2010, Nilsson's male efficiency factor (percentage of pollinated flowers divided by the percentage of flowers acting as pollen donors) was also calculated as another measure of pollinator efficiency (Nilsson et al., 1992). Complementary observations were made in 2011 at the Brazilian locality of Porto Alegre by exposing to the outdoors a cultivated specimen bearing a single inflorescence of seven flowers (Table 1). In Buenos Aires (Argentina), the pollination of C. membranacea was studied in 2011 (see Table 1) by monitoring two individuals naturally growing at the Campus of the Facultad de Ciencias Exactas y Naturales. During the observations, these plants produced a total of 27 flowers.
Pollinator behaviour was documented by means of field notes, photography and video. In general, this video record made it possible to gain a better understanding of both the pollination process and pollinator behaviour. Individuals of pollinating insects were collected and sacrificed for further taxonomic identification. These insect vouchers were deposited at the entomological didactic collections of the Zoology Department, UFRGS (Brazilian specimens) and at the Museo Argentino de Ciencias Naturales, entomological section (Argentinean specimens).
Breeding system experiments on Brazilian plants were performed in situ at Flores da Cunha, by bagging seven plants (totalling 97 flowers) in order to exclude natural pollinators (see Supplementary Data Fig. S1B). Plants cultivated in a greenhouse based in the experimental field of the Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina (13 plants totalling 112 flowers), were bagged and used for the same purpose. Four treatments were applied to these inflorescences: intact flowers (control), emasculation, hand self-pollination and cross-pollination (Table 2). Only hand self-pollinations and cross-pollinations yielded fruit in the plants of both countries (see Results and Table 2) and the percentages of fruiting success under both treatments are almost identical, rendering statistical comparisons unnecessary (Table 2). The number of plants and flowers used per treatment are detailed in Table 2.
Table 2.
Breeding system experiments; fruiting success (%) in Chloraea membranacea for control (intact flowers) and following emasculation, self- and cross-pollination treatments
| Locality/country | n | Control | Emasculation | Self-pollination | Cross-pollination |
|---|---|---|---|---|---|
| UBA (Buenos Aires, Argentina) | 13 | 0 (0/28) | 0 (0/28) | 96·43 (27/28) | 100 (28/28) |
| Flores da Cunha (RS, Brazil) | 7 | 0 (0/25) | 0 (0/24) | 100 (24/24) | 100 (24/24) |
Numbers in parentheses represent the number of fruit obtained over the number of flowers used in each treatment. n represents the number of plants used in the experiments.
RESULTS
Floral features
Only floral features involved in the pollination process will be discussed. Readers interested in a more detailed description of vegetative and floral features of C. membranacea are referred to the monograph by Correa (1969: 456–459; see also Supplementary Data Fig. S1A). The flowers at the Flores da Cunha locality had a mean life span of 22·4 d. However, flowers cultivated at Porto Alegre kept their fresh appearance for 6–7 d only (R. B. Singer, pers. observ.), while the flowers of specimens cultivated at Buenos Aires lasted for 10–17 d (A. Sanguinetti, pers. observ.). This suggests that the feature is not constant and is probably affected by environmental conditions. The flowers are greenish-white (Fig. 1A) and membranous in texture. The sepals are 16–20 mm long and 7–8 mm wide (Correa, 1969). The lateral petals are asymmetric, 15–18 mm long and 9–11 mm wide. The labellum is entire to slightly three-lobed, 13–15 mm long and 7–9 mm wide, and is articulated (hinged) at the base of the column (Fig. 1B).
Fig. 1.
Floral features and pollination of Chloraea membranacea. (A) Racemose, multi-flowered inflorescence. (B) Single flower with lateral sepal and lateral petal removed to show the column with hinged labellum attached at its base. Note the black-tipped, clavate, labellar projections (osmophores). (C) Column. Note also the ventral, dumb-bell-shaped, sticky stigmatic surface. (D) Two elongate pollinia. (E–G) Pollination by Halictid bee. (E) Bee prior to pollinarium removal. (F) Bee leaving the flower. The insect is attempting to remove the pollinarium cemented to its dorsum. (G) Pollinarium-laden bee. Note that the hind legs are loaded with pollen. Scale bars: (A, B, E–G) = 1 cm; (C, D) = 5 mm.
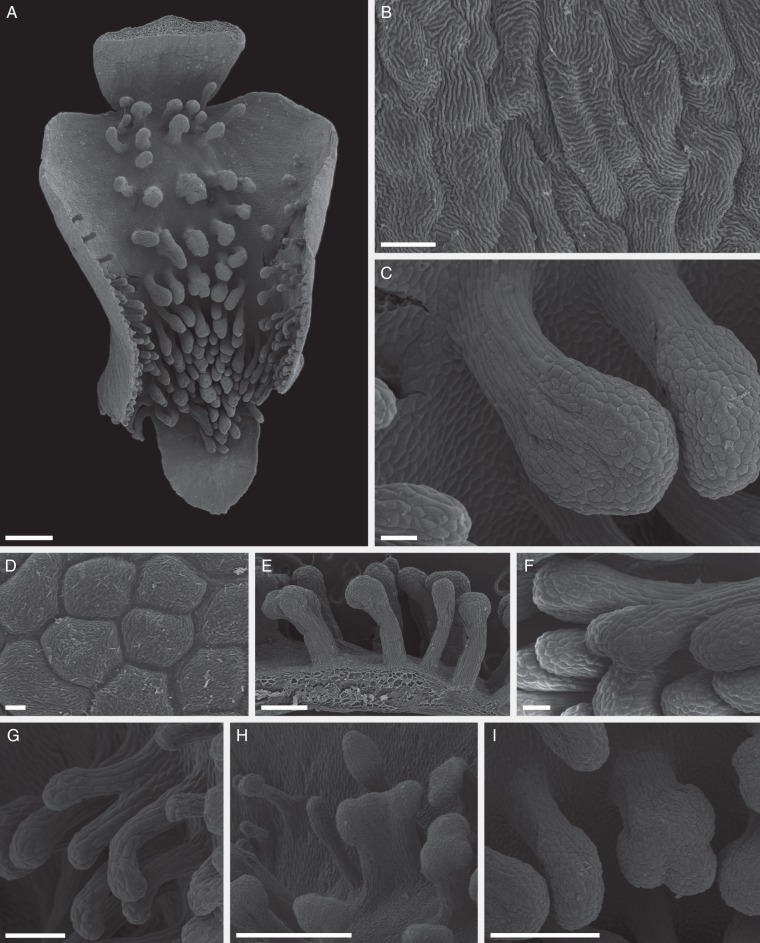
Examination of fresh material revealed that the adaxial surface of the labellum is white with numerous, clavate projections that have greyish-white stalks and black tips (Fig. 1B), the latter coated with translucent or whitish, glistening, secreted material. The projections are concentrated distally (Fig. 2A) and the epidermal cells of both labellar surface and projections have a striated cuticle (Fig. 2B, D). The projections, which may be flattened along one side, are usually unbranched and multicellular, with clavate or expanded heads (Fig. 2C). The stalk cells are narrow and elongate (Fig. 2C), whereas the head cells are more or less isodiametric and angular in surface view, often with scant amorphous residues or secretory droplets on their surface (Figs 2D and 4A). This surface material stained with Sudan III, and often accumulated between the isodiametric head cells (Fig. 4A, B). Occasionally, the projections have bifid heads (Fig. 2E-F), or are branched and deeply bifurcate (Fig. 2G), or are fused, with laterally compressed, flabelliform heads (Fig. 2H). Often, the heads consist of several confluent lobes (Fig. 2I). SEM of the adaxial labellar surface confirmed the absence of nectar-secreting cells and/or other nectariferous structures (Fig. 2).
Fig. 2.
Labellar features of C. membranacea (SEM). (A–I) Labellum bearing clavate projections (osmophores) that are concentrated distally and centrally. (B) Epidermal cells of distal, adaxial surface of labellum showing striated cuticle. (C) Detail of typical, unbranched, multicellular, clavate osmophore. Note the narrow, elongate stalk cells and the isodiametric head cells. (D) Detail of head showing angular cells with striated cuticle and scant residues of secreted material. (E,F) Osmophores with bifid heads. (G) Branched, deeply bifurcate osmophore. (H) Flabelliform, laterally compressed osmophore. (I) Osmophore with head composed of three confluent lobes. Scale bars: (A, H) = 1 mm; (B) = 20 µm; (C, F) = 100 µm; (D) = 10 µm; (E, I) = 500 µm, (G) = 200 µm.
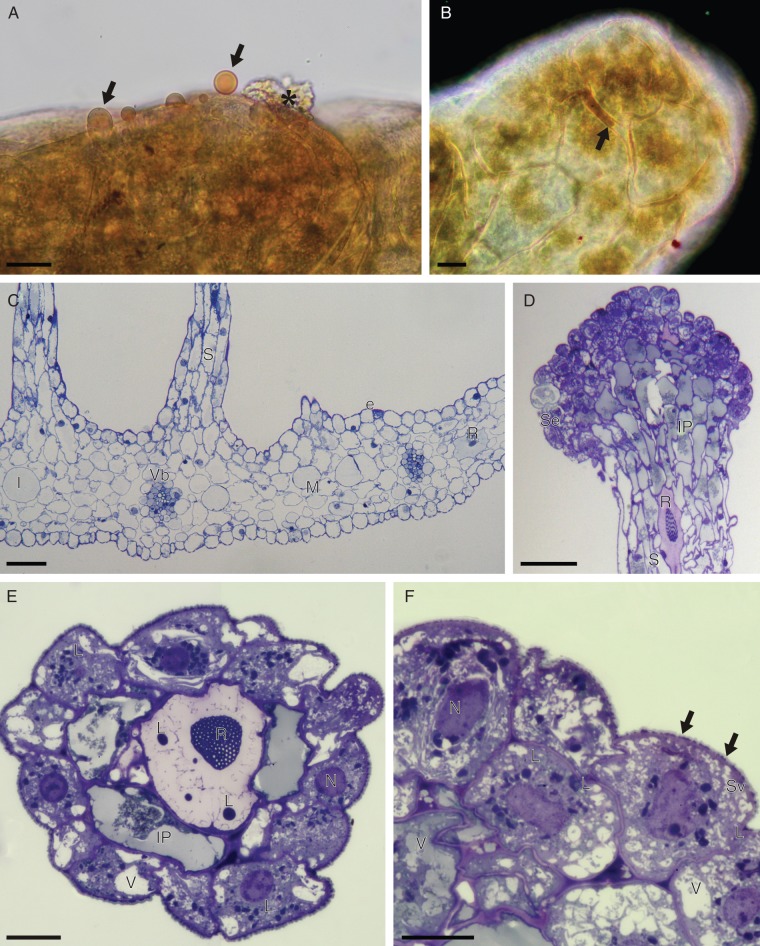
Fig. 4.
(A, B) Detail of head of labellar osmophore of C. membranacea, treated with Sudan III (LM). (A) Osmophore tissue with secreted lipid droplets (arrows) that stain orange-red with Sudan III; also note the secretory residues (asterisk) present on the osmophore surface. (B) Surface view of osmophore head showing the accumulation of secreted lipid material between isodiametric cells (arrow). Note that the cuticle also stains red with Sudan III. (C–F) Labellar anatomy of C. membranacea (LM). (C) Transverse section of labellum with bases of stalked osmophores and collateral vascular bundles. (D) Vertical section through apex of osmophore showing head comprising intensely stained secretory cells and subsecretory layer containing amorphous, intravacuolar precipitates. The stalk contains idioblasts with raphides. (E) Transverse section of osmophore showing outer layer of secretory cells, subsecretory layer with intravacuolar precipitates, and a central cell with intravacuolar raphides and spherical lipid bodies. (F) Detail of secetory cells with perinuclear plastids. Note the vesicles that aggregate close to the cell wall and the lipid material (arrows) that accumulates beneath the cuticle. Scale bars: (A, B, E, F) = 20 µm; (C, D) = 100 µm. Abbreviations: e = epidermis; I = idioblast; IP = intravacuolar precipitate; L = lipid body; M = mesophyll; N = nucleus; R = raphide; S = stalk of osmophore; Se = secretory layer; Sv = secretory vesicles; V = vacuole; Vb = vascular bundle.
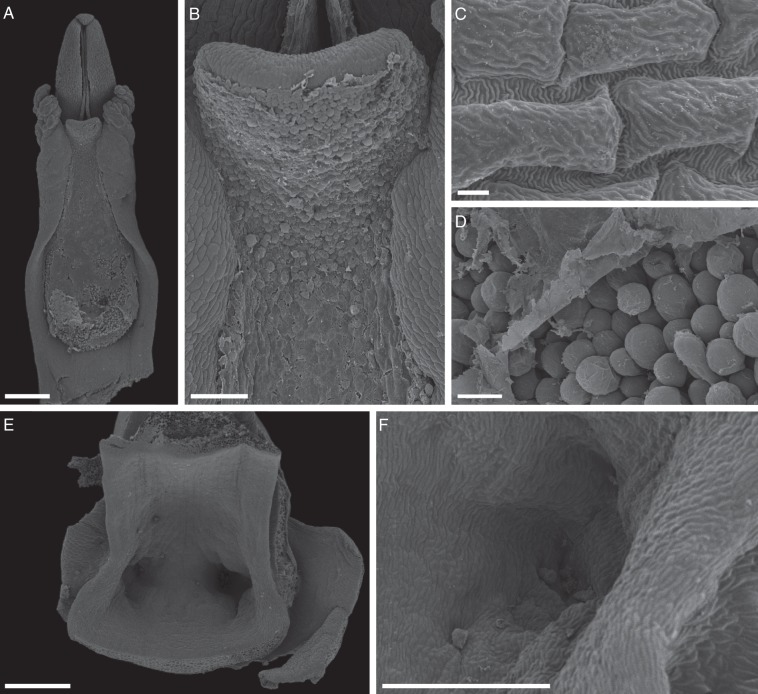
The column is arcuate, greenish, about 10 mm long, and has a well-developed column-foot (Fig. 1B, C) and two small, lateral column wings (Fig. 3A). The cuticle overlying the epidermal cells of the column is striated (Fig. 3C). The anther is erect and terminal (Figs 1C and 3A). The pollinarium comprises two, bipartite, yellow, powdery pollinia that lack pollinium stalks (Fig. 1D), and the removal of pollinia is facilitated by a rostellar secretion. No detachable viscidium (sensu Dressler, 1993) is present. The stigmatic surface is extensive, dumb-bell- or hourglass-shaped and slightly convex (Figs 1C and 3A, B). It is borne ventrally on the column, is glandular (Fig. 3A, B) and bears numerous, densely packed, unbranched papillae or trichomes. These are usually smooth-walled, unicellular, clavate and secretory (Fig. 3D). SEM revealed that the latter are occluded by a homogeneous film of stigmatic fluid (Fig. 3A, B, D). At the base of the column occur two, so-called ‘nectariferous channels’ (sensu Cisternas et al., 2012a, b, and references therein; Fig. 3E), which here are merely two shallow depressions or cavities (Fig. 3E, F). Despite the name given to these structures, the absence of nectar was confirmed. The flowers of C. membranacea emit a faint, sweet fragrance which is strongest during the warmer daylight hours (1000–1600 h), and this coincides with the greatest number of visits by pollinators. The fruit of C. membranacea specimens observed in this study dehisced along two dorsal lines of weakness (see Supplementary Data Fig. S1E), and the seeds were minute and dust-like.
Fig. 3.
Column features of C. membranacea (SEM). (A) Ventral surface of column, showing anther, lateral column wings and hourglass- or dumb-bell-shaped stigmatic surface. (B) Detail of part of stigmatic surface showing homogeneous residue film of stigmatic fluid. (C) Epidermal cells of column with striated cuticle. (D) Detail of stigmatic surface showing smooth-walled, unicellular, secretory, clavate papillae and overlying, residual, homogeneous film of stigmatic fluid. (E) ‘Nectariferous channels’ (in fact, two shallow depressions or cavities) at the base of the column. (F) Detail of one of these depressions, showing the absence of secretory cells. Scale bars: (A, E) = 1 mm; (B) = 200 µm; (C) = 10 µm; (D) = 50 µm; (F) = 500 µm.
Labellum anatomy
Transverse sections revealed that the labellum consists largely of mesophyll in which are embedded collateral vascular bundles (Fig. 4C), and is bound by a single-layered epidermis consisting of somewhat rounded and often-nucleated cells (Fig. 4C). Longitudinal and transverse sections through the labellar projections revealed that the head consists largely of a layer, some 1–4 cells deep, of isodiametric, epidermal secretory cells. At the base of the head, there is a marked distinction between these cells and the elongate, narrow cells of the stalk (Fig. 4D). The epidermis of the stalk is continuous with the adaxial epidermis of the labellum (Fig. 4C). Basally, the stalk is usually 6–10 cells wide, with a core of parenchymatous cells that is continuous with the labellar mesophyll (Fig. 4C). Like the mesophyll, the parenchymatous core of the stalk also contains idioblasts with raphides (Fig. 4D). This indicates that the projections are not epidermal, multiseriate trichomes, but rather outgrowths of the labellum. Moreover, they do not receive a direct vascular supply (Fig. 4C). Transverse sections of the projections reveal that acropetally, the secretory tissue is confined to a single layer of epidermal cells surrounding one or two core cells, the latter often containing raphides (Fig. 4E). Basipetally, however, several layers of subepidermal tissue are present, and these cells often contain amorphous, intravacuolar precipitates (Fig. 4D). LM revealed that the secretory cells are highly vesiculate, contain relatively large nuclei, numerous plastids (usually displaying perinuclear distribution) and lipid bodies (Fig. 4E, F). Starch was not detected.
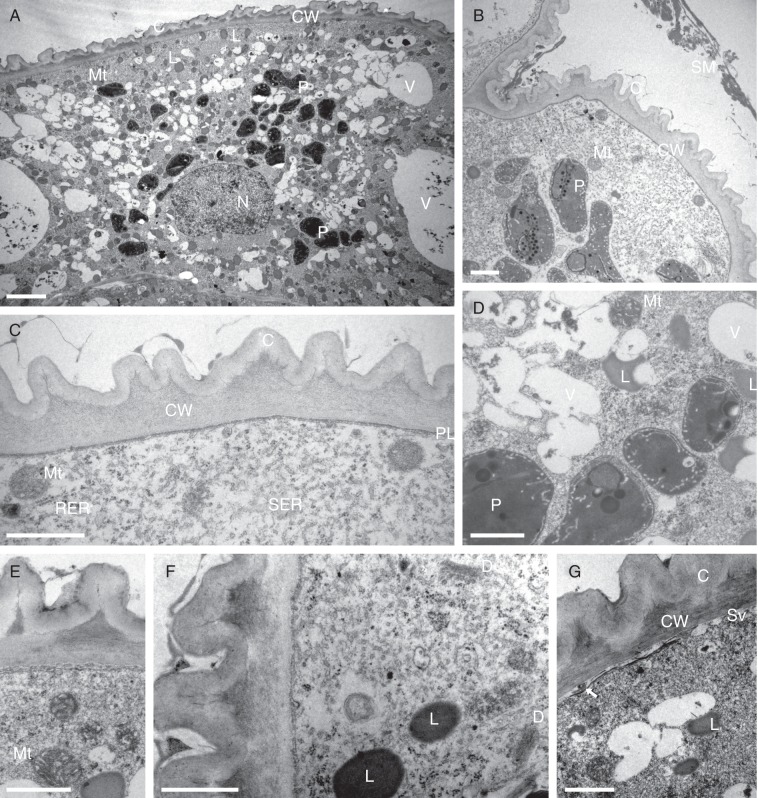
TEM confirmed LM observations. A cuticle is present on the outer tangential walls of the secretory head cells (Fig. 5A–C, E–G). This is highly folded or striated, giving the cuticle a ridged appearance in section (Fig. 5B, C, E–G). Heterogeneous, secreted material is present on the surface of the cuticle, and this often collects between the cuticular ridges (Fig. 5B, C, E–G). Both secreted material and cuticle stained with Sudan III, demonstrating that they contain lipid (Fig. 4A, B). The outer tangential cellulose cell walls of the secretory cells lack ectodesmata (Fig. 5C). However, radial walls and inner tangential walls are often pitted, the primary pit-fields containing plasmodesmata that allow communication and transfer of material between adjacent cells. The outermost region of the outer tangential wall is clearly lamellate, and the cuticle is reticulate (Fig. 5E–G). The cells of the head contain relatively large, prominent nuclei, often with nucleoli, and highly vesiculate cytoplasm with a complement of organelles consistent with that of secretory cells (Fig. 5A, B). There is a well-developed vacuome comprising small vesicles and larger vacuoles (Fig. 5A, D). Indeed, these cells often display a very characteristic and atypical type of vacuolation during which the cytoplasm appears to become divided into somewhat angular segments (Fig. 4E). Components of the vacuome may contain membranous inclusions, spherical, osmiophilic bodies and amorphous precipitates (Fig. 5A, D). The numerous, oval, dumb-bell-shaped or irregularly shaped elaioplasts generally display a perinuclear distribution (Fig. 5A, B, D). They have an osmiophilic stroma and contain lipid droplets and/or numerous plastoglobuli and membranes (Fig. 5B, D), Much greater numbers of more or less spherical, but sometimes crescent-shaped, lipid bodies are also present (Fig. 5A, D, E–G). Often, these are less intensely osmiophilic than the elaioplasts. Under LM, they stain red with Sudan III, and are interpreted as droplets of fragrance precursors (terpenoids). They often aggregate adjacent to the plasmalemma (Fig. 5A, F). The abundant mitochondria contain large numbers of well-developed cristae (Fig. 5B–E, G), reflecting the high metabolic activity of these cells during secretion. Both smooth (SER) and rough (RER) endoplasmic reticulum are present, but the former predominates (Fig. 5C, F). The SER cisternae become dilated with secretory material (Fig. 5C). Dictyosomes (Golgi apparatus), however, were rarely observed (Fig. 5F), but ribosomes were abundant. Aggregates of small vesicles often became associated with the plasmalemma and cell wall (Fig. 5G), and a darkly staining, osmiophilic layer of lipid was often visible directly beneath the plasmalemma, or in the periplasmic space (Fig. 5A, E, G), and another to the outside of the cell wall, directly beneath the cuticle (Fig. 5A, E–G). Localized sub-cuticular accumulations of osmiophilic material coincided with cuticular ridges and partial detachment of the cuticle from the outer tangential wall (Fig. 5A–C, E–G). These accumulations are triangular in section and, under LM, stain intensely with TBO (Fig. 4F). Osmiophilic extensions pass from these into the cuticle (Fig. 5E-G), strongly suggesting the presence of cuticular micro-channels, and possibly pores through which the secretion can pass. The localized presence of secreted lipoidal material on the surface of the cuticle supports this hypothesis. Although no cuticular pores were detected, this does not necessarily mean that they are absent, as the highly folded cuticle, in conjunction with the very small size of such structures and the presence of secreted material, would have frustrated our efforts to find them using SEM. Lipids, as well as occurring within plastids, ER and vesicles, or becoming associated with the plasmalemma, cell wall and cuticle, also accumulate within the larger vacuoles of the inner cells of the head, where they either occur as spherical bodies (Fig. 4E) or become associated with the tonoplast. Likewise, lipids are also present in the vacuole and along the plasmalemma and tonoplast of raphide-containing idioblasts. Some lipids found in these cells are not associated with membranes, but form numerous, free, spherical lipid bodies or droplets scattered throughout the cytosol. The innermost cells of the head are lined by a narrow layer of parietal cytoplasm enclosing well-developed and much larger vacuoles than those of the outer cells. Intravacuolar, amorphous precipitates are frequently present here (Fig. 4D–E). This combination of cellular features is typical of osmophores (scent-producing glands).
Fig. 5.
(A–G) Ultrastructure of secretory osmophore cells of C. membranacea (TEM). (A) General section through secretory cell showing ridged cuticle and distribution of organelles. Abundant, spherical, osmiophilic lipid bodies, thought to be precursors of fragrance production, are present. (B) Similar cell showing heterogeneous surface secretion and elaioplasts. (C) Detail of cell wall, cuticle and plasmalemma with associated organelles. (D) Detail of vesiculate cytoplasm showing elaioplasts containing lipid bodies and membranes. (E) Osmiophilic material accumulates between the cell wall and cuticle. (F) Secretory cytoplasm with lipid bodies and dictyosomes (Golgi apparatus). (G) Small vesicles become associated with the plasmalemma. Osmiophilic material accumulates here and in the periplasmic space (arrow). The cell wall is more obviously lamellate and osmiophilic material accumulates beneath the cuticular ridges. Micro-channels penetrate the latter. Scale bars: (A) = 2 µm; (B–G) = 1 µm. Abbreviations: C = cuticle; CW = cell wall; D = dictyosome (Golgi apparatus); L = lipid body; Mt = mitochondrion; N = nucleus; P = plastid; PL = plasmalemma; RER = rough endoplasmic reticulum; SER = smooth endoplasmic reticulum; SM = secreted material; Sv = secretory vesicles; V = vacuole.
Breeding system
The results are summarized in Table 2. Both Brazilian and Argentinean plants had a similar breeding system. They were self-compatible but pollinator-dependent. No fruit was produced either by intact or emasculated flowers, thus supporting the view that C. membranacea is not able to set fruit in the absence of pollinators. The percentage fruiting success resulting from manual self-pollination and cross-pollination was almost identical (Table 2), making statistical comparisons unnecessary.
Pollination biology
Pollinator observations are summarized in Table 3. For both Argentinean and Brazilian plants, the pollinators were male and female Augochlorini (Halictidae) bees (Fig. 1E–G, and Supplementary Data Video S1). Bees visited the flowers during the warmer daylight hours, especially when the inflorescences were receiving direct sunlight. Pollinator visits took place between 1048 and 1630 h, i.e. during the period when the flowers emit their sweet fragrance (see Floral features). In Argentina, the main pollinators were Augochloropsis multiplex (Vachal 1903) bees. In Brazil, the pollinators were also bees of the genus Augochloropsis (three morphospecies). In both countries, the pollination mechanism was similar: the bees briefly hovered in front of the flowers, alighted and entered the floral cavity. The hinged labellum moved noticeably relative to the movements of the bee inside the flower. Bees were repeatedly seen inspecting the clavate projections on the labellar surface. Remarkably, the vast majority of Halictid bees that alighted positioned themselves so that the abdomen pointed towards the column (Fig. 1E). Bees positioned in this way pressed their bodies against the rostellum when leaving the flower. The rostellum then released a sticky fluid that glued the pollinarium onto the dorsum of the bee (Fig. 1F, G). We would like to stress here that the orientation of the bee on entering the flower supports the view that the so-called ‘nectariferous channels’ are actually devoid of nectar. Also, that for all visits resulting in the removal of the pollinarium, the head of the bee projected outside the floral cavity, i.e. in the opposite direction to the ‘nectariferous channels’. In other words, Halictidae bees were not observed gathering or attempting to gather nectar from the presumed nectaries. Bees clearly spent considerable time inspecting the clavate labellar osmophores, but no attempts at nectar-collecting (or any other kind of collecting activity) at these structures were observed. The extra weight of the pollinarium upset the balance of the pollinator, and bees were often seen falling to the ground or actively attempting to remove the pollinaria with their forelegs (Fig. 1F). This latter behaviour was recorded at all three localities. Pollination occurs when a pollinarium-laden bee enters a flower and repeats the behaviour described above. As the stigmatic surface is long and broad, pollinia easily make contact with the stigmatic surface, leaving large pads of pollen.
Table 3.
Summary of pollinator observations for plants of C. membranacea in Brazil and Argentina
| Locality/country | Year | Pollinators | Period of observed insect activity | Observed pollinarium withdrawals† | Observed insect-mediated self-pollinations† | Time (s) spent by pollinators inside the flowers‡ |
|---|---|---|---|---|---|---|
| Flores da Cunha (RS, Brazil) | 2010 | Halictidae bees | 1048–1634 h | 6 | 5 (9·80 %) | 6–84 (25·33 ± 23·01) |
| Flores da Cunha (RS, Brazil) | 2011 | Halictidae bees, Vespidae wasp* | 1130–1523 h | 3 | 1 (0·47 %) | 61–79 (70 ± 12·72) |
| Porto Alegre (RS, Brazil) | 2011 | Halictidae bees | 1200–1500 h | 5 | 1 (11·11 %) | 6–65 (33 ± 19·08) |
| UBA Campus (CABA, Argentina) | 2011 | Halictidae bees | 1200–1500 h | 4 | 4 (14·81 %) | 16–49 (29 ± 11·71) |
* Observed only once.
† During video record.
‡ Values in parentheses represent mean ± s.d.
The bees may spend 6–79 s inside each flower (see Table 3 for observation details) and visit 1–3 flowers per inflorescence, and 1–3 inflorescences per visit to the population. The bees spend considerable time grooming outside the flowers and, generally, may stay at a single inflorescence for several (more than 8) minutes. It is worth noting that most bees visited more than one flower per visit to each inflorescence, promoting a variable degree of geitonogamy. The film record shows that, depending on locality, the bees self-pollinated 0·47–14·8 % of available flowers (Table 3). Remarkably, some pollinarium-laden female Halictid bees were often seen performing the same behaviour, both in Brazil and in Argentina. These bees alighted on the inflorescence and used their forelegs to transfer part of the pollen content of the pollinarium to their hind legs (see Supplementary Data Video S1). This behaviour strongly suggests that female Halictidae bees may eventually use in some way the pollen of C. membranacea during their life cycles. Pollinated flowers characteristically display a closed perianth with lateral petals hiding the column (see Supplementary Data Fig. S1C). In Brazil, unidentified flies were recorded as non-pollinating visitors to these flowers. The flies explored the outside of the flower, or licked the labellum, but made no contact with the column. An unidentified Vespidae wasp was seen once in Flores da Cunha dislodging a pollinarium (see Supplementary Data Fig. S1D, Table 3). On one occasion, in Argentina, a queen of Bombus atratus Franklin was observed to visit an inflorescence and probe three flowers, but without removing pollinia (see also Supplementary Data Video S1).
Pollination success
In 2010, of the 51 available flowers, 31 (60·78 %) developed into fruit. Individual fruiting success ranged from 22·22 to 100 %. Mean fruiting success per inflorescence was also very high (64·25 ± 31·64 %). Between 17 and 19 November 2010, 45 flowers (88·23 %) acted as pollen-donors, and 31 (60·78 %) as pollen receivers. Therefore, Nilsson's efficiency factor was 0·688, indicating that during this period, approx. 0·7 flowers were pollinated for each pollinarium removed, suggesting some pollen-loss.
In 2011, of the 213 available flowers, 98 (46 %) developed into fruit. Individual fruiting success ranged from 0 to 88·88 %. Mean fruiting success per inflorescence was also lower (42·068 ± 22·97 %). Despite the striking numerical disparity in mean fruiting success between consecutive years, the t-test revealed that this was not statistically significant (P = 0·096, t = 1·747, 20 d.f.). However, the test also revealed that a type II error was highly probable (α = 0·05; 0·262), and it is likely that this was related to sample size.
DISCUSSION
The flowers of C. membranacea display a set of morphological features that are widespread and mostly consistent with those already reported for the genus (Correa, 1969; Correa and Sánchez, 2003). However, some of our findings suggest that certain morphological features of Chloraea spp. have been incorrectly interpreted in the past. Contrary to the claim of Szlachetko & Rutkowski (2000: 62), there is no detachable viscidium, and the pollinia become glued to the pollinator by means of a rostellar secretion (see Results). Moreover, Correa (1969) states that the fruit of Chloraea is a capsule, each having six lines of dehiscence. However, our study of C. membranacea showed that the fruit of this species splits along two dorsal lines of weakness.
Absence of nectar on the labellar surface generally agrees with earlier reports of pollination in Chloraeinae (Lehnebach and Riveros, 2003; Ciotek et al., 2006; Humaña et al., 2008), and also fully agrees with previous reports relating to the pollination biology of Patagonian Chloraea spp. All Chloraea species for which pollination biology has already been studied (Clayton and Aizen, 1996; Lehnebach and Riveros, 2003; Humaña et al., 2008) presented the so-called ‘nectariferous channels’, but these lacked nectar (see also Ciotek et al., 2006). In describing the genus, Correa (1969: 380–381) remarked that these ‘nectariferous channels’ extend to ‘less than the middle of the length of the ovary’ and illustrated this (p. 381), but did not state upon which species she had based her diagram. Later, in the same text, Correa (1969: 470) claims that Chloraea praecincta Speg. & Kranzl. is the only species in the genus to lack ‘nectariferous channels’. Fortunately, one of us (A. Sanguinetti, pers. observ.) had the opportunity to examine fresh flowers of the Patagonian species C. cylindrostachya Poepp., and found no ‘nectariferous channels’. This observation, together with the fact that these structures are clearly non-secretory and almost vestigial in C. membranacea, suggests that this character is, perhaps, much more variable than expected. Recently, Cisternas et al. (2012a) have commented that similar ‘nectariferous channels’ may be present in some Chilean species of the genus Bipinnula. All this suggests that the presence of the so-called ‘nectariferous channels’ may be more homoplasious than hitherto thought.
Labellar projections such as those reported here for C. membranacea are widespread amongst Chloraea spp., and within Chloraeinae in general (Correa, 1969; Correa and Sánchez, 2003). Even so, as far as we know, this is the first time for the floral anatomy of Chloraeinae species, and the biological importance of the labellar projections in pollination, to be described. Superficially similar labellar projections are widespread in distantly related terrestrial orchids from Oceania, namely members of the genera Caladenia R. Br. (Diuridae: Caladeniinae) and Chiloglottis R. Br. (Diuridae: Thelymitrinae – see photographs and drawings in Jones, 1991, 2006), although, to our knowledge, anatomical studies of these genera have not yet been undertaken. The flowers of Chiloglottis spp. are exclusively pollinated by sexual mimicry (see Mant et al., 2005), whereas Caladenia spp. are pollinated either by food-deceit strategies or by sexual mimicry (literature reviewed by Dixon and Tremblay, 2009; Phillips et al., 2009). Similar labellar projections have also been reported for Epidendroid orchids, such as Camaridium ochroleucum Lindl. and Camaridium pulchrum Schltr. (as Maxillaria camaridii Rchb. f. and M. pulchra (Schltr.) L.O. Williams ex Correll, respectively; Singer and Koehler, 2004), where they are thought to mimic stamens (Davies and Turner, 2004; Davies, 2009).
Based on anatomical studies, we herein suggest that the labellar projections of C. membranacea are osmophores (scent-producing glands), and consequently are involved in pollinator attraction. This is supported by the presence of large numbers of intracellular, spherical, osmiophilic bodies (interpreted as droplets of a fragrance precursor) identical to those found in the osmophores of Stanhopea J. Frost ex Hook. (Epidendroideae: Stanhopeinae; Stern et al., 1987) and Brasiliorchis picta (Hook.) R. Singer, S. Koehler & Carnevali (Epidendroideae: Maxillariinae; Davies and Stpiczyńska, 2012). Moreover, the secretory cells have relatively large nuclei, often with nucleoli, together with elaioplasts containing lipid bodies and plastoglobuli. In section, the cuticle is ridged, and secretory vesicles aggregate next to the plasmalemma or within the periplasmic space. Osmiophilic material may also collect both here and between the cuticle and the outer tangential wall. Walls between secretory cells and cells of the subsecretory layer display primary pit-fields with plasmodesmata. Extensive arrays of ER are present (mainly SER, but frequently RER also), with dilated cisternae, together with mitochondria possessing well-developed cristae. The cuticle becomes distended as secreted material accumulates between it and the outer tangential wall. The presence of dictyosomes (Golgi apparatus) may change as secretion progresses (Pridgeon and Stern, 1983, 1985; Stern et al., 1987; Stpiczyńska, 1993, 2001). In many respects, the anatomical organization of the labellar secretory tissue of Chloraea membranacea thus resembles that of the fragrance-secreting tissues of several other orchid species. The osmophore tissue of orchids generally displays a granulocrine mode of fragrance secretion (i.e. with the ER producing secretory vesicles), as in Restrepia Kunth and Gymnadenia conopsea (L.) R.Br. (Pridgeon and Stern, 1983; Stpiczyńska, 2001). However, to date, eccrine fragrance secretion (involving active transport through the plasma membrane) is known to occur only in a few orchid taxa, such as Stanhopea (Stern et al., 1987). The numerous secretory vesicles associated with the plasmalemma of C. membranacea, the abundant mitochondria with well-developed cristae and the absence of visible secretory particles within the cell wall are all indicative of granulocrine secretion.
In general, our results fully agree with previous reports on the breeding system both of Chilean species of Chloraea (Clayton and Aizen, 1996; Lehnebach and Riveros, 2003; Humaña et al., 2008) and other Chloraeinae orchids (Valdivia et al., 2010). All these earlier studies indicate that Chloraea orchids are self-compatible, but pollinator-dependent (Clayton and Aizen, 1996; Lehnebach and Riveros, 2003; Humaña et al., 2008; Valdivia et al., 2010). Reiche (1910) suggested that flowers of Chloraea fonckii Phil. may be either cleistogamous or autogamous, whereas those of C. philippii Rchb. f. may also be autogamous. However, Reiche (1910) did not provide evidence in support of this claim. Within Chloraeinae, automatic self-pollination was reported for Gavilea araucana (Phil.) M.N. Correa (Valdivia et al., 2010). However, the authors did not state how this was achieved. One of us (A. Sanguinetti, pers. observ.) has documented automatic self-pollination in G. glandulifera (Poepp.) M.N. Correa. In this species, the pollinarium partially disaggregates, and pieces of the pollinia fall onto the swollen and prominent stigmatic surface. None of the floral features that are associated with automatic self-pollination were recorded for C. membranacea.
This is the first report of Halictid bees unequivocally pollinating Chloraeinae orchids. Previous reports have listed Colletidae (Lehnebach and Riveros, 2003) and Apidae (Bombus Latreille) bees, as well as Coleoptera and Diptera (Tabanidae and Sarcophagidae) as the natural pollinators of some Chilean species of Chloraea (Lehnebach and Riveros, 2003; Humaña et al., 2008). Males of Campsomeris bistrimacula (Lepeletier) (Scoliidae) are the pollinators of Bipinnula penicillata (Hoehne ex M.N. Correa) Cisternas (as Geoblasta penicillata Hoehne ex M.N. Correa) (Ciotek et al., 2006). The breeding system of some Gavilea species has been studied in detail, but the pollinators are not known (Valdivia et al., 2010). Overall, the flowers of C. membranacea present a set of floral features (relatively small, greenish-white, partially closed, and sweetly scented flowers) that are widespread amongst orchids pollinated by sweat-bees (Singer and Cocucci, 1999; Singer and Sazima, 2000). Based on floral features, it is likely that other Chloraeinae orchids, such as C. bella, are also pollinated by Halictidae bees. During our observations, some pollinarium-laden Halictidae females were recorded actively transferring pollen from the pollinarium to their hindlegs. The pollen of monandrous orchids normally occurs in pollinia and therefore is rarely used by bee pollinators. However, the pollen of Psilochilus modestus Barb. Rodr. (Epidendroideae: Triphoreae) is often gathered by its Meliponine bee pollinators (Pansarin and Amaral, 2008). On occasion, Meliponine bees were also observed collecting pollen from the pollinaria of Sauroglossum elatum Lindl. (Spiranthinae), an orchid usually pollinated by noctuid moths (Singer, 2002). Remarkably, flowers of the orchid subfamily Apostasioideae (sister-group to the remaining Orchidaceae) present their pollen loose and free (i.e. not packed in pollinia), and all available evidence to date suggests that these orchids reward their pollinators with pollen (Kocyan and Endress, 2001). The present observations indicate that C. membranacea is a rewardless plant that is pollinated by food-seeking Halictidae bees. The fact that some pollinarium-laden female bees were seen actively collecting pollen from the pollinaria should be interpreted with caution. All our evidence suggests that pollen collection is a by-product of grooming activities. As already explained, the extra weight of the pollinarium has an adverse effect on the bee which, in turn, attempts to remove it with its forelegs. It could be argued that the bees were actively seeking pollen, but our photographic and film records indicate otherwise, in that all recorded bees interacted with the clavate projections on the labellar surface and ignored the anther or the ‘nectariferous channels’. Furthermore, male bees also pollinated C. membranacea, and male bees do not collect pollen at all.
A variable degree of insect-mediated self-pollination was observed at the three locations where pollinator activities were recorded (see Table 3). In view of this, and considering that the plants are self-compatible and that there were no mechanical barriers to hinder self-pollination, we can safely assume that the observed natural fruit-set was partly a consequence of insect-mediated self-pollination.
It is important to stress that the observed fruiting percentages (60·78 and 46 % for 2010 and 2011, respectively) are remarkably high for a rewardless orchid. As a rule (Neiland and Wilcock, 1998; Tremblay et al., 2005), rewardless orchids have low fruiting success (<21 %, compared with a mean of 37 % for rewarding species). However, Humaña et al. (2008) have already reported high fruiting success for four Chilean Chloraea taxa [approx. 35 % in C. bletiodes Lindl. (white form), approx. 80 % in C. bletiodes (yellow form), approx. 90 % in C. chrysantha Poepp. and approx. 90 % in C. galeata Lindl.], despite the fact that these orchids are rewardless and pollinator-dependent. Humaña et al. (2008) have also suggested that these high percentages may be explained in terms of good availability/abundance of natural pollinators. This may also partly explain the high fruiting values observed for C. membranacea. Augochlorini Halictid bees are common and widespread, even in anthropized or urban areas, where they are common residents of gardens and orchards. However, it is our opinion that some flower features, such as pollinarium texture, may also account for the high pollination success observed. As in many terrestrial orchids (e.g. Spiranthinae; Singer and Cocucci, 1999; Singer and Sazima, 1999, 2000), the pollinarium is friable, and thus the pollen content of a single pollinarium can be transferred to the stigmatic surfaces of several flowers. Even if there is some pollen loss (as shown by the calculated Nilsson's index = 0·68), the pollen content of a single pollinarium is still able to pollinate several flowers. Lehnebach and Riveros (2003) reported a very low visitation frequency for the Chilean species C. lamellata Lindl. These authors did not provide the final fruiting success value for this species, but reported that 71·3 % of the observed flowers acted as pollen donors, whereas 28·6 % acted as pollen receivers (Lehnebach and Riveros, 2003). From these data, a Nilsson's male efficiency factor of 0·4 can be calculated for C. lamellata, a value that is considerably lower than that obtained here for C. membranacea. These data indicate that pollinator availability/ abundance is a key factor in the fruiting success of Chloraeinae. A low (approx. 12 %) fruiting success was reported for the Chilean species C. crispa Lindl. (Humaña et al., 2008), a taxon for which pollination events were only rarely observed. With the exception of Chloraea, the natural pollination success of few other Chloraeinae orchids has been studied. A fruiting success of approx. 98 % has been reported for self-pollinating Gavilea araucana (Phil.) M.N. Correa, and one of approx. 28 % for allogamous G. venosa (Lam.) Garay & Ormerod (Valdivia et al., 2010). This latter value is slightly higher than the average obtained worldwide for other non-rewarding orchids (Tremblay et al., 2005). The considerable (albeit non-significant in statistical terms) numerical difference in fruiting success between 2010 and 2011 observed at the Flores da Cunha population could have been caused by climatic differences. Climatic data gathered at a local station support this idea (full data available on request). The results of t-tests indicate that the temperature was significantly higher during the 2011 sampling period (P = 0·006, 0·033 and 0·005 for minimum, maximum and averages, respectively) and that wind speed was also higher in 2011 (P = 0·004). These factors may have influenced pollinator availability and behaviour.
Pollination strategies within the Chloraeinae orchids
All the evidence indicates that the pollination of C. membranacea flowers involves a generalized food-fraud strategy, as already proposed for other species of the genus (Lehnebach and Riveros, 2003; Humaña et al., 2008). In fact, all Chloraeinae orchids studied to date lack nectar (Lehnebach and Riveros, 2003; Ciotek et al., 2006; Humaña et al., 2008; Valdivia et al., 2010). It should be stressed, however, that the beetle pollinators of C. bletioides actually use the flowers as a shelter and may even mate inside them. Therefore, it is likely that flowers of this particular species reward pollinators by these means, and this may account for its high fruiting success (Humaña et al., 2008). Hitherto, the flowers of only two species of Gavilea have been studied (Valdivia et al., 2010), both of which are said to be nectarless. One of the most remarkable distinguishing features of this genus is the presence of two, swollen, so-called ‘nectaries’ located at the base of the column (Correa and Sánchez, 2003; Cisternas et al., 2012a,b). Among the Chloraeinae, the pollination of Bipinnula penicillata (Ciotek et al., 2006) is noteworthy. Male wasps of Campsomeris bistrimacula (Scoliidae) attempt to copulate with the insect-like labellum, thereby precipitating pollination (Ciotek et al., 2006). This is the first report of pollination by sexual mimicry in terrestrial Neotropical orchids (Ciotek et al., 2006). Even though the pollination and breeding system of fewer than 15 % of Chloraeinae orchids have been studied, all those species investigated lack nectar and most employ deception of some kind (Lehnebach and Riveros, 2003; Ciotek et al., 2006; Humaña et al., 2008; Valdivia et al., 2010). Surprisingly, recent phylogenetic analyses suggest that Chloraeinae orchids are best placed within the tribe Cranichideae, as sister-group to a clade containing the subtribes Spiranthinae, Manniellinae, Cranichidinae and Goodyerinae (Chemisquy and Morrone, 2012; Cisternas et al., 2012a). By contrast, the flowers of all these latter subtribes are nectar-secreting (Singer and Cocucci, 1999; Singer and Sazima, 1999, 2000, 2001a, b; Singer, 2002).
A greater number of studies involving more species are now necessary if we are to understand fully the evolution of pollination strategies within Chloraeeae. Fortunately, the resolution of the phylogeny of this orchid group is imminent (Chemisquy and Morrone, 2012; Cisternas et al., 2012a). Hopefully, once a complete, representative and robust phylogeny becomes available, it can be used as a framework to understand both the evolution of pollination strategies and that of floral characters (features) associated with them. There remains, however, one further important task, namely the complete reappraisal and re-evaluation of the floral characters of these orchids. Contrary to many authors (e.g. Correa, 1969; Correa and Sánchez, 2003; Chemisquy and Morrone, 2010, 2012; Cisternas et al., 2012a), we have placed certain morphological terms used in this text between quotation marks, e.g. ‘nectariferous channels’ and ‘nectary’. The reason for this is that all available bibliography relating to pollination and breeding systems, as well as this present contribution, clearly indicates that the use of these terms is misleading. Indeed, when viewed from a functional standpoint, they are blatantly incorrect, as none of the species of Chloraea and Gavilea studied so far presents nectar either at so-called ‘nectariferous channels’ (Lehnebach and Riveros, 2003; Ciotek et al., 2006; Humaña et al., 2008) or at ‘nectaries’ located at the base of the column (Valdivia et al., 2010). Only by the careful reappraisal and scrutiny of these features in representative species can their true nature and their importance in the pollination process be properly assessed. In the meantime, the incorrect use of these terms in Chloraeinae orchids is to be discouraged. More specifically, we suggest here that terms such as foveae (plural of fovea, Latin for pit) or fossae (plural of fossa, Latin for trench or ditch) be used to replace the incorrect term ‘nectariferous channels’ in Chloraea species.
Future prospects and concluding remarks
This contribution represents the first detailed report of the breeding system and pollination of a non-Patagonian species of Chloraea, as well as the first detailed report of Halictidae bees pollinating members of the subtribe Chloraeinae. To our knowledge, this is also the first detailed account of floral anatomy for that subtribe. Shortly, we intend to extend our studies to other species, so as to investigate the entire floral diversity of Chloraeinae. As stated previously, a complete resolution of the phylogeny of Chloraeinae is imminent (Chemisquy and Morrone, 2010, 2012; Cisternas et al., 2012a). When a robust, fully representative and reliable phylogeny of Chloraeae becomes available, it will be possible to plot documented pollination strategies onto phylogenetic trees and propose possible pathways for their evolution. To achieve this, a complete re-evaluation of those floral characters involved in pollination is essential. These are tasks that we intend to address in the near future.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We wish to thank the staff of the Centro de Microscopia Eletrônica/UFRGS (CME) and Greta A. Dettke (PPGBOT-UFRGS) for their help during sample preparation and Embrapa Uva e Vinho for climatic data. C.R.B. and M.P. acknowledge their CAPES grants (PhD and MSc., respectively). A.S. thanks Hector Verna for horticultural assistance and gratefully acknowledges his CONICET grant (PhD). We also thank Carlos Lehnebach (Museum of New Zealand Te Papa Tongarewa) for making rare literature on Chilean Chloraeinae orchids available to us, as well as Arturo Roig-Alsina (Museo Argentino de Ciencias Naturales Bernardino Rivadavia) for identifying specimens of Argentinean halictid bees.
LITERATURE CITED
- Almeida JA. Fatores abióticos. In: Boldrini I, editor. Biodiversidade dos campos do planalto das araucárias. Brasília: MMA, Série Biodiversidade; 2009. pp. 19–38. [Google Scholar]
- Chemisquy A, Morrone O. A phylogenetic analysis of subtribe Chloraeinae: a preliminary approach based on three chloroplast markers. Australian Systematic Botany. 2010;23:38–46. [Google Scholar]
- Chemisquy A, Morrone A. Molecular phylogeny of Gavilea (Orchidaceae: Chloraeinae) Molecular Phylogenetics and Evolution. 2012;62:889–897. doi: 10.1016/j.ympev.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Ciotek L, Giorgis P, Benitez-Vieyra S, Cocucci AA. First confirmed case of pseudocopulation in terrestrial orchids of South America: pollination of Geoblasta pennicillata (Orchidaceae) by Campsomeris bistrimacula (Hymenoptera, Scoliidae) Flora. 2006;201:365–369. [Google Scholar]
- Cisternas MA, Salazar GA, Verdugo G, Novoa P, Calderón X, Negritto MA. Phylogenetic analysis of Chloraeinae (Orchidaceae) based on plastid and molecular sequences. Botanical Journal of the Linnean Society. 2012a;168:258–277. [Google Scholar]
- Cisternas MA, Salazar GA, Verdugo G. Transfer of Geoblasta pennicillata to Bipinnula (Chloraeinae, Orchidaceae) Phytotaxa. 2012b;64:9–10. [Google Scholar]
- Clayton S, Aizen M. Effects of pollinia removal and insertion on flower longevity in Chloraea alpina (Orchidaceae) Evolutionary Ecology. 1996;10:653–660. [Google Scholar]
- Correa MN. Chloraea, genero sudamericano de Orchidaceae. Darwiniana. 1969;15:374–500. [Google Scholar]
- Correa MN, Sánchez M. Chloraeeae. In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, editors. Genera Orchidacearum, vol. 3. Orchidoideae (part two) Oxford: Oxford University Press; 2003. pp. 5–16. [Google Scholar]
- Davies KL. Food-hair form and diversification in orchids. In: Kull T, Arditti J, Sek Man Wong, editors. Orchid biology: reviews and perspectives. X. 2009. pp. 159–184. Springer Science + Business Media B.V. [Google Scholar]
- Davies KL, Stpiczyńska M. Comparative histology of floral elaiophores in the orchids Rudolfiella picta (Schltr.) Hoehne (Maxillariinae sensu lato) and Oncidium ornithorhynchum H.B.K. (Oncidiinae sensu lato) Annals of Botany. 2009;104:221–234. doi: 10.1093/aob/mcp119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Stpiczyńska M. Comparative labellar anatomy of resin-secreting and putative resin-mimic species of Maxillaria s.l. (Orchidaceae: Maxillariinae) Botanical Journal of the Linnean Society. 2012 in press. http://dx.doi.org/10.1111/j.1095-8339.2012.01278.x . [Google Scholar]
- Davies KL, Turner MP. Morphology of floral papillae in Maxillaria Ruiz & Pav. (Orchidaceae) Annals of Botany. 2004;93:75–86. doi: 10.1093/aob/mch007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DCAO-FCEN-UBA. Climatología de la Ciudad de Buenos Aires. Universidad de Buenos Aires; 2012. Departamento de Ciencias de la Atmósfera y Oceános, Facultad de Ciencias Exactas y Naturales. http://www-atmo.at.fcen.uba.ar/banco/bsas.html . [Google Scholar]
- Dixon K, Tremblay RL. Biology and natural history of Caladenia. Australian Journal of Botany. 2009;57:247–258. [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Portland, OR: Dioscorides Press; 1993. [Google Scholar]
- Gumprecht R. Orchideen in Chile. Die Orchidee. 1975;26:127–132. [Google Scholar]
- Hauman L. Notes sur les Chloraea Lindley. Mémoires publiés par l'Académie royale de Belgique, Classe des sciences, ser. 2. 1921;6:1–31. [Google Scholar]
- Hauman L. La distribución geográfica del género Chloraea Lindl. Physis. 1922;5:293–295. [Google Scholar]
- Humaña AM, Cisternas MA, Valdivia CE. Breeding system and pollination of selected orchids of Chloraea genus from central Chile. Flora. 2008;203:469–473. [Google Scholar]
- Jensen WA. Botanical histochemistry: principle and practice. San Francisco: W.H. Freeman; 1962. [Google Scholar]
- Jones DL. New taxa of Australian Orchidaceae. Australian Orchid Foundation; 1991. Australian Orchid Research 2. [Google Scholar]
- Jones DL. A complete guide to native orchids of Australia, including the island territories. Sydney: New Holland Publishers; 2006. [Google Scholar]
- Kocyan A, Endress PK. Floral structure and development in Apostasia and Neuwiedia (Orchidaceae) and their relationships to other Orchidaceae. International Journal of Plant Sciences. 2001;162:847–867. [Google Scholar]
- Lehnebach CA, Riveros M. Pollination biology of the Chilean endemic orchid Chloraea lamellata. Biodiversity and Conservation. 2003;12:1741–1751. [Google Scholar]
- Mant J, Peakall R, Weston PH. Specific pollinator attraction and the diversification of sexually deceptive Chiloglottis (Orchidaceae) Plant Systematics and Evolution. 2005;253:185–200. [Google Scholar]
- Moreno JA. Clima do Rio Grande do Sul. Porto Alegre: Secretaria da Agricultura; 1961. [Google Scholar]
- Neiland MR, Wilcock CC. Fruit set, nectar reward and rarity in the Orchidaceae. American Journal of Botany. 1998;85:1657–1671. [PubMed] [Google Scholar]
- Nilsson LA, Rabakonandrianina E, Razananaivo R, Randriamanindry JJ. Long pollinia on eyes: hawkmoth pollination of Cynorkis uniflora Lindley (Orchidaceae) in Madagascar. Botanical Journal of the Linnean Society. 1992;109:145–160. [Google Scholar]
- Nimer E. Climatologia do Brasil. Rio de Janeiro: Supren; 1989. [Google Scholar]
- Pansarin ER, Amaral MCE. Pollen and nectar as a reward in the basal epidendroid Psilochilus modestus (Orchidaceae: Triphoreae): a study on floral morphology, reproductive biology and pollination strategy. Flora. 2008;203:474–483. [Google Scholar]
- Phillips RD, Faast R, Bower CC, Brown RG, Peakall R. Implications of pollination by food and sexual deception for pollinator specificity, fruit set, population genetics and conservation of Caladenia (Orchidaceae) Australian Journal of Botany. 2009;57:287–306. [Google Scholar]
- PMPA. Prefeitura Municipal de Porto Alegre. metroclima. 2012 http://www2.portoalegre.rs.gov.br/metroclima/ (accessed June 2012) [Google Scholar]
- Pridgeon AM, Stern WL. Ultrastructure of osmophores in Restrepia (Orchidaceae) American Journal of Botany. 1983;70:1233–1243. [Google Scholar]
- Pridgeon AM, Stern WL. Osmophores of Scaphosepalum (Orchidaceae) Botanical Gazette. 1985;146:115–123. [Google Scholar]
- Reiche K. Orchidaceae chilenses: ensayo de una monografía de las orquídeas de Chile. Anales del Museo Nacional de Chile, Segunda Sección, Botánica. 1910;18:1–88. [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain for electron microscopy. Journal of Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB. The pollination biology of Sauroglossum elatum (Orchidaceae: Spiranthinae): moth-pollination and protandry in neotropical Spiranthinae. Botanical Journal of the Linnean Society. 2002;138:9–16. [Google Scholar]
- Singer RB, Cocucci AA. Pollination mechanism in southern Brazilian orchids which are exclusively or mainly pollinated by halictid bees. Plant Systematics and Evolution. 1999;217:101–117. [Google Scholar]
- Singer RB, Koehler S. Pollinarium morphology and floral rewards in Brazilian Maxillariinae (Orchidaceae) Annals of Botany. 2004;93:39–51. doi: 10.1093/aob/mch009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB, Sazima M. The pollination mechanism in the ‘Pelexia alliance’ (Orchidaceae: Spiranthinae) Botanical Journal of the Linnean Society. 1999;131:249–262. [Google Scholar]
- Singer RB, Sazima M. The pollination of Stenorrhynchos lanceolatus (Aublet) L.C. Rich. (Orchidaceae: Spiranthinae) by hummingbirds in Southeastern Brazil. Plant Systematics and Evolution. 2000;223:221–227. [Google Scholar]
- Singer RB, Sazima M. Pollination mechanism in three sympatric Prescottia (Orchidaceae: Prescottinae) species from Southeastern Brazil. Annals of Botany. 2001a;88:999–1005. [Google Scholar]
- Singer RB, Sazima M. Flower morphology and pollination mechanisms in three sympatric Goodyerinae orchids from Southeastern Brazil. Annals of Botany. 2001b;88:989–997. [Google Scholar]
- Stern WL, Curry KJ, Pridgeon AM. Osmophores of Stanhopea (Orchidaceae) American Journal of Botany. 1987;74:1323–1331. [Google Scholar]
- Stpiczyńska M. Anatomy and ultrastructure of osmophores of Cymbidium tracyanum Rolfe (Orchidaceae) Acta Societatis Botanicorum Poloniae. 1993;62:5–9. [Google Scholar]
- Stpiczyńska M. Osmophores of the fragrant orchid Gymnadenia conopsea L. (Orchidaceae) Acta Societatis Botanicorum Poloniae. 2001;70:91–96. [Google Scholar]
- Szlachetko DL, Margonska HB. Genera et species Orchidalium. 4. Polish Botanical Journal. 2001;46:123–125. [Google Scholar]
- Szlachetko DL, Rutkowski P. Gynostemia Orchidalium I. Apostasiaceae, Cypripediaceae, Orchidaceae (Thelymitroideae, Orchidoideae, Tropidioideae, Spiranthoideae, Neottioideae, Vanilloideae) Acta Botanica Fennica. 2000;169:1–379. [Google Scholar]
- Szlachetko DL, Tukałło P. Notes on subtribe Chloraeinae (Orchidaceae) Acta Societatis Botanicorum Poloniae. 2008;77:111–116. [Google Scholar]
- Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biological Journal of the Linnean Society. 2005;84:1–54. [Google Scholar]
- Valdivia CE, Cisternas MA, Verdugo SG. Reproductive biology aspects of two species of Gavilea (Orchidaceae: Chloraeinae) Gayana Botanica. 2010;67:44–51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.