Abstract
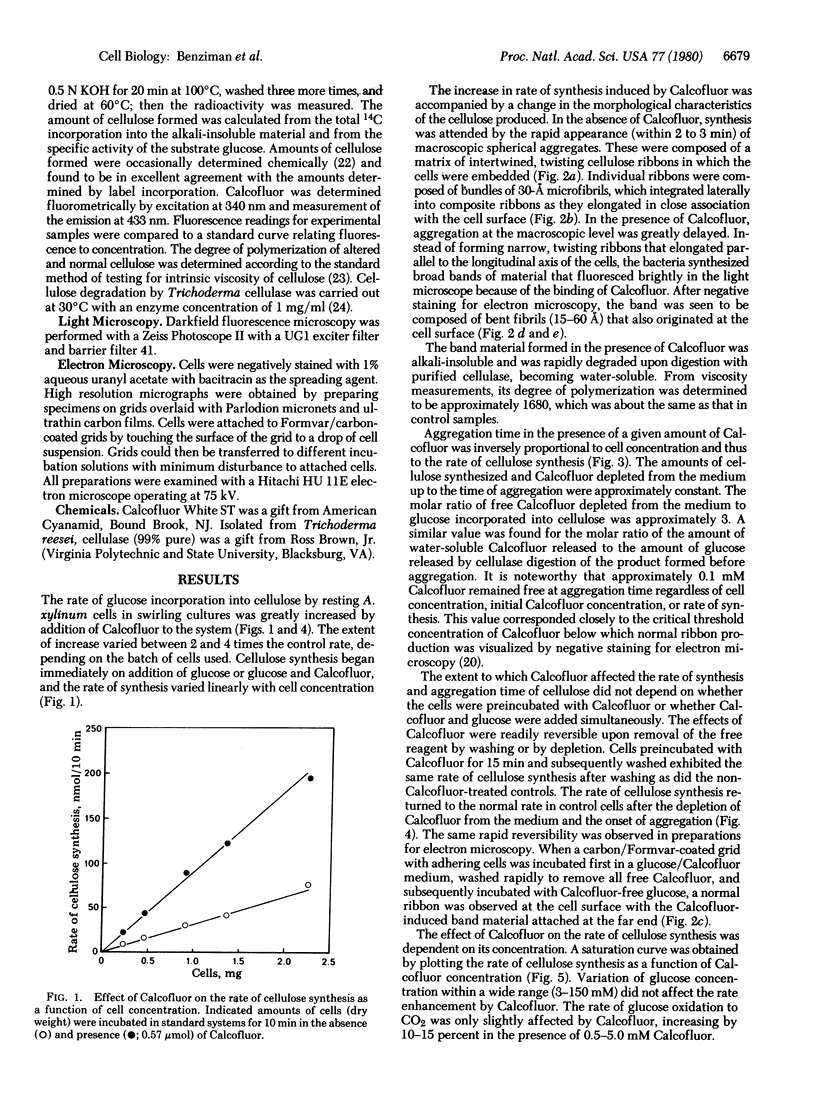
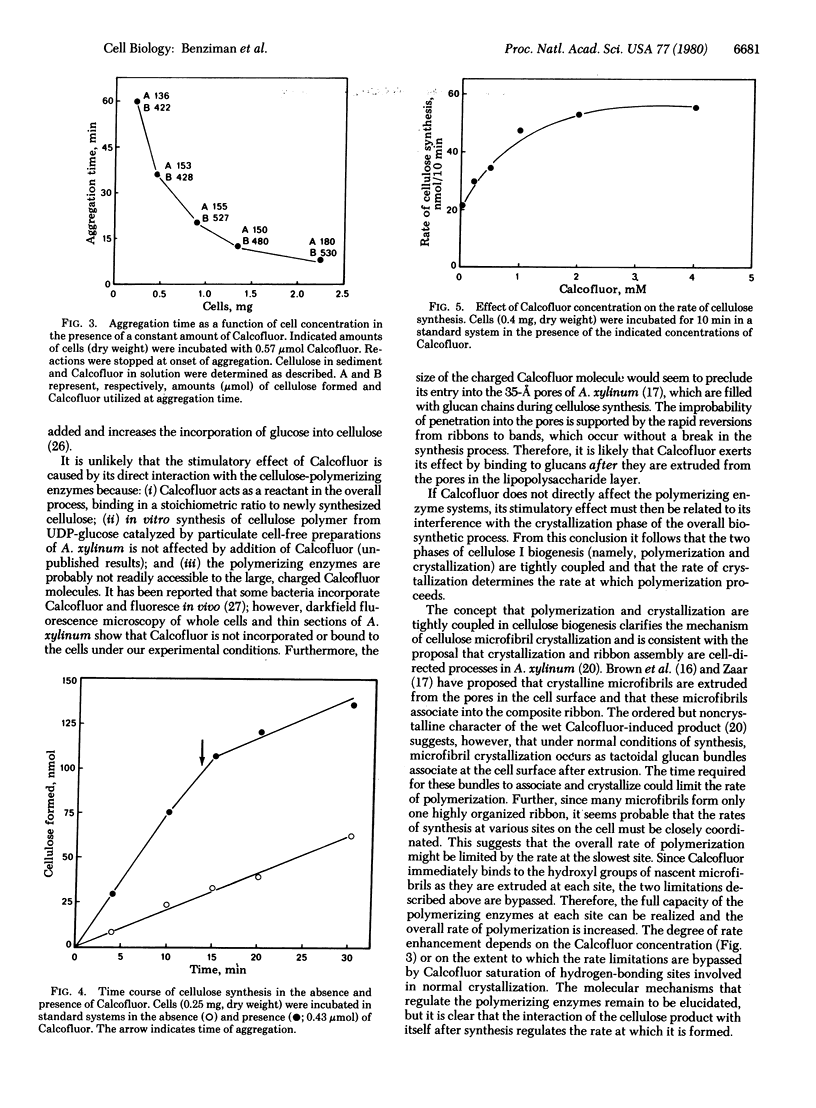
Calcofluor White ST, stilbene derivative used commerically as an optical brightener for cellulose, increased the rate of glucose polymerization into cellulose by resting cells of the gram-negative bacterium Acetobacter xylinum. This bacterium normally produces a ribbon of cellulose that is a composite of crystalline microfibrils. In concentrations above 0.1 mM, Calcofluor disrupts the assembly of crystalline cellulose I microfibrils and their integration into a composite ribbon by stoichiometric binding to glucose residues of newly polymerized glucan chains. Under these conditions, the rate of glucose polymerization increases up to 4 times the control rate, whereas oxygen uptake increases only 10-15%. These observed effects are readily reversible. If free Calcofluor is washed away or depleted below the threshold value by binding to cellulose as polymerization continues, ribbon production and the normal rate of polymerization resume. It is concluded that polymerization and crystallization are cell-directed, coupled processes and that the rate of crystallization determines the rate of polymerization. It is suggested that coupling must be maintained for biogenesis of crystalline cellulose I.
Keywords: Calcofluor White, microfibril assembly, hydrogen bonding
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell J., Parker K. D., Rudall K. M. Chitin fibres of the diatoms Thalassiosira fluviatilis and Cyclotella cryptica. J Mol Biol. 1967 Sep 14;28(2):383–385. doi: 10.1016/s0022-2836(67)80018-4. [DOI] [PubMed] [Google Scholar]
- Bracker C. E., Ruiz-Herrera J., Bartnicki-Garcia S. Structure and transformation of chitin synthetase particles (chitosomes) during microfibril synthesis in vitro. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4570–4574. doi: 10.1073/pnas.73.12.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Jr, Montezinos D. Cellulose microfibrils: visualization of biosynthetic and orienting complexes in association with the plasma membrane. Proc Natl Acad Sci U S A. 1976 Jan;73(1):143–147. doi: 10.1073/pnas.73.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Jr, Willison J. H., Richardson C. L. Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4565–4569. doi: 10.1073/pnas.73.12.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D., Manley R. S. Cellulose synthesis by Acetobacter xylinum. III. Matrix, primer and lipid requirements and heat stability of the cellulose-forming enzymes. Biochim Biophys Acta. 1975 Jan 13;381(1):109–119. doi: 10.1016/0304-4165(75)90193-2. [DOI] [PubMed] [Google Scholar]
- DARKEN M. A. Absorption and transport of fluorescent brighteners by microorganisms. Appl Microbiol. 1962 Sep;10:387–393. doi: 10.1128/am.10.5.387-393.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings T. H., Jr, Brower D. L., Staehelin L. A. Visualization of particle complexes in the plasma membrane of Micrasterias denticulata associated with the formation of cellulose fibrils in primary and secondary cell walls. J Cell Biol. 1980 Feb;84(2):327–339. doi: 10.1083/jcb.84.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herth W. The site of beta-chitin fibril formation in centric diatoms. II. The chitin-forming cytoplasmic structures. J Ultrastruct Res. 1979 Jul;68(1):16–27. doi: 10.1016/s0022-5320(79)90138-2. [DOI] [PubMed] [Google Scholar]
- Hopp H. E., Romero P. A., Daleo G. R., Pont Lezica R. Synthesis of cellulose precursors. The involvement of lipid-linked sugars. Eur J Biochem. 1978 Mar 15;84(2):561–571. doi: 10.1111/j.1432-1033.1978.tb12199.x. [DOI] [PubMed] [Google Scholar]
- Maeda H., Ishida N. Specificity of binding of hexopyranosyl polysaccharides with fluorescent brightener. J Biochem. 1967 Aug;62(2):276–278. doi: 10.1093/oxfordjournals.jbchem.a128660. [DOI] [PubMed] [Google Scholar]
- Minke R., Blackwell J. The structure of alpha-chitin. J Mol Biol. 1978 Apr 5;120(2):167–181. doi: 10.1016/0022-2836(78)90063-3. [DOI] [PubMed] [Google Scholar]
- Mueller S. C., Brown R. M., Jr Evidence for an intramembrane component associated with a cellulose microfibril-synthesizing complex in higher plants. J Cell Biol. 1980 Feb;84(2):315–326. doi: 10.1083/jcb.84.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. C., Brown R. M., Jr, Scott T. K. Cellulosic microfibrils: nascent stages of synthesis in a higher plant cell. Science. 1976 Nov 26;194(4268):949–951. doi: 10.1126/science.194.4268.949. [DOI] [PubMed] [Google Scholar]
- Swissa M., Aloni Y., Weinhouse H., Benizman M. Intermediatry steps in Acetobacter xylinum cellulose synthesis: studies with whole cells and cell-free preparations of the wild type and a celluloseless mutant. J Bacteriol. 1980 Sep;143(3):1142–1150. doi: 10.1128/jb.143.3.1142-1150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse H., Benziman M. Regulation of gluconeogenesis in Acetobacter xylinum. Eur J Biochem. 1972 Jun 23;28(1):83–88. doi: 10.1111/j.1432-1033.1972.tb01886.x. [DOI] [PubMed] [Google Scholar]
- Weinhouse H., Benziman M. Regulation of hexose phosphate metabolism in Acetobacter xylinum. Biochem J. 1974 Mar;138(3):537–542. doi: 10.1042/bj1380537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison J. H., Brown R. M., Jr Cell wall structure and deposition in Glaucocystis. J Cell Biol. 1978 Apr;77(1):103–119. doi: 10.1083/jcb.77.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaar K. Visualization of pores (export sites) correlated with cellulose production in the envelope of the gram-negative bacterium Acetobacter xylinum. J Cell Biol. 1979 Mar;80(3):773–777. doi: 10.1083/jcb.80.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]



