Abstract
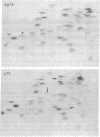
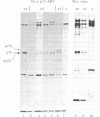
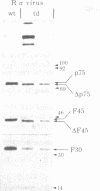
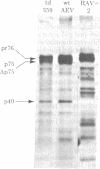
Previous studies have shown that td359 AEV, a mutant of avian erythroblastosis virus (AEV), is unable to transform erythroblasts in vitro or in vivo but is capable of transforming fibroblasts in vitro and of causing sarcomas in chicks. In this paper we show that the mutant synthesizes a gag-gene related protein (delta p75) which is about 1000 daltons smaller than the protein, p75, induced by wild-type AEV. The mutant protein lacks 3 of the approximately 53 lysine-arginine tryptic peptides resolved in p75 and also contains an additional peptide. By cleavage of delta p75 with p15 protease and analysis of the fragments for size and peptide composition, the deletion in delta p75 could be located in the non-gag region of the molecule. In contrast, with p40 AEV, a second AEV-specific protein synthesized in in vitro translation experiments, there is no change in size of translation products obtained from td359 AEV RNA. Our data provide direct evidence that p75 is required for erythroblast transformation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Graf T. Transformation parameters of chicken embryo fibroblasts infected with the ts34 mutant of avian erythroblastosis virus. Virology. 1980 Jan 30;100(2):348–356. doi: 10.1016/0042-6822(80)90526-7. [DOI] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Genetic structure of avian acute leukemia viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):801–822. doi: 10.1101/sqb.1980.044.01.086. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Structure and specific sequences of avian erythroblastosis virus RNA: evidence for multiple classes of transforming genes among avian tumor viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5023–5027. doi: 10.1073/pnas.76.10.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H., von Kirchbach A., Hayman M. J. Three new types of viral oncogenes in defective avian leukemia viruses. II. Biological, genetic, and immunochemical evidence. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1225–1234. doi: 10.1101/sqb.1980.044.01.133. [DOI] [PubMed] [Google Scholar]
- Graf T., Fink D., Beug H., Royer-Pokora B. Oncornavirus-induced sarcoma formation obscured by rapid development of lethal leukemia. Cancer Res. 1977 Jan;37(1):59–63. [PubMed] [Google Scholar]
- Graf T. In vitro transformation of chicken bone marrow cells with avian erythroblastosis virus. Z Naturforsch C. 1975 Nov-Dec;30(6):847–849. doi: 10.1515/znc-1975-11-1232. [DOI] [PubMed] [Google Scholar]
- Graf T., Royer-Pokora B., Schubert G. E., Beug H. Evidence for the multiple oncogenic potential of cloned leukemia virus: in vitro and in vitro studies with avian erythroblastosis virus. Virology. 1976 Jun;71(2):423–433. doi: 10.1016/0042-6822(76)90370-6. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Royer-Pokora B., Graf T. Defectiveness of avian erythroblastosis virus: synthesis of a 75K gag-related protein. Virology. 1979 Jan 15;92(1):31–45. doi: 10.1016/0042-6822(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kitchener G., Hayman M. J. Comparative tryptic peptide mapping studies suggest a role in cell transformation for the gag-related protein of avian erythroblastosis virus and avian myelocytomatosis virus strains CMII and MC29. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1637–1641. doi: 10.1073/pnas.77.3.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Hu S. S., Vogt P. K. Avian erythroblastosis virus: transformation-specific sequences form a contiguous segment of 3.25 kb located in the middle of the 6-kb genome. Virology. 1979 Sep;97(2):366–377. doi: 10.1016/0042-6822(79)90347-7. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Neil J. C., Vogt P. K. Cell-free translation of avian erythroblastosis virus RNA yields two specific and distinct proteins with molecular weights of 75,000 and 40,000. Virology. 1980 Jan 30;100(2):475–483. doi: 10.1016/0042-6822(80)90537-1. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Neil J. C., Vogt P. K. Cell-free translation of avian erythroblastosis virus RNA yields two specific and distinct proteins with molecular weights of 75,000 and 40,000. Virology. 1980 Jan 30;100(2):475–483. doi: 10.1016/0042-6822(80)90537-1. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Martin G. S. Cell-free translation of avian erythroblastosis virus RNA. J Virol. 1980 Apr;34(1):280–284. doi: 10.1128/jvi.34.1.280-284.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Anderson S. M., Riemen M. W., Hanafusa H. gag-Related polypeptides encoded by replication-defective avian oncoviruses. J Virol. 1979 Dec;32(3):749–761. doi: 10.1128/jvi.32.3.749-761.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Grieser S., Beug H., Graf T. Mutant avian erythroblastosis virus with restricted target cell specificity. Nature. 1979 Dec 13;282(5740):750–752. doi: 10.1038/282750a0. [DOI] [PubMed] [Google Scholar]