Abstract
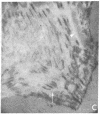
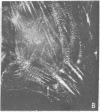
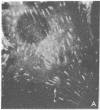
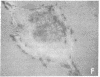
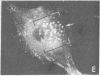
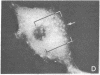
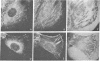
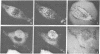
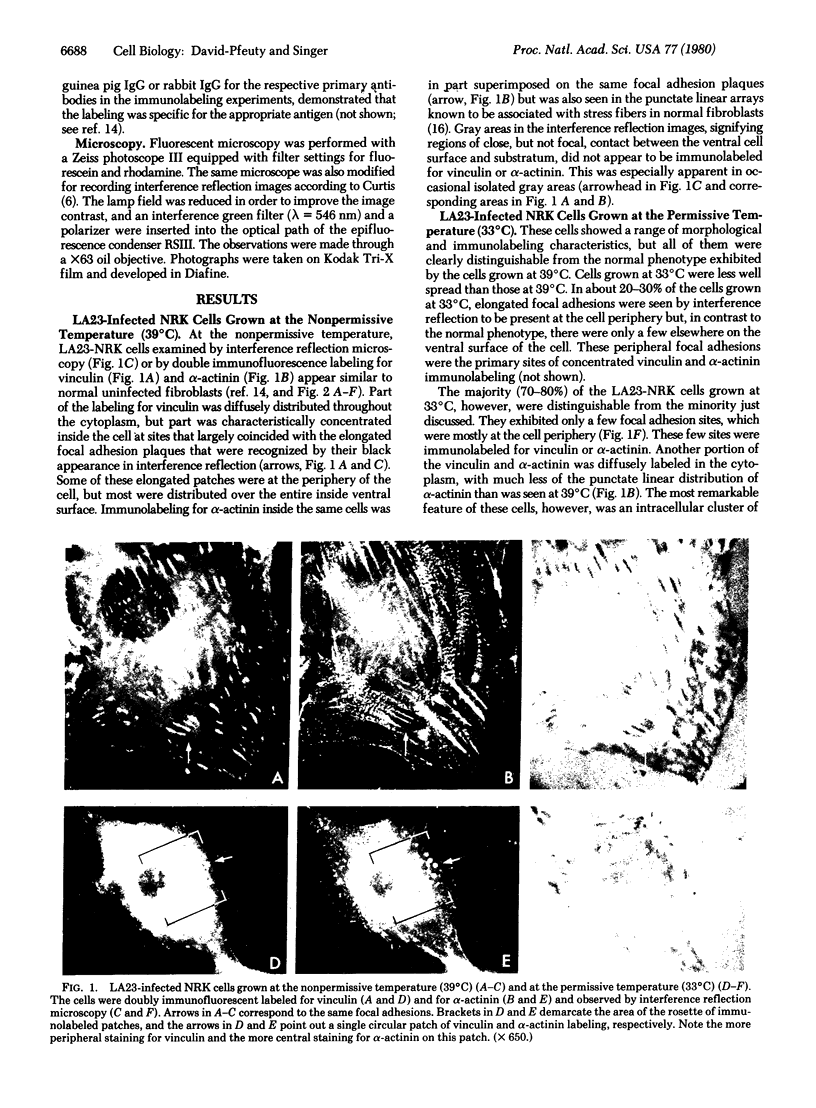
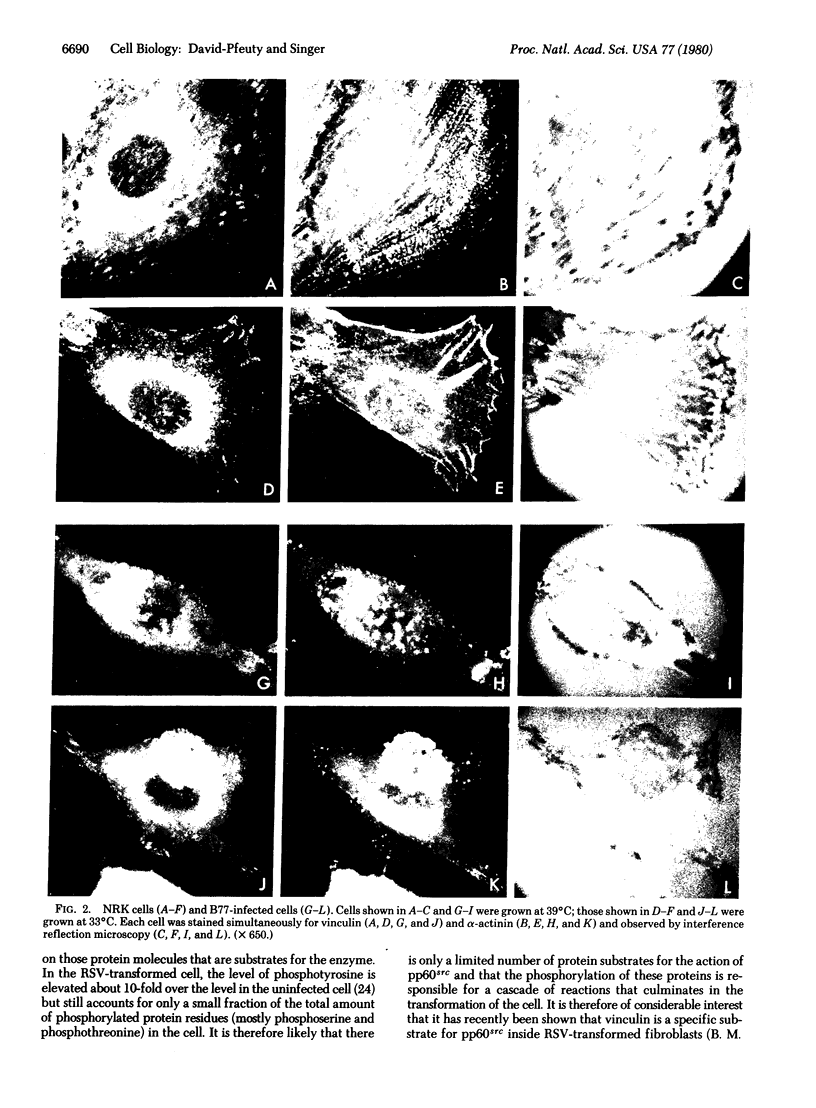
It was recently shown by combined immunofluorescence and interference reflection microscopy that a protein named vinculin, along with alpha-actinin, is concentrated at focal adhesion plaques inside cultured normal fibroblasts [Geiger, B. (1979) Cell 18, 193-205]. These plaques are the discrete, isolated sites of strong adhesions formed between the ventral surfaces of the cells and the substrata on which they are grown. We show that after transformation of fibroblasts by Rous sarcoma virus a majority of the cells have many fewer focal adhesion plaques and now exhibit a cluster of small patches that are immunolabelled for both vinculin and alpha-actinin. Such a cluster (rosette) is located near the ventral surface of the cell, usually partly under the nucleus. The significance that these altered distributions of vinculin and alpha-actinin may have for the rounding up and loss of adherence of transformed cells is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Dunn G. A. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp Cell Res. 1975 Apr;92(1):57–62. doi: 10.1016/0014-4827(75)90636-9. [DOI] [PubMed] [Google Scholar]
- Ash J. F., Vogt P. K., Singer S. J. Reversion from transformed to normal phenotype by inhibition of protein synthesis in rat kidney cells infected with a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3603–3607. doi: 10.1073/pnas.73.10.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter-Hahn J., Fox C. H., Thorell B. Quantitative reflection contrast microscopy of living cells. J Cell Biol. 1979 Sep;82(3):767–779. doi: 10.1083/jcb.82.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Steinbaugh P. J., Erikson R. L. Characterization of the avian sarcoma virus protein p60src. Virology. 1978 Nov;91(1):130–140. doi: 10.1016/0042-6822(78)90361-6. [DOI] [PubMed] [Google Scholar]
- Burridge K., Feramisco J. R. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell. 1980 Mar;19(3):587–595. doi: 10.1016/s0092-8674(80)80035-3. [DOI] [PubMed] [Google Scholar]
- CURTIS A. S. THE MECHANISM OF ADHESION OF CELLS TO GLASS. A STUDY BY INTERFERENCE REFLECTION MICROSCOPY. J Cell Biol. 1964 Feb;20:199–215. doi: 10.1083/jcb.20.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A. H., Tokuyasu K. T., Singer S. J. Iron-dextran antibody conjugates: General method for simultaneous staining of two components in high-resolution immunoelectron microscopy. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3392–3396. doi: 10.1073/pnas.76.7.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Geiger B., Tokuyasu K. T., Dutton A. H., Singer S. J. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D., Yerna M. J., Schloss J. A. Localization and organization of microfilaments and related proteins in normal and virus-transformed cells. J Supramol Struct. 1976;5(2):155–183. doi: 10.1002/jss.400050206. [DOI] [PubMed] [Google Scholar]
- Heath J. P., Dunn G. A. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. J Cell Sci. 1978 Feb;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. J Cell Sci. 1976 Jun;21(1):129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J Cell Biol. 1973 Feb;56(2):412–428. doi: 10.1083/jcb.56.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. A., Lloyd C. W., Thom D. Control of grip and stick in cell adhesion through lateral relationships of membrane glycoproteins. Nature. 1977 May 12;267(5607):124–128. doi: 10.1038/267124a0. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L. R. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R. Immunofluorescence on avian sarcoma virus-transformed cells: localization of the src gene product. Cell. 1979 Jan;16(1):11–24. doi: 10.1016/0092-8674(79)90183-1. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M. Mechanisms of morphogenesis in cell cultures. Int Rev Cytol. 1977;50:159–274. doi: 10.1016/s0074-7696(08)60099-6. [DOI] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J., Hale A. H., Losasso L. Decreased adherence to the substrate in Rous sarcoma virus-transformed chicken embryo fibroblasts. Cell. 1977 Jan;10(1):45–51. doi: 10.1016/0092-8674(77)90138-6. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Jay G., Pastan I. Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1979 Sep;18(1):125–134. doi: 10.1016/0092-8674(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Wyke J. A. The selective isolation of temperature-sensitive mutants of Rous sarcoma virus. Virology. 1973 Apr;52(2):587–590. doi: 10.1016/0042-6822(73)90357-7. [DOI] [PubMed] [Google Scholar]