Abstract
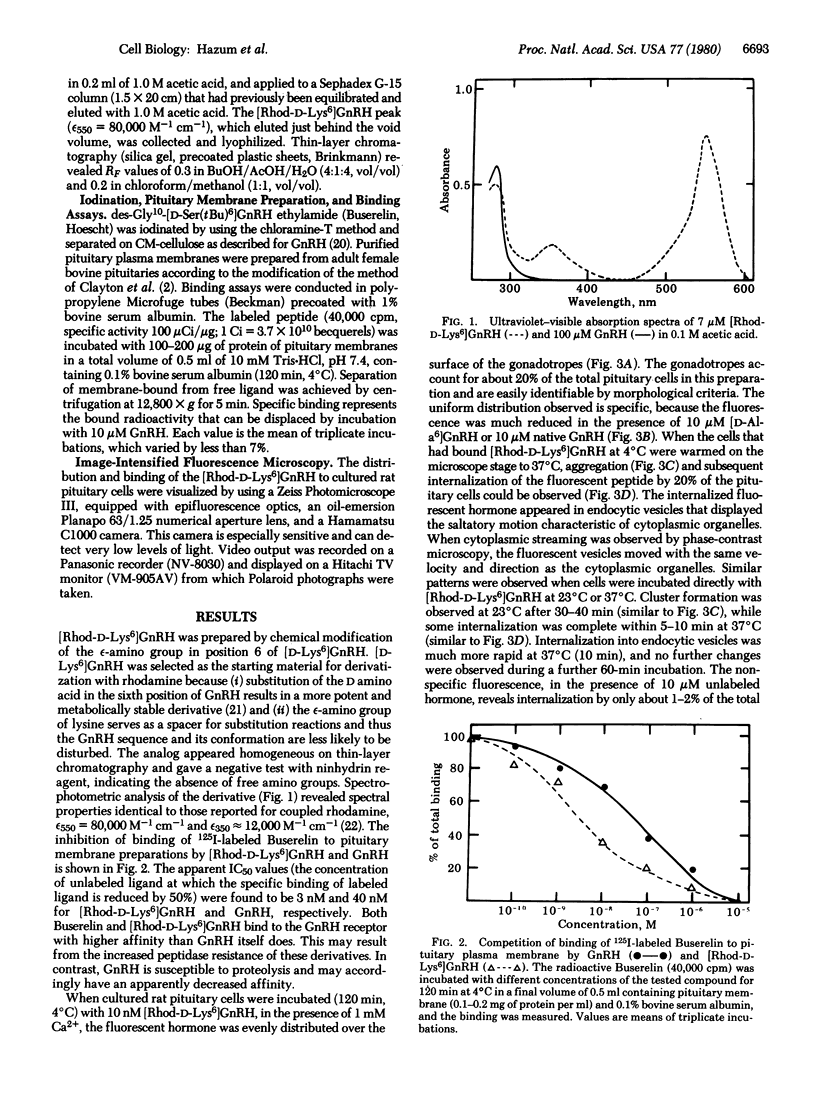
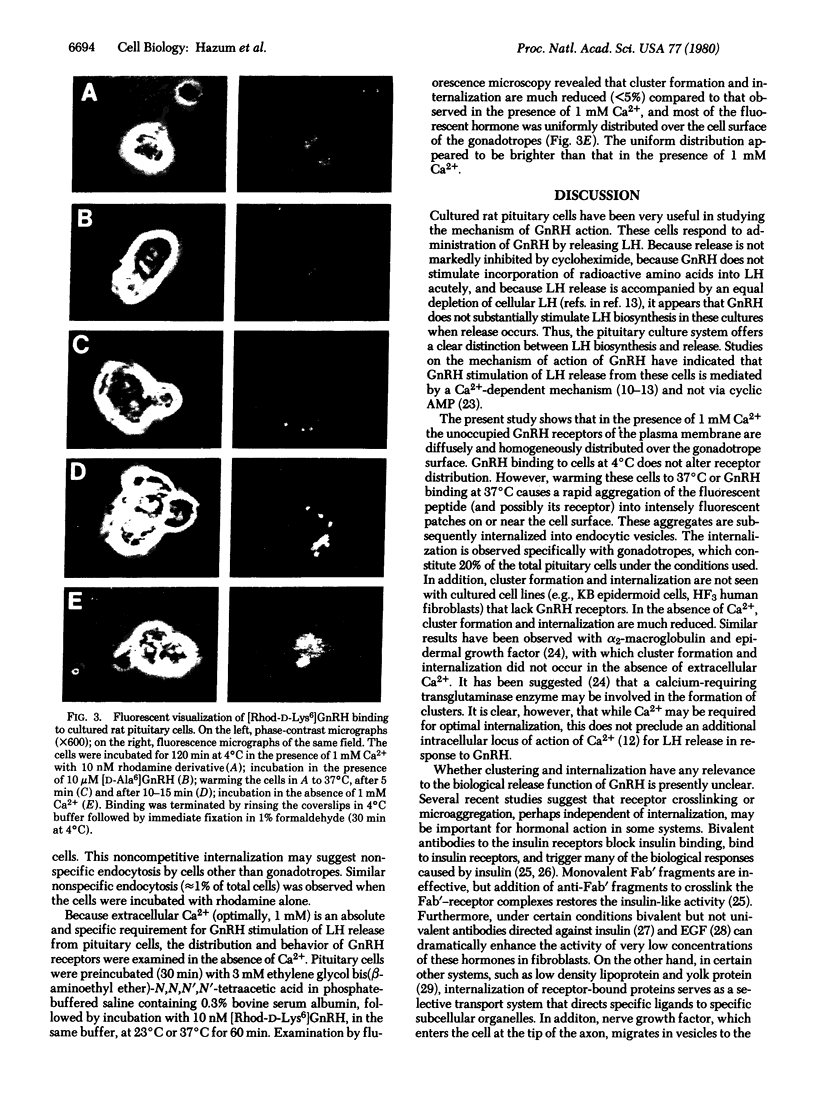
A bioactive, fluorescent derivative of gonadotropin-releasing hormone, < Glu-His-Trp-Ser-Tyr-D-Lys(N epsilon-tetramethylrhodamine)-Leu-Arg-Pro-Gly-NH2, was prepared. This peptide retained high-affinity binding (apparent dissociation constant, 3 nM) to the receptor for gonadotropin-releasing hormone and was utilized for microscopic visualization and localization of gonadotropin-releasing hormone receptors in cultured rat pituitary cells. The fluorescently labeled receptors were initially distributed uniformly on the cell surface and formed patches, which subsequently internalized (at 37 degrees C) into endocytic vesicles. These processes were dependent on specific binding sites for the rhodamine-labeled peptide to gonadotrope cells. Cluster formation and internalization were markedly reduced in the absence of Ca2+, which is required for gonadotropin secretion. It is possible that cluster formation, microaggregation, and internalization of gonadotropin-releasing hormone receptors may be important in eliciting biological effects or for the observed loss of tissue responsiveness after desensitization due to exposure to the homologous hormone.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgeat P., Chavancy G., Dupont A., Labrie F., Arimura A., Schally A. V. Stimulation of adenosine 3':5'-cyclic monophosphate accumulation in anterior pituitary gland in vitro by synthetic luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2677–2681. doi: 10.1073/pnas.69.9.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw R. A. Nerve growth factor. Annu Rev Biochem. 1978;47:191–216. doi: 10.1146/annurev.bi.47.070178.001203. [DOI] [PubMed] [Google Scholar]
- Clayton R. N., Shakespear R. A., Duncan J. A., Marshall J. C., Munson P. J., Rodbard D. Radioiodinated nondegradable gonadotropin-releasing hormone analogs: new probes for the investigation of pituitary gonadotropin-releasing hormone receptors. Endocrinology. 1979 Dec;105(6):1369–1376. doi: 10.1210/endo-105-6-1369. [DOI] [PubMed] [Google Scholar]
- Clayton R. N., Shakespear R. A., Marshall J. C. LH-RH binding to purified pituitary plasma membranes: absence of adenylate cyclase activation. Mol Cell Endocrinol. 1978 Jun;11(1):63–78. doi: 10.1016/0303-7207(78)90033-3. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Morrell D. V., Dufau M. L., Catt K. J. Gonadotropin-releasing hormone action in cultured pituicytes: independence of luteinizing hormone release and adenosine 3',5'-monophosphate production. Endocrinology. 1979 Feb;104(2):448–453. doi: 10.1210/endo-104-2-448. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Rogers D. C. Restoration of responsiveness to gonadotropin releasing hormone (GnRH) in calcium-depleted rat pituitary cells. Life Sci. 1979 Jun 25;24(26):2461–2465. doi: 10.1016/0024-3205(79)90456-9. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Rogers D. C., Sandhu F. S. Alteration of the intracellular calcium level stimulates gonadotropin release from cultured rat anterior pituitary cells. Endocrinology. 1979 Nov;105(5):1122–1127. doi: 10.1210/endo-105-5-1122. [DOI] [PubMed] [Google Scholar]
- Conne B. S., Aubert M. L., Sizonenko P. C. Quantification of pituitary membrane receptor sites to LHRH: use of a superactive analog as tracer. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1249–1256. doi: 10.1016/0006-291x(79)91171-9. [DOI] [PubMed] [Google Scholar]
- Fraser H. M., Laird N. C., Blakeley D. M. Decreased pituitary responsiveness and inhibition of the luteinizing hormone surge and ovulation in the stumptailed monkey (Macaca arctoides) by chronic treatment with an agonist of luteinizing hormone-releasing hormone. Endocrinology. 1980 Feb;106(2):452–457. doi: 10.1210/endo-106-2-452. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Hazum E., Chang K. J., Cuatrecasas P. Opiate (Enkephalin) receptors of neuroblastoma cells: occurrence in clusters on the cell surface. Science. 1979 Nov 30;206(4422):1077–1079. doi: 10.1126/science.227058. [DOI] [PubMed] [Google Scholar]
- Hazum E., Chang K. J., Cuatrecasas P. Role of disulphide and sulphydryl groups in clustering of enkephalin receptors in neuroblastoma cells. Nature. 1979 Dec 6;282(5739):626–628. doi: 10.1038/282626a0. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Gregory H. Topographical localization of the receptors for luteinizing hormone-releasing hormone on the surface of dissociated pituitary cells. J Cell Biol. 1977 Nov;75(2 Pt 1):528–540. doi: 10.1083/jcb.75.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch Y., Baram T., Hazum E., Fridkin M. Resistance to enzymic degradation of LH-RH analogues possessing increased biological activity. Biochem Biophys Res Commun. 1977 Jan 24;74(2):488–491. doi: 10.1016/0006-291x(77)90330-8. [DOI] [PubMed] [Google Scholar]
- Labrie F., Borgeat P., Lemay A., Lemaire S., Barden N., Drouin J., Lemaire I., Jolicoeur P., Bélanger A. Role of cyclic AMP in the action of hypothalamic regulatory hormones. Adv Cyclic Nucleotide Res. 1975;5:787–801. [PubMed] [Google Scholar]
- Marchisio P. C., Naldini L., Calissano P. Intracellular distribution of nerve growth factor in rat pheochromocytoma PC12 cells: evidence for a perinuclear and intranuclear location. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1656–1660. doi: 10.1073/pnas.77.3.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian J., Conn P. M. Gonadotropin releasing hormone stimulation of cultured pituitary cells requires calcium. Mol Pharmacol. 1979 Jul;16(1):196–201. [PubMed] [Google Scholar]
- Marian J., Conn P. M. The calcium requirement in GnRH-stimulated LH release is not mediated through a specific action on receptor binding. Life Sci. 1980 Jul 7;27(1):87–92. doi: 10.1016/0024-3205(80)90022-3. [DOI] [PubMed] [Google Scholar]
- Marshall J. C., Odell W. D. Preparation of biologically active 125-I LH-RH suitable membrane-binding studies. Proc Soc Exp Biol Med. 1975 Jun;149(2):351–355. doi: 10.3181/00379727-149-38806. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., Schlessinger J., Shechter Y., Pastan I., Willingham M. C. Collection of insulin, EGF and alpha2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978 Aug;14(4):805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., Willingham M. C., Davies P. J., Pastan I. Amines inhibit the clustering of alpha2-macroglobulin and EGF on the fibroblast cell surface. Nature. 1979 Feb 22;277(5698):661–663. doi: 10.1038/277661a0. [DOI] [PubMed] [Google Scholar]
- Naor Z., Koch Y., Chobsieng P., Zor U. Pituitary cyclic AMP production and mechanism of luteinizing hormone release. FEBS Lett. 1975 Oct 15;58(1):318–321. doi: 10.1016/0014-5793(75)80288-2. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kahane I., Cuatrecasas P. Receptor-mediated internalization of fluorescent chemotactic peptide by human neutrophils. Science. 1979 Sep 28;205(4413):1412–1414. doi: 10.1126/science.472759. [DOI] [PubMed] [Google Scholar]
- Schechter Y., Hernaez L., Schlessinger J., Cuatrecasas P. Local aggregation of hormone-receptor complexes is required for activation by epidermal growth factor. Nature. 1979 Apr 26;278(5707):835–838. doi: 10.1038/278835a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Chang K. J., Jacobs S., Cuatrecasas P. Modulation of binding and bioactivity of insulin by anti-insulin antibody: relation to possible role of receptor self-aggregation in hormone action. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2720–2724. doi: 10.1073/pnas.76.6.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Petrali J. P. Quantitative immunocytochemistry of pituitary receptors for luteinizing hormone-releasing hormone. Cell Tissue Res. 1975 Sep 17;162(2):141–176. doi: 10.1007/BF00209204. [DOI] [PubMed] [Google Scholar]
- Sundberg D. K., Fawcett C. P., McCann S. M. The involvement of cyclic-3',5'-AMP in the release of hormones from the anterior pituitary in vitro. Proc Soc Exp Biol Med. 1976 Jan;151(1):149–154. doi: 10.3181/00379727-151-39163. [DOI] [PubMed] [Google Scholar]
- Tang L. K., Spies H. G. Effect of synthetic LH-releasing factor (LRF) on LH secretion in monolayer cultures of the anterior pituitary cells of cynomolgus monkeys. Endocrinology. 1974 Apr;94(4):1016–1021. doi: 10.1210/endo-94-4-1016. [DOI] [PubMed] [Google Scholar]







