Abstract
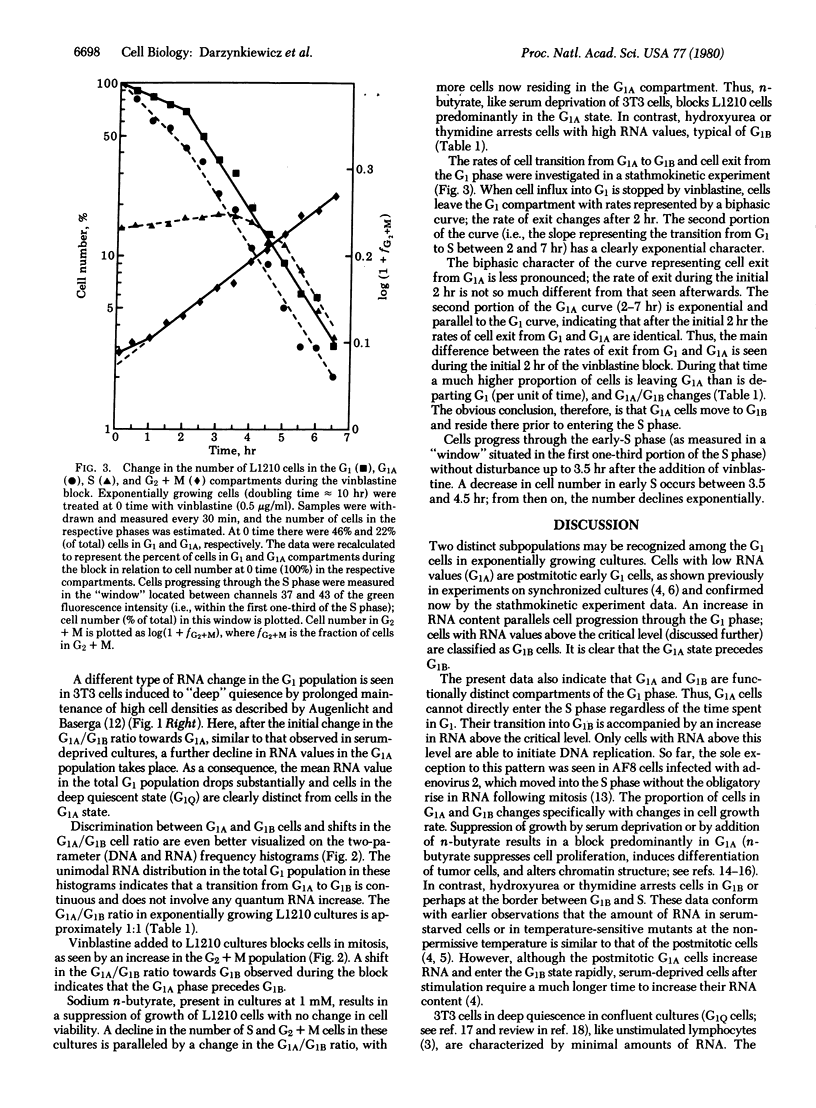
Cellular DNA and RNA were simultaneously quantitated in individual cells of cycling and quiescent populations by flow cytometry. Based on differences in RNA content, two distinct subcompartments, G1A and G1B, were recognized in the G1 phase of exponentially growing cells. After mitosis, cells reside in the low-RNA, G1A compartment from which they cannot enter the S phase directly. An increase in RNA above a critical level is required for G1 cells to be able to initiate DNA replication; G1 cells with RNA values above this level are classified as G1B. Cell transition from G1A to G1B and from G1B to S was analyzed in a stathmokinetic experiment on L1210 cells with a doubling time of 10 hr. The half-time of cell residence in the indeterminate state of G1 was found to be 1 hr, and the duration of the deterministic portion of G1 phase was 2 hr. The indeterminate state, although not identical with G1A, is most likely located in G1A. Cell quiescence induced by serum deprivation (3T3 cells) or by addition of n-butyrate (L1210) results in cell arrest at a state which, judged by RNA content, is similar to that of G1A of exponentially growing cells. The exit from this state, however, is much slower after stimulation of these blocked cells than the transition of G1A to G1B in cells growing exponentially. Thymidine or hydroxyurea arrest cells in G1B or perhaps at the border between G1B and S. Prolonged incubation of 3T3 cells to confluence results in a marked loss of cellular RNA below the level of the G1A state. This deep quiescent state (G1Q) is distinctly different from the G1A state of cycling cells or cells blocked in G1A by serum deprivation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashihara T., Traganos F., Baserga R., Darzynkiewicz Z. A comparison of cell cycle-related changes in postmitotic and quiescent AF8 cells as measured by cytofluorometry after acridine orange staining. Cancer Res. 1978 Aug;38(8):2514–2518. [PubMed] [Google Scholar]
- Augenlicht L. H., Baserga R. Changes in the G0 state of WI-38 fibroblasts at different times after confluence. Exp Cell Res. 1974 Dec;89(2):255–262. doi: 10.1016/0014-4827(74)90789-7. [DOI] [PubMed] [Google Scholar]
- Bauer K. D., Dethlefsen L. A. Total cellular RNA content: correlation between flow cytometry and ultraviolet spectroscopy. J Histochem Cytochem. 1980 Jun;28(6):493–498. doi: 10.1177/28.6.6156196. [DOI] [PubMed] [Google Scholar]
- Coulson P. B., Bishop A. O., Lenarduzzi R. Quantitation of cellular deoxyribonucleic acid by flow microfluorometry. J Histochem Cytochem. 1977 Oct;25(10):1147–1153. doi: 10.1177/25.10.72097. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Evenson D. P., Staiano-Coico L., Sharpless T. K., Melamed M. L. Correlation between cell cycle duration and RNA content. J Cell Physiol. 1979 Sep;100(3):425–438. doi: 10.1002/jcp.1041000306. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Evenson D., Staiano-Coico L., Sharpless T., Melamed M. R. Relationship between RNA content and progression of lymphocytes through S phase of cell cycle. Proc Natl Acad Sci U S A. 1979 Jan;76(1):358–362. doi: 10.1073/pnas.76.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Melamed M. R. New cell cycle compartments identified by multiparameter flow cytometry. Cytometry. 1980 Sep;1(2):98–108. doi: 10.1002/cyto.990010203. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T. K., Melamed M. R. Cell cycle-related changes in nuclear chromatin of stimulated lymphocytes as measured by flow cytometry. Cancer Res. 1977 Dec;37(12):4635–4640. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Conformation of RNA in situ as studied by acridine orange staining and automated cytofluorometry. Exp Cell Res. 1975 Oct 1;95(1):143–153. doi: 10.1016/0014-4827(75)90619-9. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Lymphocyte stimulation: a rapid multiparameter analysis. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2881–2884. doi: 10.1073/pnas.73.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora M., Darzynkiewicz Z., Baserga R. DNA synthesis and cell division in a mammalian cell mutant temperature sensitive for the processing of ribosomal RNA. Exp Cell Res. 1980 Jan;125(1):241–249. doi: 10.1016/0014-4827(80)90208-6. [DOI] [PubMed] [Google Scholar]
- Pochron S., Rossini M., Darzynkiewicz Z., Traganos F., Baserga R. Failure of accumulation of cellular RNA in hamster cells stimulated to synthesize DNA by infection with adenovirus 2. J Biol Chem. 1980 May 25;255(10):4411–4413. [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Sharpless T., Traganos F., Darzynkiewicz Z., Melamed M. R. Flow cytofluorimetry: discrimination between single cells and cell aggregates by direct size measurements. Acta Cytol. 1975 Nov-Dec;19(6):577–581. [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B., Puck T. T., Wenger L. The role of butyrate in the reverse transformation reaction in mammalian cells. J Cell Physiol. 1978 Jan;94(1):69–75. doi: 10.1002/jcp.1040940109. [DOI] [PubMed] [Google Scholar]
- Traganos F., Evenson D. P., Staiano-Coico L., Darzynkiewicz Z., Melamed M. R. Action of dihydroxyanthraquinone on cell cycle progression and survival of a variety of cultured mammalian cells. Cancer Res. 1980 Mar;40(3):671–681. [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Bradbury E. M., Allfrey V. G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978 May;75(5):2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. V., Chambers S. H. Fluorescence discrimination between diploid cells on their RNA content: a possible distinction between clonogenic and non-clonogenic cells. Br J Cancer. 1977 Nov;36(5):592–600. doi: 10.1038/bjc.1977.236. [DOI] [PMC free article] [PubMed] [Google Scholar]