Abstract
Super-macromolecular complexes play many important roles in eukaryotic cells. Classical structural biological studies focus on their complicated molecular structures, physical interactions and biochemical modifications. Recent advances concerning intracellular electric fields generated by cell organelles and super-macromolecular complexes shed new light on the mechanisms that govern the dynamics of mitosis and meiosis. In this review we synthesize this knowledge to provide an integrated theoretical model of these cellular events. We suggest that the electric fields generated by synchronized oscillation of microtubules, centrosomes, and chromatin fibers facilitate several events during mitosis and meiosis, including centrosome trafficking, chromosome congression in mitosis and synapsis between homologous chromosomes in meiosis. These intracellular electric fields are generated under energy excitation through the synchronized electric oscillations of the dipolar structures of microtubules, centrosomes and chromosomes, three of the super-macromolecular complexes within an animal cell.
Keywords: Polar wind, Chromosome, Microtubule, Centrosome, Spindle body
Background
The choreography of microtubules, centrosomes and chromosomes during mitosis and meiosis is beautifully designed by nature. Finely regulated and synchronized movements of these super-macromolecular complexes against the entropic forces within a dividing cell ensure the fidelity of the genetic material in both daughter cells. Currently, several models exist for the mechanisms of chromosome congression and spindle body assembly during M phase such as the search and capture model, kinetochore-mediated k-fibre formation, kinetochore motors contributing to congression, and the polar wind model. The mechanisms evoked by these models probably overlap, so there is redundancy among them, since mutations in the genes involved have only mild effects on chromosome congression during mitosis [1]. Many open questions remain within these models. In the polar-wind model, an unknown force (also known as the ejection force) generated by the spindle poles is considered to push the chromosomes to the spindle equator. Laser microsurgery experiments show that chromosome fragments without kinetochores are invariably expelled from the spindle, and chromosomes without kinetochores can still move from the vicinity of the spindle pole to the spindle equator [1-3]. The ejection force of the spindle body is dependent on the polymerization of spindle body microtubules, as depolymerization of astral microtubules by nocodazole or colcemid prevents the expulsion of severed chromosome arms from the spindle, whereas stabilization of microtubules by taxol drives chromosomes to the periphery of the astral array [4]. In addition, the driving force responsible for the pole-ward flux of spindle microtubules during metaphase remains uncharacterized [5].
Cellular electric fields have been studied in various cell types, and several studies have reported the existence of dielectrophoretic forces around cells [6-8]; electromagnetic interactions between cells have also been studied [9-11]. Cifra et al. proposed that microtubules, which comprise heterodimers polymerized into a helical structure, can generate an electric field under intracellular energy excitation [12-15]. Inhibition of microtubule polymerization by an external electromagnetic field has been reported by Kirson et al. [16,17]. Pokorny´ et al. detected four peaks of electric field activity around yeast cells during M phase, which correlated with spindle body assembly, kinetochore microtubule capture, and mitotic spindle elongation during anaphase A and B, visualized by fluorescence microscopy [18]. Comparing synchronized and unsynchronized tubulin mutants of yeast cells, Pokorny´ et al. verified that synchronized yeast cells show more electric activity during M phase than non-synchronized yeasts [19]. Direct measurements of electric resonant oscillations in microtubules have been presented at conferences by A. Bandyopadhyay. The technical aspects of direct detection of electric fields within a living cell have been discussed in a recent review [20]. Resonance absorption of external electromagnetic fields by cancers has been reported by Vedruccio et al. [21], and Zimmerman et al. reported that cancer cell proliferation is inhibited by specific modulation frequencies [22].
Coherent oscillations in microtubules can be explained by Fröhlich’s theory, which describes a system of oscillators with energy supply, linear and nonlinear coupling with a heat bath. If a sufficient energy supply is provided to this system, condensation of energy occurs in the lowest mode leading to its coherent excitation [23,24]. Electrostatic but not electrodynamic interactions are screened over long distances (Debye Screening). Given an intracellular salt concentration of ~ 150 mM, the effectiveness of electrostatic interaction is shortened to the nanometer range (the Debye length is ~ 0.7-0.8 nm). However, resonant electrodynamic interactions, such as the electromagnetic interactions generated by electric oscillations within the cell, may play a role in the long-distance recruitment of biomolecules. Following Fröhlich, Preto et al. suggested that long-range electrodynamic interactions can be triggered only under resonance conditions, and such interactions are effective when one normal mode is statistically privileged, typically out of thermal equilibrium, which could be the case in the intracellular context [25,26].
In this article, we integrate research from several disciplines to provide an ‘electric’ view of the dynamics of these super-macromolecular complexes in mitosis, meiosis and other relevant cellular events. From our theoretical point of view, many of the unidentified forces regulating major cellular dynamic events during mitosis are probably electric forces generated by the synchronized oscillation of the electric dipoles within these super-macro organelle structures. Chronic exposure to extremely low frequency electric fields could affect several key steps of mitosis and neuronal cell physiology, resulting in an increased risk for cancer.
The electrical properties of microtubules and centrosomes
The electric field of the microtubule is generated by the synchronized oscillation of α and β tubulins. These tubulins form electric dipoles during microtubule polymerization; under intracellular energy excitation, synchronized oscillation of α and β tubulin subunits generates a longitudinal electric field around the microtubule [12-15] (Figure 1). Cifra et al. suggested that the source of the energy excitation could be hydrolysis of guanosine triphosphate (GTP) during the process of dynamic instability of microtubules, and also energy transferred from the movement of motor proteins or released from mitochondria as “wasted” energy from the citric acid cycle. We propose that the overall entropic environment within a living cell could be the source of energy for electric oscillation of microtubules. Cancer cells have different entropic states from normal cells as a result of the Warburg effect, which can cause mitochondrial malfunctions and further lead to alteration of cytoskeleton-based cellular elastoelectrical oscillations [27]. The microtubule networks of cancer cells generate an electromagnetic field with different frequencies. Thus, specific electromagnetic frequencies have been used to diagnose specific cancers [21,28], and tumor-specific modulating electromagnetic fields have been used to treat patients with advanced cancer with positive results [22,29].
Figure 1.
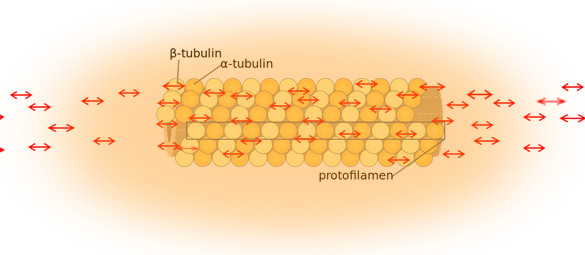
The red arrow illustrates the electric field of the microtubule under intracellular energy excitation .
The centriole of the centrosome is composed of α, β and γ tubulins organized differently from the subunits of microtubules; each centrosome comprises two centrioles, which are composed of nine triplets of microtubules. The two centrioles are arranged perpendicularly and surrounded by an amorphous mass of dense material (the pericentriolar material) [30]. As in microtubules, an electric field would be generated by synchronized oscillation between the α and β tubulins within the microtubule triplet of the centrioles (Figure 2).
Figure 2.
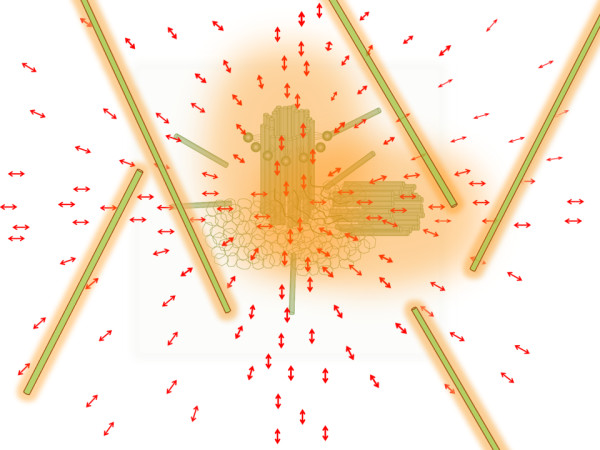
The red arrows illustrate the electric field of a centrosome under intracellular energy excitation .
Electric fields in centrosome separation and bipolar spindle body assembly
Mechanisms of centrosome separation and bipolar spindle body assembly have been discussed in a recent review [31]. The process is still incompletely understood. Plus end-directed motor proteins such as kinesin 5 and minus end-directed motor proteins such as dynein are known to play dominant roles in centrosome separation and spindle assembly. However, centrosomal microtubules and microtubules of the nuclear envelope (NE) and cellular cortex need to move into close proximity for motor proteins to attach to both so they can generate the pulling forces. The current models assume a randomized mode of microtubule interaction, which is quite inefficient. For example, at a certain point a centrosome would have to stop moving until certain microtubules had grown sufficiently for appropriate bridging by motor proteins, particularly during prophase, when the centrosomes do not have many associated microtubules. When the electric fields of microtubules and centrosomes are considered, these structures are mutually attractive. Thus, centrosome movement along the microtubule networks of the cellular cortex and NE is more efficient. We can also envision a more autonomous mode of microtubule lattice formation within the cellular cortex and NE.
Electrical properties of duplicated chromosomes
Andrews et al. have studied the effects of high frequency (range 2 to 50 MHz) electric fields on mammalian (human and Chinese hamster) chromosomes in vitro. They showed that such chromosomes can be oriented, aligned and translated by an oscillating electrical force. They also observed that above certain threshold field strengths the chromosomes orient themselves with their long axes along the field direction. The dependence of this threshold on frequency was measured and was found to be much larger at low than at high frequencies [32]. Using electric dichroism experiments, Crothers reported permanent dipole moments in dinucleosomes linked by 140 and 175 base pairs of DNA [33]. Jian Sun et al. suggested an electrostatic mechanism of nucleosomal array folding, revealed by computer simulation, which explains the salt-dependent chromatin fiber conformations [34]. Schalch et al. reported that the X-ray structure of an oligonucleosome revealed that linker DNA elements zigzag back and forth between two stacks of nucleosome cores, forming a truncated two-start helix, and do not follow a path compatible with a one-start solenoidal helix [35]. Grigoryev et al. reported evidence for heteromorphic chromatin fibers, showing that the 2-start zigzag topology and the type of linker DNA bending that defines solenoid models may be simultaneously present in a structurally heteromorphic chromatin fiber with a uniform 30 nm diameter [36].
However, the physical mechanisms that regulate higher order packaging of M phase chromosomes are still not well characterized. Here we present a hypothesis of chromosome compaction. We apply a pulse-coupled oscillation clustering model to the dynamic events of chromosome packaging and inter-/intra-chromosomal organization. During chromosomal packaging, differentially compacted regions form partially synchronized electric oscillators interacting with an elastic electromagnetic field. According to the physical pulse-coupled oscillator model, unsynchronized pulse-coupled oscillators with proximal natural frequencies form synchronized oscillation clusters at a given coupling strength. As the coupling strength increases, these synchronized oscillation clusters merge with each other [37-40].
During M phase chromosome compaction, the 30 nm chromatin fiber is initially formed by the electrostatic forces between neighboring nucleosomes. Under intracellular stochastic energy excitation, electric dipolar oscillation would be generated between neighboring nucleosomes. After oscillation synchronization and coupling, regulated electric oscillation is generated along the 30 nm chromatin fiber, and the oscillation coupling process further compacts that fiber [12-14,41]. This facilitates further packing into the 300 nm fiber; the electric field bends according to the physical curvature of the compacting 30 nm fiber, generating an oscillating electromagnetic field that goes through the 300 nm chromatin fiber. After the second round of oscillation synchronization and coupling, the 300 nm fiber becomes compacted and coiled into the 250 nm chromatin fiber, along which the third order of electromagnetic field is generated; this round of oscillation coupling and clustering facilitates the packing of the 250 nm chromatin fiber into the 700 nm chromosome arms. The coiling electromagnetic field of the 250 nm chromatin fiber generates the electromagnetic field of a chromosome arm [42,43] (Figure 3). We speculate that the source of the dipolar electric oscillation between neighboring nucleosomes is the variety of intracellular entropic forces, and the direction of oscillation primarily depends on the zigzag arrangement of neighboring nucleosomes along the 30 nm chromatin fiber. Each of the M phase chromosomal arms can be viewed as a partially synchronized oscillation cluster. Under intracellular energy excitation, partially synchronized electric fields can be generated for each chromosomal arm, and these orient the dynamics of the chromosome when they interact with the electric field generated by the spindle microtubules.
Figure 3.
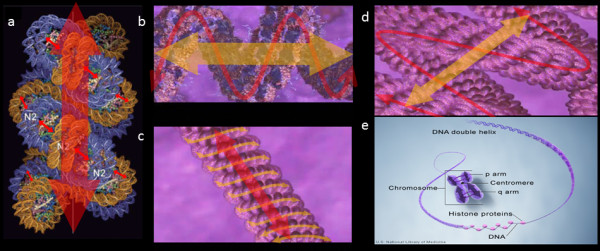
(a) The small red arrows indicate the electric oscillations generated between the neighboring histone octamers by excitation of entropic energy within the cell nucleus. The big red arrow represents the electric field generated by the electric oscillation along the 30 nm chromatin fiber. (b-d) Schematic illustration of several orders of oscillation coupling and clustering of EMFs in chromatin fibers, which facilitate the multi-step event of M phase chromosome packaging. The red and orange arrows indicate the multiple orders of EMFs generated during chromosome packaging. (e) The purple arrows indicate the EMFs of compacted M phase chromosome arms; the purple cycles indicate coupling of EMFs. The duplicated chromosome arms hold a juxtaposed position.
Theoretically, the oscillation clustering model explains the closely juxtaposed configuration of duplicated chromosomes during M phase, which is counter-intuitive from the perspective of electrostatic repulsion between duplicated chromosome arms. As the homologous chromosomal regions develop synchronized oscillation clusters with identical natural frequencies, they tend to cluster together. The same scenario could apply to synapsis during meiosis; the electric oscillations of homologous chromosomes couple with each other, preventing synapsis between non-homologous chromosomes.
Electric interactions during mitosis and meiosis
The intracellular electric fields described in the foregoing sections could facilitate several cellular events during mitosis and meiosis. First, the metaphase bipolar spindle microtubules are formed through microtubule nucleation from the γ tubulin ring complexes (γ-TuRCs) at the centrosomes and retrograde delivery of peripheral microtubules by motor proteins [31,44-46]. The electric fields of microtubules and centrosomes could facilitate spindle microtubule assembly through electric interactions. Secondly, the unidentified polar-wind or ejection force of the spindle body is likely to be generated by interactions between the electric fields of the spindle body and chromosomes [1-3]. In this case, the bipolar spindle body and chromosomes can be viewed as two oscillating clusters with different average oscillating frequencies. Given the oscillation clustering model, a partially entrained system of oscillators with similar frequencies preferentially cluster with each other at a given coupling strength [37-40]. So the clustering of electric fields of spindle microtubules would result in the repulsion of the electric field of the chromosomes, which pushes the chromosomes from the proximal regions of centrosomes out of the spindle body. Congression at the central plate regions of a dividing cell can also be viewed as oscillation clustering of the electric fields of chromosomes. The processes may also be viewed as a dynamic electric phase, which indicates that the intensity of the electric field changes in different subcellular regions during metaphase. Cell organelles with different electric field intensities would automatically locate themselves according to the electric phase. In addition, the pole-ward flux of spindle microtubules during metaphase could be driven by the electric locking of those microtubules within the spindle body, which means that the synchronized electric fields of the spindle body would hold the physical position of a spindle microtubule growing at the plus end and depolymerizing at the minus end at the same time within a dividing cell [5] (Figure 4).
Figure 4.
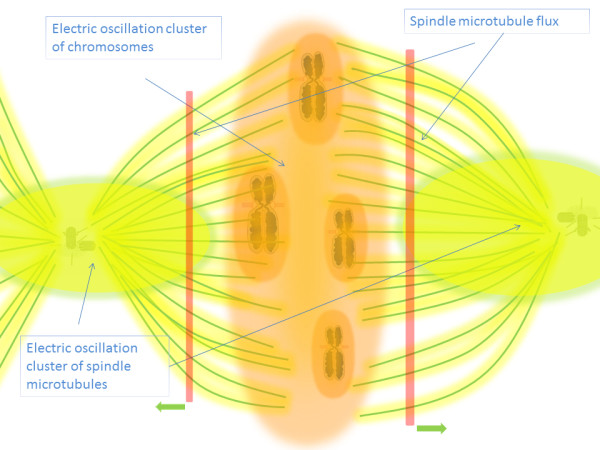
Schematic illustration of the electric interactions between spindle body microtubules and chromosomes facilitating congression through oscillation clustering, and spindle body pole-ward flux during mitosis; the green arrows indicate the direction of the pole-ward flux .
According to the physical organization of the duplicated chromosome arms, the condensed electric chromosomal fields around the centromeric regions could attract microtubule fragments to the sister kinetochores through electric interaction, which is consistent with observations of kinetochore movement along uncaptured microtubules, forming K-fibers (kinetochore associated microtubules) [47]. Thus, electric interactions between chromosomes and microtubules may also facilitate K-fiber capture by kinetochores. The chromosome oscillation observed during congression could be explained as the turbulence of chromosome arms passing through the chaotic electric landscape of two astral microtubule networks.
In meiosis, the kinetochores are positioned at one side of the duplicated chromosome dimers, so the two sister chromosomes do not separate. The electric oscillation clustering between homologous chromosomal regions results in synapsis and recombination between homologous chromosomes; the electric fields generated by two duplicated homologous chromosomes can be viewed as two identical partially-entrained electric oscillation clusters, constituted by sub-chromosomal clusters throughout the chromosome arms. Such clusters in homologous chromosomes share identical electric frequencies, so the close juxtaposition between homologous chromosomes at synapsis is achieved through electric clustering and coupling among them. Synapsis does not occur during mitosis probably because the chromosome configuration caused by the opposing outward-pulling forces of the kinetochores at the opposite sides of duplicated centromere disfavors inter-chromosomal electric attraction. In addition, this event may be regulated by synaptonemal complex proteins [48].
Magidson et al. reported that chromosomes adopt a toroidal/ring shape organization of after NE breakdown, which facilitates spindle assembly during M phase [47]. Their observation matches the electric model at several points: the ring shape organization could be generated by the electric interaction between M phase chromosomes and the spindle body, and the interplay between the electric fields of the chromosome ring and spindle body microtubules promotes the capture of microtubules by kinetochores.
Discussion
Numerous reports indicate that extremely low frequency electric fields can increase the risks of certain types of cancer [49]. Micronuclei (MN) in buccal mucosal cells, comprising acentric fragments or complete chromosomes that fail to attach to the mitotic spindle during cytokinesis, are increased in people chronically exposed to extremely low frequency electric fields [50]. Research by Hardell et al. indicates increased brain tumor risks with latency time and cumulative mobile or cordless phone use [51]. Volkow et al. reported that 50-minute cell phone exposure was associated with increased brain glucose metabolism in the region closest to the antenna [52]. However, the exact cellular biophysical pathways that relay very low frequency electric radiations to genetic alterations that lead to cancer are not well characterized. From our theoretical point of view, chronic exposure to extremely low frequency electric fields would intervene in several key steps of mitosis and neuronal cell physiology, potentially resulting in an increased risk for cancer.
To characterize these intracellular electric fields and study their cellular functions further, biophysicists should develop more detailed mathematical and physical models for chromosome electric fields and their role in M phase chromosome compaction, to allow these fields to be described and calculated more precisely and to predict the dynamics of related cellular events. The dynamic of changes of the electric fields in a living cell during mitosis and other cellular processes could be visualized using live cell imaging technologies such as nano-sized voltmeters [53]. It would be particularly interesting to observe microtubule self-organization under energy excitation in vitro, which would allow us to observe the dynamics of microtubule movements directly through electric interactions. These insights will help us to understand the molecular mechanisms of signal pathways better and to elucidate cellular super-macromolecular behavior, cell organelle organization and functions, intra- and inter-cellular communications, tissue morphogenesis, embryo development, neurobiology, and oncogenesis, and finally to advance our knowledge about life to a new level.
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
Yue Zhao conceived the general concepts and wrote the article; Qimin Zhan shared insights and gave advice. Both authors read and approved the final version of the article.
Contributor Information
Yue Zhao, Email: alexanderyz@gmail.com.
Qimin Zhan, Email: Zhanqimin@pumc.edu.cn.
Acknowledgments
We thank Dr. Michal Cifra of Institute of Photonics and Electronics, Academy of Sciences of the Czech Republic and Dr. Yujie Sun of Biodynamic Optical Imaging Center of Peking University for their helpful discussion and insights about electric fields in live intracellular organelles. We thank Dr. Lennart Hardell of University Hospital, Sweden for providing us some of the latest epidemiological data linked with EMF and cancer. We also thank Editor-in-Chief of Theoretical Biology and Medical Modelling Dr. Paul Agutter for language editing of the article.
References
- Walczak CE, Cai S, Khodjakov A. Mechanisms of chromosome behavior during mitosis. Nat Rev Mol Cell Bio. 2009;11:91–102. doi: 10.1038/nrm2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Davison EA, Jensen LCW, Cassimeris L, Salmon ED. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and the half-spindle. J Cell Biol. 1986;103:581–591. doi: 10.1083/jcb.103.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. Kinetochores moving away from their associated pole do not exert a significant pushing force on the chromosome. J Cell Biol. 1996;135:315–327. doi: 10.1083/jcb.135.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault JG, DeMarco AJ, Salmon ED, Rieder CL. Studies on the ejection properties of asters: astral microtubule turnover influences the oscillatory behavior and positioning of mono-oriented chromosomes. J Cell Sci. 1991;99:701–710. doi: 10.1242/jcs.99.4.701. [DOI] [PubMed] [Google Scholar]
- Kwok BH, Kapoor TM. Microtubule flux: drivers wanted. Curr Opin Cell Biol. 2007;19:36–42. doi: 10.1016/j.ceb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Pohl J, Christophers E. Photo inactivation and recovery in skin fibroblasts after formation of mono- and bifunctional adducts by furocoumarins-plus-UVA. J Invest Dermatol. 1980;75(4):306–310. doi: 10.1111/1523-1747.ep12530921. [DOI] [PubMed] [Google Scholar]
- Hölzel R, Lamprecht I. Electromagnetic fields around biological cells. Neural Net World. 1994;4(3):327. [Google Scholar]
- Hölzel R. Electric Activity of Non-Excitable Biological Cells at Radio Frequencies. Electro- and Magnetobiol. 2001;20:1. [Google Scholar]
- Albrecht-Buehler G. Surface extensions of 3 T3 cells towards distant infrared sources. J Cell Biol. 1991;114:493–502. doi: 10.1083/jcb.114.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht-Buehler G. A rudimentary form of cellular vision. Proc Natl Acad Sci USA. 1992;89:8288–8292. doi: 10.1073/pnas.89.17.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht-Buehler G. A Long-Range Attraction Between Aggregating 3T3 Cells Mediated By Near-Infrared Light Scattering. Proc Natl Acad Sci USA. 2005;102(14):5050–5055. doi: 10.1073/pnas.0407763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelka D, Cifra M. Calculation of the electromagnetic field around microtubule. Acta Polytechnica Czech Technical University in Prague. CTU Publishing House. 2009;49(2–3):58–63. ISSN 1210–2709. [Google Scholar]
- Cifra M, Havelka D, Deriu MA. Electric Field Generated by Longitudinal Axial Microtubule Vibration Modes with High Spatial Resolution Microtubule Model. Electrodynamic Activity of Living Cells. J Physics: Conference Series. 2011;329:012013. [Google Scholar]
- Cifra M, Pokorný J, Havelka D, Kucera O. Electric field generated by axial longitudinal vibration modes of microtubule. Biosystems. 2010;100(2):122–131. doi: 10.1016/j.biosystems.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Havelka D, Cifra M, Kučera O, Pokorný J, Vrba J. High-frequency electric field and radiation characteristics of cellular microtubule network. J Theor Biol. 2011;286(1):31–40. doi: 10.1016/j.jtbi.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, Schatzberger R, Palti Y. Disruption of Cancer Cell Replication by Alternating Electric Fields. Cancer Res. 2004;64:3288–3295. doi: 10.1158/0008-5472.CAN-04-0083. [DOI] [PubMed] [Google Scholar]
- Kirson ED, Dbalý V, Tovarys F, Vymazal J, Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S, Gurvich Z, Schneiderman R, Wasserman Y, Salzberg M, Ryffel B, Goldsher D, Dekel E, Palti Y. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci USA. 2007;104(24):10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny J, Hasek J, Jelınek F, Saroch J, Palan B. Electric activity of yeast cells in the M phase. Electro Magnetobiol. 2001;20:371–396. [Google Scholar]
- Jelínek F, Cifra M, Pokorny J, Vanis J, Simsa J, Hasek J, Frydlová I. Measurement of Electrical Oscillations and Mechanical Vibrations of Yeast Cells Membrane Around 1kHz. Electric Biology and Medicine. 2009;28(2):223–232. doi: 10.1080/15368370802710807. [DOI] [PubMed] [Google Scholar]
- Kučera O, Cifra M, Pokorný J. Technical aspects of measurement of cellular electric activity. Eur Biophys J. 2010;39(10):1465–1470. doi: 10.1007/s00249-010-0597-8. [DOI] [PubMed] [Google Scholar]
- Vedruccio C, Meessen A. EM Cancer Detection by Means of Non Linear Resonance Interaction. Proceedings of PIERS 2004, Progress in Electromagnetics Research Simposium, Pisa, Italy; 2004. pp. 909–912. [Google Scholar]
- Zimmerman JW, Pennison MJ, Brezovich I, Yi N, Yang CT, Ramaker R, Absher D, Myers RM, Kuster N, Costa FP, Barbault A, Pasche B. Cancer cell proliferation is inhibited by specific modulation frequencies. Br J Cancer. 2012;106:307–313. doi: 10.1038/bjc.2011.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich H. Long-range coherence and energy storage in biological systems. International Journal of Quantum Chemistry. 1968;2:641–649. doi: 10.1002/qua.560020505. [DOI] [Google Scholar]
- Fröhlich H. The biological effects of microwaves and related questions. Advances in Electronics and Electron Physics. 1980;53:85–152. [Google Scholar]
- Preto J, Floriani E, Nardecchia I, Ferrier P, Pettini M. Experimental assessment of the contribution of electrodynamic interactions to long-distance recruitment of biomolecular partners, theoretical basis. Phys Rev E Stat Nonlin Soft Matter Phys. 2012;85(4–1):041904. doi: 10.1103/PhysRevE.85.041904. PMID 22680495. [DOI] [PubMed] [Google Scholar]
- Preto J, Pettini M. Long-range resonant interactions in biological systems. arXiv. p. 5187v1.
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Pokorný J, Vedruccio C, Cifra M, Kučera O. Cancer physics: diagnostics based on damped cellular elastoelectrical vibrations in microtubules. Eur Biophys J. 2011;40(6):747–759. doi: 10.1007/s00249-011-0688-1. [DOI] [PubMed] [Google Scholar]
- Barbault A, Costa FP, Bottger B, Munden RF, Bomholt F, Kuster N, Pasche B. Amplitude-modulated electromagnetic fields for the treatment of cancer: discovery of tumor-specific frequencies and assessment of a novel therapeutic approach. J Exp Clin Cancer Res. 2009;28(1):51. doi: 10.1186/1756-9966-28-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey SJ. Re-evaluating centrosome function. Nature Rev Molec Biol. 2001;2:688–699. doi: 10.1038/35089575. [DOI] [PubMed] [Google Scholar]
- Tanenbaum ME, Medema RH. Mechanisms of centrosome separation and bipolar spindle assembly. Dev Cell. 2010;14, 19(6):797–806. doi: 10.1016/j.devcel.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Andrews MJ, McClure JA. Effects of high frequency electric fields on mammalian chromosomes in vitro. J Biol Phys. 1979;6(1–2):69–86. doi: 10.1007/BF02311220. [DOI] [Google Scholar]
- Crothers DM, Dattagupta N, Hogan M, Klevan L, Lee KS. Transient electric dichroism studies of nucleosomal particles. Biochemistry. 1978;17(21):4525–4533. doi: 10.1021/bi00614a026. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang Q, Schlick T. Electrostatic mechanism of nucleosomal array folding revealed by computer simulation. Proc Natl Acad Sci U S A. 2005;102(23):8180–8185. doi: 10.1073/pnas.0408867102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalch T, Duda S, Sargent DF, Richmond TJ. X-ray structure of a tetranucleosome and its implications for the chromatin fibre. Nature. 2005;436:138–141. doi: 10.1038/nature03686. [DOI] [PubMed] [Google Scholar]
- Grigoryev SA, Arya G, Correll S, Woodcock CL, Schlick T. Evidence for heteromorphic chromatin fibers from analysis of nucleosome interactions. Proc Natl Acad Sci USA. 2009;106(32):13317–13322. doi: 10.1073/pnas.0903280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strogatz SH, Stewart I. Coupled oscillators and biological synchronization. Sci Am. 1993;269(6):102–109. doi: 10.1038/scientificamerican1293-102. [DOI] [PubMed] [Google Scholar]
- Morelli LG, Cerdeira H, Zanette DH. Frequency clustering of coupled phase oscillators on small-world networks. Eur Phys J B. 2005;43:243–250. doi: 10.1140/epjb/e2005-00046-2. [DOI] [Google Scholar]
- Smet FD, Aeyels D. Clustering in a network of non-identical and mutually interacting agents. Proc R Soc A. 2009;465:745–768. doi: 10.1098/rspa.2008.0259. [DOI] [Google Scholar]
- Aeyels D, Smet FD. Emergence and evolution of multiple clusters of attracting agents. Physica D. 2010;239:1026–1037. doi: 10.1016/j.physd.2010.02.012. [DOI] [Google Scholar]
- Cifra M, Fields JZ, Farhadi A. Electromagnetic cellular interactions. Prog Biophys Mol Biol. 2011;105(3):223–246. doi: 10.1016/j.pbiomolbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Olins DE, Olins AL. Chromatin history: our view from the bridge. Nat Rev Mol Cell Biol. 2003;4:809–814. doi: 10.1038/nrm1225. [DOI] [PubMed] [Google Scholar]
- Annunziato A. DNA packaging: Nucleosomes and chromatin. Nature Education. 2008;1(1) [Google Scholar]
- Kollman JM, Merdes A, Mourey L, Agard DA. Microtubule nucleation by γ-tubulin complexes. Nat Rev Mol Cell Biol. 2011;12:709–721. doi: 10.1038/nrm3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusan NM, Tulu US, Fagerstrom C, Wadsworth P. Reorganization of the microtubule array in prophase/prometaphase requires cytoplasmic dynein-dependent microtubule transport. J Cell Biol. 2002;158:997–1003. doi: 10.1083/jcb.200204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulu US, Rusan NM, Wadsworth P. Peripheral, non-centrosome-associated microtubules contribute to spindle formation in centrosome-containing cells. Curr Biol. 2003;13:1894–1899. doi: 10.1016/j.cub.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Magidson V, O'Connell CB, Lončarek J, Paul R, Mogilner A, Khodjakov A. The Spatial Arrangement of Chromosomes during Prometaphase Facilitates Spindle Assembly. Cell. 2011;146(4):555–567. doi: 10.1016/j.cell.2011.07.012. PMID: 21854981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M, Siomos MF, Nasmyth K. Un ménage à quatre: the molecular biology of chromosome segregation in meiosis. Cell. 2003;112(4):423–40. doi: 10.1016/S0092-8674(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Baan R, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Islami F, Galichet L, Straif K. WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of radiofrequency electric fields. Lancet Oncol. 2011;12(7):624–626. doi: 10.1016/S1470-2045(11)70147-4. [DOI] [PubMed] [Google Scholar]
- Carbonari K, Gonçalves L, Roth D, Moreira P, Fernández R, Martino-Roth MG. Increased micronucleated cell frequency related to exposure to radiation emitted by computer cathode ray tube video display monitors. Genet Mol Biol. 2005;28(3):469–474. doi: 10.1590/S1415-47572005000300024. [DOI] [Google Scholar]
- Hardell L, Carlberg M, Mild KH. Pooled analysis of case–control studies on malignant brain tumours and the use of mobile and cordless phones including living and deceased subjects. Int J Oncol. 2011;38(5):1465–1474. doi: 10.3892/ijo.2011.947. 10.3892. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Vaska P, Fowler JS, Telang F, Alexoff D, Logan J, Wong C. Effects of Cell Phone Radiofrequency Signal Exposure on Brain Glucose Metabolism. JAMA. 2011;305(8):808–813. doi: 10.1001/jama.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner KM, Kopelman R, Philbert MA. “Nanosized Voltmeter” Enables Cellular-Wide Electric Field Mapping. Biophys J. 2007;93:1163–1174. doi: 10.1529/biophysj.106.092452. [DOI] [PMC free article] [PubMed] [Google Scholar]


