Abstract
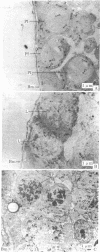
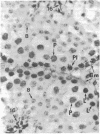
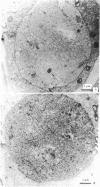
Male mice were injected intraperitoneally with 125 microCi (1 Ci = 3.7 X 10(10) becquerels) of [3H]thymidine at 1-hr intervals and killed 1 hr after the second injection. Testes were prepared for bright-field and electron microscopic autoradiography. Primary spermatocytes, identified by light microscopy to be at the premeiotic interphase stage, were found to be heavily labeled. Electron microscopic examination disclosed the coincidental occurrence of synaptonemal complexes and label within the nuclei of premeiotic interphase spermatocytes, indicating synapses of homologues had begun during the S phase. The significance of this finding for the traditional view of meiosis is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter A. T. Electron microscopy of meiosis in Drosophila melanogaster females. I. Structure, arrangement, and temporal change of the synaptonemal complex in wild-type. Chromosoma. 1975;51(2):157–182. doi: 10.1007/BF00319833. [DOI] [PubMed] [Google Scholar]
- Day J. W., Grell R. F. Synaptonemal complexes during premeiotic DNA synthesis in oocytes of Drosophila melanogaster. Genetics. 1976 May;83(1):67–79. doi: 10.1093/genetics/83.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAWCETT D. W. The fine structure of chromosomes in the meiotic prophase of vertebrate spermatocytes. J Biophys Biochem Cytol. 1956 Jul 25;2(4):403–406. doi: 10.1083/jcb.2.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell R. F. Heat-induced exchange in the fourth chromosome of diploid females of Drosophila melanogaster. Genetics. 1971 Dec;69(4):523–527. doi: 10.1093/genetics/69.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell R. F. Time of recombination in the Drosophila melanogaster oocyte: evidence from a temperature-sensitive recombination-deficient mutant. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3351–3354. doi: 10.1073/pnas.75.7.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassir Y., Simchen G. Meiotic recombination and DNA synthesis in a new cell cycle mutant of Saccharomyces cerevisiae. Genetics. 1978 Sep;90(1):49–68. doi: 10.1093/genetics/90.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch E. A., Smith P. A., King R. C. The division and differentiation of Drosophila cystocytes. J Morphol. 1967 Jan;121(1):55–70. doi: 10.1002/jmor.1051210106. [DOI] [PubMed] [Google Scholar]
- MONESI V. Autoradiographic study of DNA synthesis and the cell cycle in spermatogonia and spermatocytes of mouse testis using tritiated thymidine. J Cell Biol. 1962 Jul;14:1–18. doi: 10.1083/jcb.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott A. Human male meiosis: chromosome behaviour at pre-meiotic and meiotic stages of spermatogenesis. Can J Genet Cytol. 1971 Sep;13(3):536–549. doi: 10.1139/g71-079. [DOI] [PubMed] [Google Scholar]
- OAKBERG E. F. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat. 1956 Nov;99(3):391–413. doi: 10.1002/aja.1000990303. [DOI] [PubMed] [Google Scholar]
- SWIFT H. H. The desoxyribose nucleic acid content of animal nuclei. Physiol Zool. 1950 Jul;23(3):169–198. doi: 10.1086/physzool.23.3.30152074. [DOI] [PubMed] [Google Scholar]