Abstract
Most patients with gastric or gastroesophageal junction (gej) cancer are diagnosed with inoperable advanced or metastatic disease. In these cases, chemotherapy is the only treatment demonstrating survival benefit. The present study compares clinicopathologic characteristics and survival outcomes for patients with advanced esophageal, gej, and gastric adenocarcinoma treated with first-line chemotherapy [epirubicin–cisplatin–5-fluorouracil (ecf), epirubicin–cisplatin–capecitabine (ecx), or etoposide–leucovorin–5-fluorouracil (elf)] or best supportive care (bsc) at our institution with those for historical controls.
Methods
We retrospectively reviewed medical information for 401 patients with newly diagnosed advanced esophageal, gej, or gastric adenocarcinoma treated with first-line chemotherapy (ecf, ecx, or elf) or bsc from January 1, 2004, through December 31, 2010. Descriptive statistics were used to compare the data collected with data for historical control patients.
Results
Of the study patients, 93% were diagnosed with metastatic disease (n = 374), and 63% received bsc only (n = 251). The main reasons that patients received bsc only included poor Eastern Cooperative Oncology Group performance status (55%), patient decision (31%), and comorbidities (14%). Of the remaining patients, 98 (24%) received ecf or ecx and 52 (13%) received elf as first-line treatment. Median overall survival was significantly longer in patients treated with ecf or ecx or with elf than in those receiving bsc (12.7 months vs. 12.7 months vs. 5.5 months respectively). Chemotherapy also significantly reduced the risk of death (64% reduction with ecf or ecx, 58% with elf).
Conclusions
We confirmed the substantial overall survival benefit of combination chemotherapy compared with bsc, with better survival in our patient population than in historical controls. However, novel treatment options are still warranted to improve outcomes in this patient population.
Keywords: Esophagus, gastroesophageal junction, gastric adenocarcinoma, survival outcomes
1. INTRODUCTION
Gastric cancer is the fourth most common malignancy and the second leading cause of cancer-related death worldwide, with 989,000 new cases and 738,000 deaths occurring annually1. Approximately 95% of all malignant gastric neoplasms are adenocarcinoma, which can be divided by the Lauren classification into either an intestinal or a diffuse form2. Esophageal cancer is the eighth most common cancer and the sixth most common cause of cancer death in the world1. Most esophageal malignancies are classified as either squamous cell carcinoma or adenocarcinoma, with the latter subtype emerging as the more common in developed countries.
Most patients with gastric or gastroesophageal junction (gej) cancer are diagnosed with inoperable advanced or metastatic disease. In such cases, chemotherapy is the only treatment demonstrating survival benefit, with a 63% reduction in the risk of death in comparison to best supportive care (bsc), while also improving overall quality of life3. Although there is no globally standardized regimen, fluoropyrimidine and platinum combinations form the backbone of chemotherapy for patients with advanced disease. Furthermore, compared with doublet combinations, the addition of an anthracycline or a taxane to a fluoropyrimidine and platinum agent significantly improves overall survival (os)3,4.
At our institution, the combination of either epirubicin, cisplatin, and 5-fluorouracil (ecf) or epirubicin, cisplatin, and capecitabine (ecx) is the first-line chemotherapy of choice in advanced esophageal, gej, and gastric adenocarcinoma patients with an Eastern Cooperative Oncology Group (ecog) performance status (ps) of 0–2. This practice is based on the real-2 study5. At our institution, ecx became an approved regimen in October 2007. Furthermore, given its clinical benefit in advanced gastric cancer and its tolerable toxicity, the elf regimen (etoposide, leucovorin, and 5-fluorouracil) can alternatively be considered in patients with medical contraindications to an anthracycline-based regimen or refusal of the central venous catheter required for ecf use6. However, elf has been considered inferior to triplet combinations3.
Most recently, the addition of molecularly targeted therapy in combination with chemotherapy has been tested in patients with advanced gastric and gej cancer. The toga (Trastuzumab for Gastric Cancer) study was the first randomized phase iii study that showed a significant os benefit from the addition of trastuzumab, a her2-directed monoclonal antibody, to a platinum-plus-fluoropyrimidines (5-fluorouracil or capecitabine) regimen, compared with platinum plus fluoropyrimidines alone in patients with her2 overexpression7. However, a trastuzumab-containing regimen is still not considered an accepted treatment option for patients with her2 overexpression at our institution because the comparison arm uses only doublet chemotherapy and not our standard triplet combination containing an anthracycline.
The objective of the present study was to determine the clinicopathologic characteristics and survival outcomes of patients with advanced lower esophageal, gej, and gastric adenocarcinoma treated either with first-line chemotherapy (ecf, ecx, or elf) or with bsc at our institution from January 1, 2004, to December 31, 2010, and to compare the results with results for historical control patients.
2. METHODS
We undertook a retrospective chart review for all patients with newly diagnosed advanced esophageal, gej, or gastric adenocarcinoma treated with first-line chemotherapy (ecf, ecx, or elf) or with bsc at the Cross Cancer Institute in Edmonton, Alberta, from January 1, 2004, through December 31, 2010. Major exclusion criteria were a non-adenocarcinoma histology and diagnosis with a secondary malignancy after treatment for advanced esophageal, gej, or gastric cancer. The source of medical information included the Cross Cancer Institute electronic health record system, paper charts, and Alberta Netcare, a provincial Web-based electronic health records system. The variables extracted from the database included patient information (date of birth, sex, vital status, date of death or date of last follow-up, and cause of death), diagnosis (primary site and date of diagnosis), primary treatment (chemotherapy or bsc), and progression (date of progression, site of progression, and treatment at progression). Ethics approval was obtained through our local tumour board, and all patient information was de-identified. Figure 1 illustrates the chemotherapy protocols.
FIGURE 1.
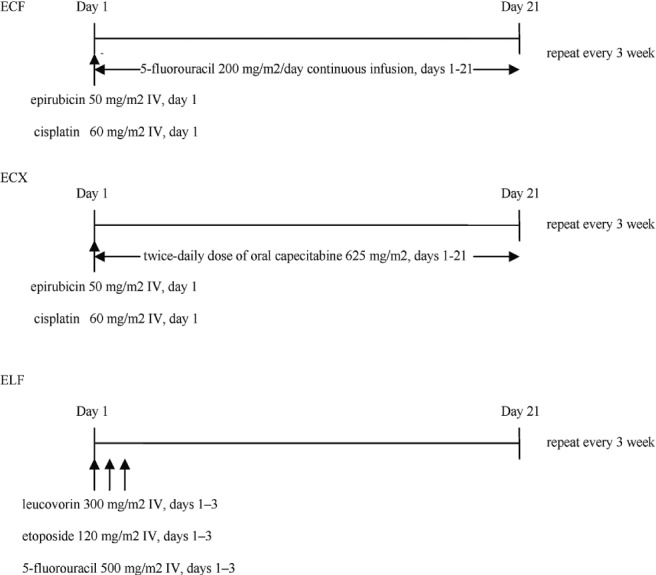
Dose and administration of chemotherapy regimens. ecf = epirubicin–cisplatin–5-fluorouracil; IV = intravenously; ecx = epirubicin–cisplatin–capecitabine; elf = etoposide–leucovorin–5-fluorouracil.
2.1. Statistical Analysis
Descriptive statistics were used to compare the data we collected with data from historical control patients. Overall survival was calculated from the date of diagnosis to date of death from any cause. Progression-free survival was calculated from the date of diagnosis to the first date of documented progressive disease or death from any cause. Data from patients who were alive and from those who were free of progression were censored at the last date of follow-up visit (for os and for progression-free survival respectively). Survival was calculated using the Kaplan–Meier method. Log-rank statistics were used to compare the survival curves. A Cox proportional hazards model was used to estimate hazard ratios (hrs) and the corresponding 95% confidence (ci) intervals. A p value of 0.05 or less was considered statistically significant. All analyses were performed using the SAS software application (version 9.1.3: SAS Institute, Cary, NC, U.S.A.).
3. RESULTS
From January 1, 2004, to December 31, 2010, 401 patients at the Cross Cancer Institute were newly diagnosed with advanced lower esophageal, gej, or gastric adenocarcinoma and received either first-line chemotherapy or bsc. The last date of follow-up for censored patients was October 13, 2011. The median period of follow-up was 5.73 months (range: 0.20–64.10 months).
Table i summarizes demographics and baseline disease characteristics for the patient cohort, 79% of whom were men. Median age at the time of diagnosis was 60, 63, and 67 years in the groups of patients receiving ecf or ecx, elf, and bsc respectively. Most patients in the chemotherapy groups had an ecog ps of 0–1 (62% in the ecf or ecx group and 58% in the elf group); 80% of patients receiving bsc had an ecog ps of 2 or more. Gastric adenocarcinoma was the most common primary tumour (47%). At the time of diagnosis, 93% of patients were observed to have metastatic disease. Most patients (52%) had only 1 metastatic site at first presentation.
TABLE I.
Patient demographics and baseline characteristics
| Variable |
Treatment group
|
||
|---|---|---|---|
| ecf/ecx | elf | bsc | |
| Patients (n) | 98 | 52 | 251 |
| Age (years) | |||
| Median | 60 | 63 | 67 |
| Range | 29–83 | 39–79 | 29–93 |
| ecog ps [n (%)] | |||
| 0–1 | 61 (62) | 30 (58) | 51 (20) |
| 2 | 7 (7) | 9 (17) | 27 (11) |
| 3–4 | 1 (1) | 0 (0) | 72 (29) |
| Missing | 29 (30) | 13 (25) | 101 (40) |
| Sex [n (%)] | |||
| Male | 78 (80) | 42 (81) | 195 (78) |
| Female | 20 (20) | 10 (19) | 56 (22) |
| Primary tumour site [n (%)] | |||
| Esophagus | 28 (29) | 12 (23) | 82 (33) |
| gej | 22 (22) | 12 (23) | 56 (22) |
| Stomach | 48 (49) | 28 (54) | 113 (45) |
| Pathology type (Lauren) | |||
| Intestinal | 11 (11) | 11 (21) | 51 (20) |
| Diffuse | 6 (6) | 3 (6) | 4 (2) |
| Mixed | 1 (1) | 0 (0) | 2 (1) |
| Non-classified | 80 (82) | 38 (73) | 194 (77) |
| Extent of disease | |||
| Locally advanced | 6 (6) | 6 (12) | 15 (6) |
| Metastatic | 92 (94) | 46 (88) | 236 (94) |
| Histologic grade | |||
| 1 | 6 (6) | 1 (2) | 9 (4) |
| 2 | 29 (30) | 18 (35) | 71 (28) |
| 3 | 49 (50) | 24 (46) | 146 (58) |
| Missing | 14 (14) | 9 (17) | 25 (10) |
| Metastatic sites | |||
| 0 or 1 | 62 (63) | 32 (61) | 114 (46) |
| 2 | 23 (24) | 16 (31) | 81 (32) |
| ≥3 | 13 (13) | 4 (8) | 56 (22) |
| Previous treatments | |||
| Surgery | 4 (4) | 3 (6) | 23 (9) |
| Radiation | 0 (0) | 0 (0) | 5 (2) |
| Chemotherapy | 2 (2) | 0 (0) | 5 (2) |
| Chemoradiation | 3 (3) | 3 (6) | 9 (4) |
| Other palliative modality | |||
| Surgery | 10 (10) | 6 (12) | 8 (3) |
| Radiation | 13 (13) | 8 (15) | 9 (4) |
ecf = epirubicin–cisplatin–5-fluorouracil; ecx = epirubicin–cisplatin–capecitabine; elf = etoposide–leucovorin–5-fluorouracil; bsc = best supportive care; ecog ps = Eastern Cooperative Oncology Group performance status; gej = gastroesophageal junction.
In the study cohort, 63% of patients received only bsc (n = 251). Of the patients who received bsc, 206 had data available on the reason for not receiving palliative chemotherapy, which included poor ecog ps (55%, n = 113), patient decision (30%, n = 62), and comorbidities (15%, n = 31). First-line palliative chemotherapy was ecf or ecx in 98 patients (24%); 52 (13%) received elf. The patients receiving ecf or ecx underwent a median 5 cycles of chemotherapy (range: 1–26 cycles); those receiving elf underwent a median of 3 cycles (range: 1–14 cycles; p = 0.005). Data were available for 39 patients who received elf. The main reasons for not receiving ecf or ecx included comorbidities or contraindication to cisplatin or anthracycline chemotherapy (54%) and patient decision concerning toxicity or requirement for a central line (46%). Second-line chemotherapy after disease progression was given to 14 patients (14%) in the ecf or ecx group and to 6 patients (12%) in the elf group. Third-line chemotherapy was administered to 4 patients in the study cohort, 2 patients per group. Palliative surgery for advanced disease was used for 6% of patients, and palliative radiotherapy was used for 7%.
At the time of analysis, 382 deaths had occurred: 84 in the ecf or ecx group, 50 in the elf group, and 248 in the bsc group. Median os was 12.7 months (95% ci: 10.7 to 14.8 months) in patients who received ecf or ecx, 12.7 months (95% ci: 9.4 to 16.0 months) in those who received elf, and 5.5 months (95% ci: 4.6 to 6.4 months) in those who received bsc (Figure 2). A significant benefit with respect to os was seen both in the ecf or ecx group and in the elf group compared with the bsc group [hazard ratio (hr): 0.36; p < 0.001; and hr: 0.42; p < 0.001 respectively]. The hr for death in the chemotherapy groups remained similar when adjusted for age, ecog ps, and number of metastatic sites. We observed no difference in os between patients treated with ecf or ecx and those treated with elf (hr: 0.88; p = 0.63).
FIGURE 2.
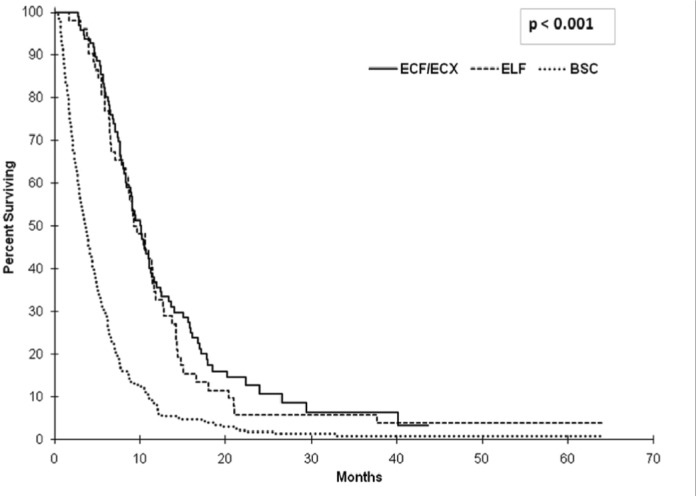
Overall survival (os) in the study patients. Median os was 12.7 months [95% confidence interval (ci): 10.7 to 14.8 months] in patients who received ecf (epirubicin–cisplatin–5-fluorouracil) or ecx (epirubicin–cisplatin–capecitabine), 12.7 months (95% ci: 9.4 to 16.0 months) in those who received elf (etoposide–leucovorin– 5-fluorouracil), and 5.5 months (95% ci: 4.6 to 6.4 months) in those who received best supportive care (bsc). The hazard ratio for death was 0.36 (p < 0.001) in the ecf/ecx group and 0.42 (p < 0.001) in the elf group compared with the bsc group.
Median progression-free survival in the chemotherapy arms was 8.48 months (95% ci: 6.87 to 9.86 months) for ecf or ecx and 8.87 months (95% ci: 6.67 to 10.32 months) for elf (Figure 3). Analysis of os for patients receiving bsc alone was also performed. The analysis demonstrated that, within that group, the patients who declined chemotherapy despite having a good ecog ps had a statistically better median os than did the patients with other reasons for not undergoing treatment: 6.11 months (95% ci: 4.44 to 6.47 months) and 2.83 months (95% ci: 2.43 to 3.32 months) respectively (hr: 0.62; p = 0.001; Figure 4).
FIGURE 3.
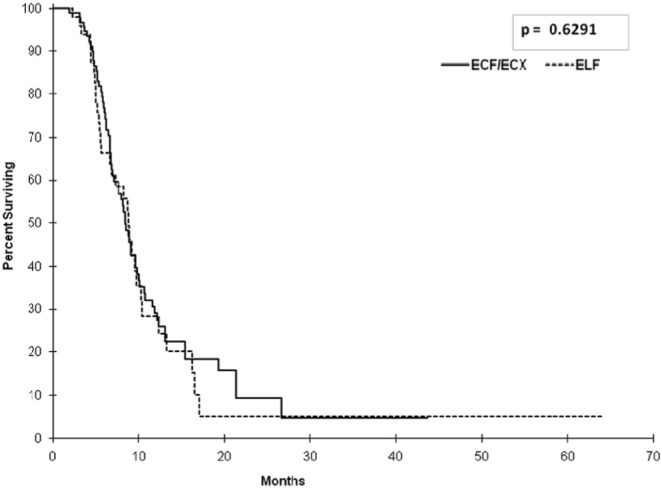
Progression-free survival (pfs) among patients receiving chemotherapy. Median pfs in the ecf (epirubicin–cisplatin– 5-fluorouracil) or ecx (epirubicin–cisplatin–capecitabine) group was 8.48 months [95% confidence interval (ci): 6.87 to 9.86 months]; it was 8.87 months (95% ci: 6.67 to 10.32) in the elf (etoposide–leucovorin–5-fluorouracil) group (p = 0.6291).
FIGURE 4.
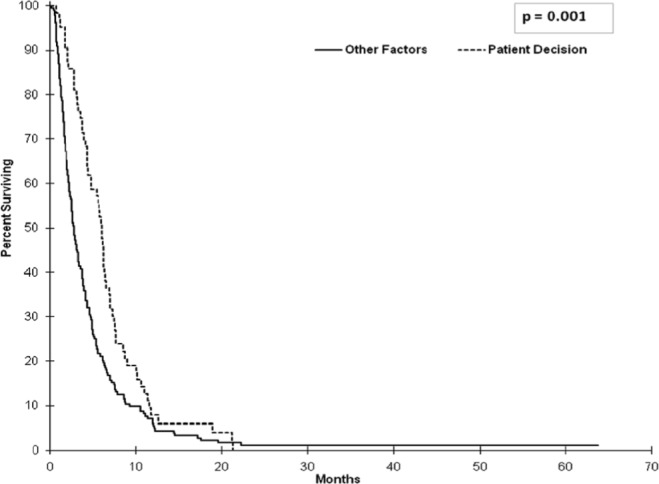
Overall survival (os) in patients receiving best supportive care depending on patient decision or other factors [poor Eastern Cooperative Oncology Group (ecog) performance status (ps) or comorbidities]. Median os in patients who declined chemotherapy despite having a good ecog ps was 6.11 months [95% confidence interval (ci): 4.44 to 6.47 months]; it was just 2.83 months (95% ci: 2.43 to 3.32 months) in patients with other reasons for not undergoing treatment (hazard ratio: 0.62; p = 0.001).
4. DISCUSSION
In the present study, we compared clinicopathologic characteristics and survival outcomes for patients with advanced esophageal, gej, and gastric adenocarcinoma treated with first-line chemotherapy (ecf or ecx; or elf) or with bsc at our institution with data for historical control patients. Of our patient population, 79% were men, which accords with the sex distribution found in previous reports5,8. The most common primary tumour site in our cohort was the stomach, with only 18% of patients having intestinal-type disease, which is well known to carry a more favourable outcome than diffuse-type tumours9–11. That proportion was clearly lower than the proportion in a previous report from the United States, which showed that 74% of gastric cancers were classified as the intestinal type8. The low proportion of intestinal-type tumours in the present study might be explained by an associated large number of non-classified tumours (78%).
At our institution, anthracycline-based chemotherapy (ecf and ecx) is used as the first-line chemotherapy option in patients with a good ecog ps. Our study confirms the substantially superior os benefit of ecf or ecx over bsc, with a 64% reduction in risk of death (hr: 0.36; p < 0.001). Among patients treated with ecf or ecx, the median os (12.7 months) was longer than those reported from landmark trials, including the study conducted in the United Kingdom, in which the median os was 8.9 months with ecf12, and the real-2 trial, in which the median os was 9.9 months for the ecf and ecx arms alike5. Although we observed no obvious differences in clinical characteristics between the patients in the present study and those in the real-2 trial (Table ii), a major difference was the histologic tumour type. Specifically, only the adenocarcinoma subtype met our inclusion criteria, and approximately 10% of the patients who participated in real-2 had squamous cell tumours or undifferentiated carcinoma. Other histologic characteristics that might affect survival, such as histologic grade and Lauren classification, were not evaluated in the real-2 trial.
TABLE II.
Demographics and baseline characteristics of patients receiving ecf/ecx at the Cross Cancer Institute (cci) and during the real-2 study5
| Variable | cci | real-2 |
|---|---|---|
| Median age (years) | 60 | 64 |
| ecog ps (%) | ||
| 0–1 | 62 | 88 |
| 2 | 7 | 12 |
| 3–4 | 1 | — |
| Missing | 30 | — |
| Sex (%) | ||
| Male | 80 | 81 |
| Female | 20 | 19 |
| Primary tumour site (%) | ||
| Esophagus | 29 | 32 |
| gej | 22 | 29 |
| Stomach | 49 | 39 |
| Extent of disease (%) | ||
| Locally advanced | 6 | 22 |
| Metastatic | 94 | 78 |
| Type of tumour (%) | ||
| Adenocarcinoma | 100 | 90 |
| Squamous-cell carcinoma | — | 9 |
| Undifferentiated carcinoma | — | 1 |
| Metastatic sites (%) | ||
| 0 or 1 | 63 | 61 |
| ≥2 | 37 | 39 |
ecog ps = Eastern Cooperative Oncology Group performance status; gej = gastroesophageal junction.
Perhaps more importantly, the earlier studies allowed a maximum of only 8 cycles of chemotherapy, whereas at our institution, we continue treatment until disease progression or intolerance of therapy. However, the median number of cycles of chemotherapy in the present study is less than that reported in the real-2 trial. We postulate that continuation of chemotherapy beyond 8 cycles where clinically appropriate is an important factor allowing for longer median survival. For future clinical trials in this patient population, consideration should be given to allowing chemotherapy to continue until disease progression or therapy intolerance. Limiting the number of chemotherapy cycles may affect patient survival and might be considered a design flaw in future trials. In addition, for our patients, a palliative care team tended to be involved early in the disease course, which is another factor that might have allowed for longer median survival. However, a prospective study aimed at determining the survival impact of early palliative care in patients with advanced gastric cancer would be warranted.
Though elf has never been directly compared with ecf or ecx, a previous study demonstrated comparable clinical benefit for elf and a comparator arm of 5-fluorouracil, doxorubicin, and methotrexate (famtx) in advanced adenocarcinoma of the stomach, with the toxicity profile favoring elf6. Using cross-trial comparisons, elf has since been considered inferior to ecf, because the survival advantage of ecf compared with famtx was greater than that of elf compared with famtx12. Nonetheless, elf has been used at our institution for patients who have comorbidities or contraindications to anthracycline- or cisplatin-based combinations. Our study validates use of elf, given that we demonstrated a significant os benefit of elf over bsc, with a 58% reduction in the risk of death (hr: 0.42; p < 0.001). Furthermore, the median os of patients treated with elf in our study (12.7 months) is longer than that previously reported (7.2 months)6. Interestingly, progression-free survival (hr: 0.90) and os (hr: 0.88) were both comparable between patients receiving ecf or ecx and those receiving elf in the present study, even though patients received a statistically significantly higher number of cycles of ecf or ecx. However, the elf group had a higher percentage of patients with intestinal-type tumours, more locally advanced disease, and a smaller percentage of patients having 3 or more metastatic sites, all of which carry a more favorable prognosis.
Our study is limited by its retrospective design, unavoidable missing data (particularly for clinicopathologic characteristics), and also the relatively small number of patients in the elf group. Nevertheless, those aspects are not likely to have affected the survival analyses affirming the survival benefit of combination chemotherapy over bsc. Furthermore, the survival data are certainly reliable because of the completely documented records from our database.
5. CONCLUSIONS
Our analysis demonstrates that palliative combination chemotherapy substantially improved os in patients with advanced esophageal, gej, and gastric adenocarcinoma, with better survival than that seen in historical controls. Anthracycline-based chemotherapy is the standard first-line regimen, but the elf regimen can be considered an alternative in patients with contraindications to an anthracycline-based regimen. Moreover, elf is more comfortably administered than ecf given the lack of a central line requirement, although its use declined after the availability of ecx. Importantly, investigation in prospective studies of novel targeted therapies, especially her2 inhibition strategies, is still warranted to improve outcomes in these patients. We plan to follow this study by looking at the survival data in our patients who are her2 overexpressors and who were treated with anthracycline-based chemotherapy. We plan to compare their outcomes with outcomes from historical trial data in which patients received a regimen containing a her2 inhibitor7.
6. ACKNOWLEDGMENTS
The work reported here was supported by the Department of Oncology and the Faculty of Medicine, University of Alberta; Alberta Health Services; and the Faculty of Medicine, Prince of Songkla University.
7. CONFLICT OF INTEREST DISCLOSURES
No author has a financial conflict of interest with respect to this project.
8. REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: globocan 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Pisters P, Kelsen DP, Tepper JE. Cancer of the stomach. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 8th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams and Wilkins; 2008. pp. 1043–79. [Google Scholar]
- 3.Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010:CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. on behalf of the V325 Study Group Phase iii study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Starling N, Rao S, et al. on behalf of the Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 6.Vanhoefer U, Rougier P, Wilke H, et al. Final results of a randomized phase iii trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648–57. doi: 10.1200/JCO.2000.18.14.2648. [DOI] [PubMed] [Google Scholar]
- 7.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of her-2 positive advanced gastric or gastroesophageal junction cancer (toga): a phase 3, open-label, randomized controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev. 2009;18:1945–52. doi: 10.1158/1055-9965.EPI-09-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita K, Sakuramoto S, Katada N, et al. Diffuse type advanced gastric cancer showing dismal prognosis is characterized by deeper invasion and emerging peritoneal cancer cell: the latest comparative study to intestinal advanced gastric cancer. Hepatogastroenterology. 2009;56:276–81. [PubMed] [Google Scholar]
- 10.Zheng H, Takahashi H, Murai Y, et al. Pathobiological characteristics of intestinal and diffuse-type gastric carcinoma in Japan: an immunostaining study on the tissue microarray. J Clin Pathol. 2007;60:273–7. doi: 10.1136/jcp.2006.038778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyahara R, Niwa Y, Matsuura T, et al. Prevalence and prognosis of gastric cancer detected by screening in a large Japanese population: data from a single institute over 30 years. J Gastroenterol Hepatol. 2007;22:1435–42. doi: 10.1111/j.1440-1746.2007.04991.x. [DOI] [PubMed] [Google Scholar]
- 12.Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–7. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]


