Abstract
Background
Timeliness of care (rapid initiation of treatment after definitive diagnosis) is a key component of high-quality cancer treatment. The present study evaluated factors influencing timeliness of care for U.S. Medicare enrollees.
Methods
Data for Medicare enrollees diagnosed with breast, colorectal, lung, or prostate cancer while living in U.S. seer (Surveillance, Epidemiology and End Results) regions in 2000–2002 were analyzed. Patients were classified as experiencing delayed treatment if the interval between diagnosis and treatment was greater than the 95th percentile for each cancer site. The impacts of patient sociodemographic, clinical, and area-based factors on the likelihood of delayed treatment were analyzed using multivariate logistic regression.
Results
Black patients (compared with white patients) and patients initially treated with radiation therapy or chemotherapy (rather than surgery) had a greater likelihood of treatment delays across all four cancer sites. Hispanic status, dual Medicare–Medicaid status, location of initial treatment (inpatient vs. outpatient), and stage at diagnosis also affected timeliness of care for some cancer sites. Surprisingly, area-based factors reflecting availability of cancer care services were not significantly associated with timeliness of care or were associated with greater delays in areas with greater numbers of service providers.
Conclusions
Multiple factors affected receipt of timely cancer care for members of the study population, all of whom had coverage of medical care services through Medicare. Because delays in treatment initiation can increase morbidity, decrease quality of life, shorten survival, and result in greater costs, prospective studies and tailored interventions are needed to address those factors among at-risk patient groups.
Keywords: Neoplasms, access to health care, Medicare, health care disparities
1. INTRODUCTION
Multiple sociodemographic factors affect cancer care patterns, including age, sex, race or ethnicity, insurance status, household income, level of education, and usual source of health care1–9. Timeliness of care (that is, rapid initiation of treatment after definitive diagnosis) is a key component of high-quality cancer treatment. For many types of cancer, timely treatment after diagnosis can improve morbidity, quality of life, and survival.
Only a few studies have examined the influence of patient sociodemographic factors on the receipt of timely cancer care. Both Gwyn et al.10 and Elmore et al.11 found that, compared with white women, black women were more likely to experience treatment delays. In contrast, among prostate cancer patients treated in the U.S. military health care system (that is, an “equal-access medical care system”), no significant differences in wait time were observed between black and white men12.
A number of studies have used data from the U.S. National Cancer Institute’s Surveillance, Epidemiology and End Results (seer) program cancer registry linked to Medicare claims data (seer–Medicare). Based on such data, Gorin et al.13 reported that, compared with white women, black women with breast cancer experienced increased odds of treatment delays. Other studies using seer–Medicare data found that older patients or those residing in rural areas had an increased likelihood of delay in initiation of adjuvant chemotherapy for breast cancer14, and older patients or those with more comorbid conditions had an increased likelihood of delays in starting adjuvant chemotherapy for colorectal cancer15.
One recent study used data from the National Cancer Data Base, a hospital-based cancer registry jointly sponsored by the American Cancer Society and the American College of Surgeons, to examine the impact of patient sociodemographic factors on timeliness of cancer care16. The study reported that, compared with white patients, black and Hispanic patients with cancer were at greater risk of experiencing delays in care. In addition, insurance status, geographic region, and type of treatment hospital were associated timelines of care. The study concluded that additional research was needed to examine the role of health system, physician, clinical, and patient characteristics in treatment delays.
To provide further information about factors that may affect treatment delays for common types of cancer, particularly among older people, who experience the greatest rates of cancer incidence, we used seer-Medicare claims data to examine disparities in timeliness of care. Our study assesses the role of individual-level and area-level characteristics on timeliness of care for individuals with the four most common types of cancer in the United States.
2. METHODS
2.1. Study Population
For this application, we included seer–Medicare data for patients diagnosed with breast (female only), colorectal, lung, or prostate cancer during 2000–2002 (the most recent seer–Medicare data at the time of study initiation) in all available seer regions17. Of patients with a cancer diagnosis listed in the seer registry, we included only those who received chemotherapy, radiation therapy, or surgical cancer treatment within 12 months after the diagnosis and who were continuously enrolled in Medicare Part A and B for 12 months after the diagnosis. This approach allowed us to exclude patients who did not receive cancer therapy with curative intent because of comorbidities, patient preference, or other factors. Further, because the date of cancer diagnosis might be set to the date of Medicare enrollment for individuals diagnosed with cancer before age 65, only patients diagnosed with cancer at age 66 or older were included.
2.2. Study Variables
We used seer registry data to identify the site and stage of cancer, age at diagnosis, date of diagnosis, race and ethnicity, county of residence, and rural or urban status of residence. Because the American Joint Committee on Cancer stage was missing for many patients (particular those with prostate cancer), seer stage categories of in situ, local, regional, distant, and unstaged were used. Because very few breast cancer patients were unstaged (<1%), those unstaged patients were eliminated from the study population. Further, because almost no lung cancer patients were diagnosed with in situ disease, the patients classified as in situ were combined with those diagnosed with local disease. Similarly, because of small sample sizes, prostate cancer patients with in situ or local disease at diagnosis were combined with patients having regional disease at diagnosis.
Using seer data, race and ethnicity were coded as white, black, Hispanic, Asian, and other (the latter combining the seer categories “Native American,” “unknown,” and “other”). Rural or urban status for the patients’ county of residence was coded based on definitions in the 2004 area resource file (arf), which ranged from “counties of metro areas of 1 million population or more” (referent group) to “completely rural or less than 2,500 urban population.” The smallest two categories of the continuum (completely rural or less than 2,500 urban population adjacent to a metro area, and the corresponding population not adjacent to a metro area) were combined because of small numbers. Marital status was included in the model as single, married, divorced, or widowed.
Medicare enrollment information was used to assess dual Medicare–Medicaid coverage status (indicating the low-income status of Medicare enrollees). Part A and B claims were used to determine the date and type of initial cancer therapy [based on selected cpt (Current Procedural Terminology), icd-9 (International Classification of Diseases, 9th Revision), and hcpcs (Healthcare Common Procedure Coding System) procedure codes] and the level of patient comorbidities (scored based on the modified Charlson comorbidity index as adapted by Deyo et al.18). Patients were classified into four comorbidity groups based on Charlson scores of 0–3 (fewest comorbidities), 4–6, 7–9, and 10 or more (most comorbidities).
The seer–Medicare dataset includes limited information directly assessing patient socioeconomic status and ease of access to medical care. Because both of those factors may affect timeliness of care, we used a number of variables measuring characteristics of the patients’ census track or county of residence as proxies. Using information on the county of residence (Federal Information Processing Standard code, also included in the seer file), we merged data from the arf to assess county-specific medical care access variables: number of hospitals offering oncology services or radiation therapy services, and four cancer site–specific access variables. These county-based site-specific variables were number of hospitals offering breast cancer screening or mammography services (for breast cancer patients), number of colorectal cancer surgeons engaged in patient care (for colorectal cancer patients), number of thoracic surgeons engaged in patient care (for lung cancer patients), and number of urologists engaged in patient care (for prostate cancer patients). Neither the number of breast cancer surgeons nor the number of medical oncologists was available in the arf data by county. Each county-based cancer site–specific variable was included only in the regressions for the relevant patient group. We also used arf data to control for the percentage of Medicare managed-care penetration in each county. For all variables, arf data from 2002 or the most recent year of data before 2002 were used.
2.3. Outcome Measure
The study evaluated factors affecting timeliness of care, defined as the time from cancer diagnosis to initiation of first treatment. Date of cancer diagnosis was obtained from the seer “first diagnosis date at age 65 or older” field, specified as month and year. Date of treatment initiation was determined by examining Medicare Part A and B claims for procedure codes (cpt, icd-9, or hcpcs; see Appendix A) corresponding to chemotherapy, radiation therapy, or surgery specific to the indicated cancer site. Time from diagnosis to initial treatment was determined as the number of months from diagnosis to the first Medicare treatment claim.
To evaluate patient characteristics associated with significant delays in therapy, we used delays greater than the 95th percentile of time from diagnosis to treatment initiation as the dependent variable. The 95th percentile was chosen to represent delays that would correspond to a statistically significant one-tailed time period (that is, p < 0.05) from diagnosis to treatment initiation. Our intent in using the 95th percentile was to identify relative “outliers” in terms of delays in treatment for each cancer site, particularly given that the United States has no widely accepted guidelines for wait times from diagnosis to treatment. Part A and B claims were also used to categorize the venue of initial treatment (hospital inpatient vs. hospital outpatient vs. physician office), which was included in the multivariate regression analyses as discussed later.
2.4. Statistical Analysis
Multivariate logistic regression was used to assess the likelihood of a patient’s being in the top 5% of time from diagnosis to treatment initiation, controlling for age group (quartiles of the study population), sex (when appropriate), race or ethnicity, dual Medicare–Medicaid status, marital status, level of comorbidities, urban or rural residence status, year of diagnosis (2000, 2001, 2002), venue of initial treatment (hospital inpatient vs. hospital outpatient vs. physician office), and the area-based variables described earlier. For all analyses, patient age, Charlson score, and values of area-based variables were categorized in quartiles by cancer site, with the lowest quartile being used as the reference group. A single multivariate logistic model was run for each cancer type, incorporating the specified demographic, clinical, and area-based variables. All analyses were performed using the SAS software application (version 9.1: SAS Institute, Cary, NC, U.S.A.).
3. RESULTS
3.1. Study Population Characteristics
A total of 161,274 patients met the study’s inclusion criteria. Table i presents details of the study population (all patients included in the analyses). Prostate cancer patients constituted the largest population, followed by colorectal, breast, and lung cancer patients. Most patients were non-Hispanic white. Between 9% and 16% of patients were covered by dual Medicare–Medicaid insurance, depending on the cancer site. Level of comorbidities (based on Charlson score) varied substantially between the patients for the four cancer sites, with approximately 15% of prostate cancer patients compared with more than 56% of lung cancer patients being in the group with the most comorbidities. Almost half of the colorectal cancer patients and more than 70% of the breast cancer patients were diagnosed with in situ or local-stage disease. In contrast, only 23% of lung cancer patients were diagnosed with local-stage disease.
TABLE I.
Characteristics of the study population
| Variable |
Cancer site
|
|||
|---|---|---|---|---|
| Breast | Colorectal | Lung | Prostate | |
| Patients (n) | 39,762 | 39,951 | 32,899 | 47,749 |
| Mean age at diagnosis (years) | 75.9 | 77.7 | 74.6 | 74.4 |
| Race/ethnicity (%) | ||||
| White | 88.8 | 86.3 | 87.8 | 84.4 |
| Black | 6.6 | 7.7 | 7.3 | 9.3 |
| Hispanic | 1.2 | 1.4 | 1.1 | 1.9 |
| Asian | 1.7 | 2.7 | 2.2 | 2.6 |
| Other | 1.6 | 1.9 | 1.5 | 1.8 |
| Female | 100.0 | 55.1 | 46.6 | 0.0 |
| Marital status (%) | ||||
| Married | 41.8 | 47.9 | 53.2 | 68.7 |
| Single | 7.0 | 7.7 | 6.4 | 6.4 |
| Divorced | 6.7 | 5.5 | 7.5 | 4.1 |
| Widowed | 39.4 | 32.9 | 27.2 | 9.5 |
| Unknown | 5.1 | 6.0 | 5.7 | 11.3 |
| Dual Medicare–Medicaid coverage (%) | 13.0 | 15.7 | 15.0 | 8.9 |
| Comorbidities (%) | ||||
| Charlson score 0–3 | 35.6 | 19.5 | 6.7 | 41.6 |
| Charlson score 4–6 | 22.2 | 21.5 | 13.2 | 28.7 |
| Charlson score 7–9 | 21.3 | 24.5 | 23.4 | 15.1 |
| Charlson score 10+ | 20.9 | 34.5 | 56.6 | 14.6 |
| Urban/rural residence (%) | ||||
| Metro county | ||||
| With >1 million population | 56.0 | 56.0 | 55.1 | 56.3 |
| With 250,000 to 1 million population | 19.5 | 18.3 | 17.7 | 18.2 |
| With <250,000 population | 9.6 | 9.3 | 10.3 | 9.8 |
| Urban county (not) adjacent to metro area | ||||
| Adjacent with >20,000 population | 3.9 | 3.9 | 4.4 | 3.9 |
| Not adjacent with >20,000 population | 2.1 | 2.2 | 2.3 | 2.1 |
| Adjacent with 2,500–19,999 population | 4.0 | 4.7 | 4.6 | 4.7 |
| Not adjacent with 2,500–19,999 population | 3.2 | 3.8 | 3.6 | 3.2 |
| Completely rural county or urban county | ||||
| With <2,500 population | 1.6 | 1.9 | 2.0 | 1.7 |
| Stage at diagnosis (%) | ||||
| In situ | 15.1 | 5.7 | na | na |
| Local | 58.0 | 40.9 | 22.7 | na |
| Regional | 22.7 | 35.1 | 39.7 | 91.9 |
| Distant | 4.3 | 14.2 | 33.1 | 4.2 |
| Unstaged | na | 4.1 | 4.5 | 3.9 |
| Location of initial therapy (%) | ||||
| Inpatient facility | 47.7 | 80.9 | 58.6 | 28.7 |
| Hospital outpatient facility | 47.9 | 8.5 | 33.8 | 60.3 |
| Physician office | 4.4 | 10.6 | 7.6 | 11.0 |
| Type of initial therapy (%) | ||||
| Surgery | 95.6 | 95.4 | 26.8 | 32.5 |
| Chemotherapy | 2.3 | 2.1 | 33.4 | 45.6 |
| Radiation therapy | 2.2 | 2.5 | 39.8 | 21.8 |
| Time from diagnosis to treatment (months) | ||||
| 95th percentile | 2 | 1 | 3 | 5 |
na = not applicable.
Table i also presents the study outcome measure, the 95th percentile of time (in months) from diagnosis to initial treatment. Colorectal cancer patients experienced the shortest time from diagnosis to treatment initiation, with the 95th percentile being 1 month. The 95th percentile was 2 months for breast cancer patients, 3 months for lung cancer patients, and 5 months for prostate cancer patients.
3.2. Patient Factors Associated with Timeliness of Care
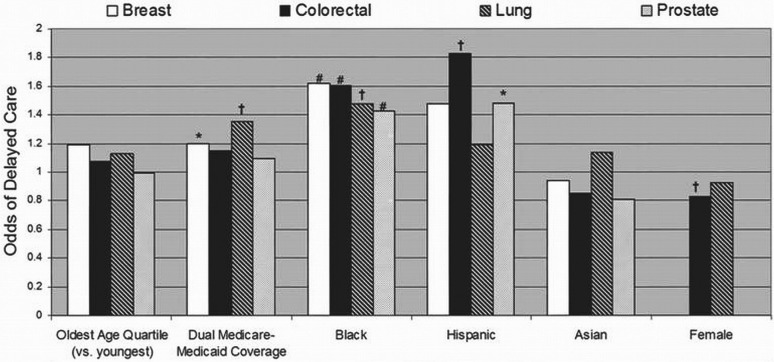
Figure 1 presents results from the multivariate logistic regression analyses examining the association between timeliness of care (that is, treatment starting at or beyond the 95th percentile of time from diagnosis) and patient characteristics by cancer site. Age did not significantly affect delays in care. Compared with patients not having Medicaid coverage, dual Medicare–Medicaid patients with breast or lung cancer were more likely to experience delays. Compared with white patients, black patients with any of the four cancers studied were very significantly more likely to experience delays in care (p < 0.0001 or 0.0005); Hispanic patients with colorectal or prostate cancer were similarly more likely to experience delayed initiation of treatment (p < 0.0005 or 0.05). No significant differences were observed between Asian and white patients. Comparisons of patients in the “other” race and ethnicity category with white patients were also nonsignificant (data not shown). Compared with men, women with colorectal cancer were significantly less likely to experience treatment delays, but no differences by sex were observed for lung cancer patients.
FIGURE 1.
Patient factors affecting timeliness of care: odds ratios from the multivariate logistic regression models for the likelihood of delays in initiation of cancer treatment for patients with breast cancer, colorectal cancer, lung cancer, and prostate cancer. Increased likelihood of delay was observed among black patients (compared with white patients) for all four cancer types, and among dual Medicare–Medicaid patients (compared with those enrolled in Medicare only) and among Hispanic patients (compared with white patients) for certain cancer sites. The likelihood of delay was observed to be decreased for women (compared with men) having colorectal cancer. * p < 0.05. † p < 0.0005. # p < 0.0001.
Table ii presents additional results from individual-level variables included in regression analyses that are not illustrated in Figure 1. Although a small number of significant associations were observed between residence status and delays in care, results were not consistent across the four cancer sites. Residence in the most rural areas was significantly associated with increased treatment delays only among prostate cancer patients.
TABLE II.
Additional multivariate regression results1
| Variable |
Cancer site
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
Breast
|
Colorectal
|
Lung
|
Prostate
|
|||||
| or | p Value | or | p Value | or | p Value | or | p Value | |
| Residence (reference: metro county with >1 million population) | ||||||||
| Metro county | ||||||||
| With 250,000 to 1 million population | 1.09 | ns | 0.74 | <0.005 | 0.94 | ns | 1.09 | ns |
| With <250,000 population | 0.85 | ns | 0.68 | <0.005 | 0.65 | <0.005 | 1.22 | ns |
| Urban county (not) adjacent to metro area | ||||||||
| Adjacent with >20,000 population | 1.41 | ns | 0.90 | ns | 1.09 | ns | 1.35 | <0.05 |
| Not adjacent with >20,000 population | 1.32 | ns | 0.65 | ns | 1.18 | ns | 0.86 | ns |
| Adjacent with 2,500–19,999 population | 1.12 | ns | 0.64 | <0.05 | 0.99 | ns | 0.89 | ns |
| Not adjacent with 2,500–19,999 population | 1.54 | ns | 0.79 | ns | 1.05 | ns | 1.40 | <0.05 |
| Completely rural county or urban county | ||||||||
| With <2,500 population | 1.30 | ns | 0.74 | ns | 0.94 | ns | 1.53 | <0.05 |
| Marital status (reference: married) | ||||||||
| Single | 1.33 | <0.05 | 1.07 | ns | 1.05 | ns | 1.21 | <0.05 |
| Divorced | 1.23 | ns | 1.09 | ns | 1.35 | <0.005 | 1.13 | ns |
| Widowed | 1.21 | <0.05 | 1.10 | ns | 1.19 | <0.05 | 1.00 | ns |
| Stage at diagnosis | ||||||||
| Reference | Local | Local | Local | Locoregional | ||||
| In situ | 1.10 | ns | 0.56 | <0.0005 | — | — | — | — |
| Regional | 1.06 | ns | 0.89 | ns | 0.59 | <0.0001 | — | — |
| Distant | 0.76 | <0.05 | 1.08 | ns | 0.29 | <0.0001 | 0.88 | ns |
| Unstaged | — | — | 2.81 | <0.0001 | 1.15 | ns | 1.43 | <0.005 |
| Type of initial treatment (reference: initial surgery) | ||||||||
| Chemotherapy | 6.74 | <0.0001 | 6.50 | <0.0001 | 1.32 | <0.0005 | 1.39 | <0.0001 |
| Radiation therapy | 8.76 | <0.0001 | 5.39 | <0.0001 | 2.06 | <0.0001 | 3.52 | <0.0001 |
ns = statistically nonsignificant.
3.3. Clinical Factors Associated with Timeliness of Care
Table iii presents regression results (odds ratios and statistical significance) for selected clinical factors associated with timeliness of care. Comorbidities had a significant impact on treatment delays only for women with breast cancer. The venue of initial treatment was strongly associated with delays in care for three of the four types of cancer studied—that is, significant delays in treatment initiation were observed for colorectal, lung, and prostate cancer patients whose initial treatment episode occurred in an outpatient setting (either a physician office or a hospital outpatient clinic) rather than as an inpatient episode. However, venue of treatment was not significantly associated with delays for breast cancer patients. That observation may reflect the high rate of surgery as initial treatment (Table i) for breast cancer, and the experience of clinicians in performing breast cancer surgeries, including mastectomies, in outpatient settings.
TABLE III.
Multivariate regression results for clinical and area-based factors1
| Variable |
Cancer site
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
Breast
|
Colorectal
|
Lung
|
Prostate
|
|||||
| or | p Value | or | p Value | or | p Value | or | p Value | |
| Clinical factors | ||||||||
| Most comorbidities (vs. fewest) | 1.43 | 0.0005 | 1.13 | ns | 1.23 | ns | 1.12 | ns |
| Year of diagnosis | ||||||||
| 2001 | 0.93 | ns | 1.18 | 0.05 | 1.00 | ns | 1.04 | ns |
| 2002 | 1.27 | 0.005 | 1.20 | 0.005 | 1.03 | ns | 1.00 | ns |
| Treatment site | ||||||||
| Hospital outpatient clinic | 1.13 | ns | 1.34 | 0.0005 | 1.36 | 0.0001 | 1.17 | 0.05 |
| Physician office | 1.25 | ns | 1.23 | 0.005 | 1.40 | 0.005 | 1.53 | 0.0001 |
| Area-based factorsa | ||||||||
| Number of hospitals with | ||||||||
| Oncology services | 1.83 | ns | 0.73 | ns | 0.79 | ns | 0.72 | ns |
| Radiation therapy services | 1.11 | ns | 1.40 | 0.05 | 0.95 | ns | 1.08 | ns |
| Percentage Medicare managed care penetration | 1.82 | 0.0001 | 1.11 | ns | 1.00 | ns | 1.28 | 0.05 |
| Number of cancer-site specific providers or centres | 0.62 | ns | 0.90 | ns | 1.52 | 0.05 | 1.41 | 0.05 |
| Census tract | ||||||||
| Percentage non-English speakers | 1.20 | ns | 1.02 | ns | 1.26 | 0.05 | 1.08 | ns |
| Percentage with high school only or less education | 0.98 | ns | 1.04 | ns | 1.00 | ns | 0.91 | ns |
Impact of highest quartile compared with lowest quartile.
ns = statistically nonsignificant.
Table ii presents a number of other clinical factors associated with timeliness of care. Among breast cancer patients, those with distant-stage disease at diagnosis (compared with the reference category, local-stage disease) were significantly less likely to experience delays. Lung cancer patients with regional- or distant-stage disease were also less likely to experience delays. In contrast, colorectal cancer patients with in situ disease (compared with local-stage disease) at diagnosis were less likely to experience delays (perhaps because the in situ cancer was removed at the time of diagnostic colonoscopy), and delays for those with regional- or distant-stage disease were not statistically different from delays for patients with local-stage disease. For both colorectal cancer and prostate cancer patients, individuals with unstaged disease were significantly more likely to experience delays.
Across all four cancer sites, patients who received chemotherapy or radiation therapy as initial treatment were significantly more likely to experience treatment delays than were the patients who initially received surgery. These delays were statistically significant for all four groups (p < 0.0001), independent of the effect of stage at diagnosis (which was separately controlled for). Further, the observed effects were not related to possible delays resulting from surgery needed before commencement of radiation therapy or chemotherapy, because the patients did not receive prior cancer-specific surgeries. The magnitude of the association was greatest for breast and colorectal cancer patients. That finding may reflect the treatment patterns presented in Table i: Nearly all breast and colorectal cancer patients (>95%) received surgery as initial therapy, suggesting complicating factors or unusual circumstances that could delay care among the patients receiving initial chemotherapy or radiation therapy.
3.4. Area-Based Factors Associated with Timeliness of Care
Table iii also presents results from regression analyses of area-based factors. We expected that greater availability of oncology care in geographic proximity to a study patient would be associated with more timely care. However, the presence of more hospitals offering oncology services or radiation therapy services in a patient’s county of residence had little significant association with delays in treatment initiation. Greater numbers of thoracic surgeons or urologists in a county were associated with significantly increased likelihoods of delays in treatment initiation for lung and prostate cancer patients respectively.
We also found that breast cancer and prostate cancer patients residing in counties with the highest quartile of Medicare managed-care penetration (compared with those residing in counties in the lowest quartile) were more likely to experience delays. Residing in census tracts with a greater proportion of individuals having only high school or lower education was not significantly associated with treatment delays for any of the four cancers.
4. DISCUSSION
Our analyses indicate that delays in treatment initiation after definitive diagnosis for breast, colorectal, lung, and prostate cancer patients are significantly associated with a variety of patient, clinical, and area-level factors. However, our results indicate that those factors affect timeliness of care differently across the four cancer sites. Patient race or ethnicity (particularly for black patients), location of initial treatment (that is, inpatient vs. outpatient), and type of initial treatment (surgery vs. radiation therapy or chemotherapy), were strongly associated with timeliness of care for most or all of the cancer sites. In contrast, other factors such as dual Medicare–Medicaid status were significantly associated with delays in care for only one or two of the cancer sites. That observation likely reflects differences both in treatment patterns and in factors influencing treatment decisions by patients and clinicians across the four cancer sites.
Greater likelihood of delayed treatment initiation was also observed for three of the four cancer sites (colorectal, lung, and prostate) for patients receiving initial therapy as an outpatient rather than as an inpatient. To our knowledge, that observation has not previously been reported. This treatment location affect is unlikely to be a result of stage at diagnosis or patient comorbidities, because the analyses controlled for both of those factors separately. Discussions with oncologists suggest that this finding may be a result of scheduling issues—that is, finding an available time and bringing together the necessary health care providers to initiate treatment may be more difficult in outpatient settings (for example, private practices) than in inpatient environments. As minimally invasive surgeries and other interventions (including multimodality therapies) that do not require inpatient stays become more common, additional steps will be needed to ensure that patient preference for outpatient treatment does not result in delayed care.
The present study has a number of limitations. First, only individuals age 66 or older residing in seer regions at the time of diagnosis were included. Furthermore, the outcome measure (time from diagnosis to treatment initiation) is based on data from the seer and Medicare datasets and is therefore limited to information available in those datasets. For example, the data contain limited information on patient-level characteristics. Beyond information on dual Medicare–Medicaid status, no data on socioeconomic status were available for the patients. Gwyn et al.10 reported that the poverty index (annual household income adjusted by the number of individuals in the household) was significantly associated with delayed treatment initiation for women with breast cancer, suggesting that individual-level socioeconomic variables may be significantly associated with timeliness of care. In preliminary analyses, we found that ZIP code–based median household income was not significantly associated with timeliness of care (data not shown), and the study regressions therefore did not include that variable. Collection by seer cancer registries of individual socioeconomic status variables, such as household income or years of education, would permit greater assessment of the impacts of those important factors.
Our study population included only individuals who received treatment, defined as surgery, chemotherapy, or radiation therapy, within 1 year of diagnosis. Although this choice likely includes most Medicare enrollees diagnosed with cancer while residing in seer regions, there are almost certainly individuals who chose to receive other treatments or no treatment at all. Particularly for men diagnosed with prostate cancer, oral hormonal therapy or expectant management (“watchful waiting”) may be appropriate treatment alternatives. Others may choose best supportive care that does not involve treatments captured in our study. Those possibilities may limit the generalizability of our results to individuals who receive more “standard” and potentially curative treatments for cancer. Further, some patients with late-stage diagnosis of their cancer may have short waits because they require emergent or salvage treatment (for example, locally advanced breast cancer, colorectal cancer with perforation). Adjustment for stage will not necessarily account for that possibility, which therefore may also affect the results. However, it is likely that such patients represent a small proportion of the total study population.
The present study focused on what is essentially an intermediate outcome: delay in treatment initiation. Delays in the timeliness of care matter only if they are associated with other outcomes affecting patient morbidity and mortality, such as the need for more invasive or intensive treatment, increased treatment-associated toxicities, decreased quality of life, and decreased survival. Most studies have reported clinical impacts associated with delays in cancer treatment. For example, Hershman et al.14,15 reported that delays in initiation of adjuvant chemotherapy were associated with increased mortality for breast and colorectal cancer patients. Colleoni et al.19 reported that early initiation of chemotherapy among estrogen receptor–negative breast cancer patients was associated with increased survival, but early initiation did not significantly affect patients with estrogen receptor–positive breast cancer. In a study of 129 veterans with lung cancer, time to treatment initiation did not significantly affect survival, although a trend toward greater survival among individuals with solitary pulmonary nodules who were treated in a more timely manner was observed20. Recently, a meta-analysis presented by Raphael et al.21 found that increased delays in the receipt of adjuvant chemotherapy were associated with significant decreases in both overall survival and disease-free survival. More work is needed to delineate the effects of timeliness of care on outcomes other than survival, including quality of life, functional status, and costs.
5. CONCLUSIONS
Our study indicates that multiple factors are associated with disparities in timeliness of care for Medicare enrollees with cancer. Race or ethnicity, dual Medicare–Medicaid status, venue and type of treatment initiation, and stage at diagnosis were all significantly associated with delay in treatment initiation for certain cancer sites. Results from our study accord with studies assessing timeliness of care for individuals diagnosed with cancer in the 1980s and 1990s10,11,13,14 and with a recent hospital registry-based study16. Interventions to address patient, provider, and health system factors that may contribute to delays in care are needed to achieve optimal health outcomes for all cancer patients.
APPENDIX A: PROCEDURE CODES USED TO IDENTIFY CANCER TREATMENT
| Treatment type | Cancer site |
Procedure code authority
|
||
|---|---|---|---|---|
| cpt | icd-9 | hcpcs | ||
| Chemotherapy | All | 96401–96549 | 99.25, 99.28 | J1825, J1830, J9xxx |
| Radiation therapy | All | 77401–77525, 77750–77799, 79000–79999 | 92.2x, 92.3x | A9600, A9605, Q3001 |
| Surgery | Breast | 19120, 19125, 19126, 19160, 19162, 19180, 19182, 19200, 19220, 19240 | 85.20, 85.21, 85.22, 85.23, 85.33, 85.34, 85.35, 85.36, 85.4x | na |
| Prostate | 52601, 52612, 52614, 52647, 52648, 53850–53853, 55801–55845, 55866, 55873 | 60.2x, 60.3, 60.4, 60.5, 60.6x, 60.96, 60.97 | na | |
| Colorectal | 44140–44160, 44204–44212, 45110–45190, 45308–45315, 45320, 45333, 45338, 45339, 45383–45385, 45395, 45397 | 45.4x, 45.7x, 45.8x, 48.3x, 48.4x, 48.5x, 48.6x, 48.82 | na | |
| Lung | 32440–32504, 32657, 32662, 32663 | 32.0x, 32.1, 32.31, 32.28, 32.29, 32.3, 32.4, 32.5, 32.6, 32.9 | na | |
cpt = Current Procedural Terminology; icd-9 = International Classification of Diseases, 9th Revision; hcpcs = Healthcare Common Procedure Coding System; x = any additional digit or no additional digit.
6. CONFLICT OF INTEREST DISCLOSURES
Neither of the authors has potential financial conflicts of interest regarding this manuscript. No external funding was received for performance of the study.
7. REFERENCES
- 1.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–6. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 2.Hahn KM, Bondy ML, Selvan M, et al. Factors associated with advanced disease stage at diagnosis in a population-based study of patients with newly diagnosed breast cancer. Am J Epidemiol. 2007;166:1035–44. doi: 10.1093/aje/kwm177. [DOI] [PubMed] [Google Scholar]
- 3.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9:222–31. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 4.Halpern MT, Chen AY, Marlow NS, Ward E. Disparities in receipt of lymph node biopsy among early stage female breast cancer patients. Ann Surg Oncol. 2009;16:562–70. doi: 10.1245/s10434-008-0205-7. [DOI] [PubMed] [Google Scholar]
- 5.Harlan LC, Greene AL, Clegg LX, Mooney M, Stevens JL, Brown ML. Insurance status and the use of guideline therapy in the treatment of selected cancers. J Clin Oncol. 2005;23:9079–88. doi: 10.1200/JCO.2004.00.1297. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi M, Thacker HL, Litaker DG, Kippes C. Differences in breast cancer screening rates: an issue of ethnicity or socioeconomics? J Womens Health Gend Based Med. 2000;9:1025–31. doi: 10.1089/15246090050200060. [DOI] [PubMed] [Google Scholar]
- 7.Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999;91:1409–15. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 8.Sambamoorthi U, McAlpine DD. Racial, ethnic, socioeconomic, and access disparities in the use of preventive services among women. Prev Med. 2003;37:475–84. doi: 10.1016/S0091-7435(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 9.Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 10.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100:1595–604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 11.Elmore JG, Nakano CY, Linden HM, Reisch LM, Ayanian JZ, Larson EB. Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Med Care. 2005;43:141–8. doi: 10.1097/00005650-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Optenberg SA, Thompson IM, Friedrichs P, Wojcik B, Stein CR, Kramer B. Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA. 1995;274:1599–605. doi: 10.1001/jama.1995.03530200035033. [DOI] [PubMed] [Google Scholar]
- 13.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166:2244–52. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 14.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006;99:313–21. doi: 10.1007/s10549-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 15.Hershman D, Hall MJ, Wang X, et al. Timing of adjuvant chemotherapy initiation after surgery for stage iii colon cancer. Cancer. 2006;107:2581–8. doi: 10.1002/cncr.22316. [DOI] [PubMed] [Google Scholar]
- 16.Fedewa SA, Edge SB, Stewart AK, Halpern MT, Marlow NM, Ward EM. Race and ethnicity are associated with delays in breast cancer treatment (2003–2006) J Health Care Poor Underserved. 2011;22:128–41. doi: 10.1353/hpu.2011.0006. [DOI] [PubMed] [Google Scholar]
- 17.United States; National Cancer Institute (nci) Surveillance, Epidemiology and End Results (seer) Home > About seer > seer Registries > List of seer Registries [Web page] Bethesda, MD: NCI; n.d. [Available online at: http://seer.cancer.gov/registries/list.html; cited February 21, 2012] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with icd-9-cm administrative databases. J Clin Epidemiol. 1992;45:613–19. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Colleoni M, Bonetti M, Coates AS, et al. Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. The International Breast Cancer Study Group. J Clin Oncol. 2000;18:584–90. doi: 10.1200/JCO.2000.18.3.584. [DOI] [PubMed] [Google Scholar]
- 20.Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133:1167–73. doi: 10.1378/chest.07-2654. [DOI] [PubMed] [Google Scholar]
- 21.Raphael M, Biagi JJ, Mackillop WJ, et al. The impact of time to adjuvant chemotherapy (ac) on survival in colorectal cancer (crc): a systematic review and meta-analysis [abstract 6125] J Clin Oncol. 2011;29 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=102&abstractID=84808; cited September 7, 2012] [Google Scholar]