Abstract
Wheat end-use quality mainly derives from two interrelated characteristics: the compositions of gluten proteins and grain hardness. The composition of gluten proteins determines dough rheological properties and thus confers the unique viscoelastic property on dough. One group of gluten proteins, high molecular weight glutenin subunits (HMW-GS), plays an important role in dough functional properties. On the other hand, grain hardness, which influences the milling process of flour, is controlled by Puroindoline a (Pina) and Puroindoline b (Pinb) genes. However, little is known about the combined effects of HMW-GS and PINs on dough functional properties. In this study, we crossed a Pina-expressing transgenic line with a 1Ax1-expressing line of durum wheat and screened out lines coexpressing 1Ax1 and Pina or lines expressing either 1Ax1 or Pina. Dough mixing analysis of these lines demonstrated that expression of 1Ax1 improved both dough strength and over-mixing tolerance, while expression of PINA detrimentally affected the dough resistance to extension. In lines coexpressing 1Ax1 and Pina, faster hydration of flour during mixing was observed possibly due to the lower water absorption and damaged starch caused by PINA expression. In addition, expression of 1Ax1 appeared to compensate the detrimental effect of PINA on dough resistance to extension. Consequently, coexpression of 1Ax1 and PINA in durum wheat had combined effects on dough mixing behaviors with a better dough strength and resistance to extension than those from lines expressing either 1Ax1 or Pina. The results in our study suggest that simultaneous modulation of dough strength and grain hardness in durum wheat could significantly improve its breadmaking quality and may not even impair its pastamaking potential. Therefore, coexpression of 1Ax1 and PINA in durum wheat has useful implications for breeding durum wheat with dual functionality (for pasta and bread) and may improve the economic values of durum wheat.
Introduction
Wheat is one of the “big-three” cereal crops in the world. Its success results from not only its adaptability to a wide range of climatic conditions but also its unique end-use quality which allows it to be processed into a range of flour-based food, such as bread, pasta and noodles [1]. Wheat end-use quality mainly derives from interrelated characteristics: the contents and compositions of gluten proteins and grain hardness [2]. On one hand, wheat gluten proteins predominantly determine the rheological properties of dough and thus confer the unique viscoelastic properties on dough [3]. On the other hand, grain hardness determines the milling process of flour and the physical nature of the milled products, and therefore strongly influences a bundle of quality traits [4].
Gluten proteins consist of monomeric gliadins and polymeric glutenins. In particular, the high molecular weight glutenin subunits (HMW-GS) are especially important as they are major determinants of the functional properties of wheat dough. Hexaploid wheat contains six HMW-GS genes, with tightly linked pairs of genes encoding x-type and y-type subunits located at Glu-A1, Glu-B1 and Glu-D1 loci on the chromosomes of 1A, 1B and 1D, respectively. Particularly, HMW-GS subunits 1Ax1, 1Dx5 and 1Dy10 are associated with strong dough and good breadmaking quality in bread wheat, durum wheat and Tritordeum, respectively, by transgenic technology [5], [6], [7], [8].
Grain hardness is controlled by two closely linked genes, Pina and Pinb, located at the Hardness locus (Ha) on the short arm of chromosome 5D [9], [10], [11]. PINA and PINB compose the grain hardness marker protein, friabilin, which is found in larger amounts on soft wheat starch than that on hard wheat, and absent in durum wheat [12], [13]. Recent results have demonstrated that mutations in either the Pina-D1a allele or the Pinb-D1a allele are associated with hard texture, with both wild-type Pin genes (the Pina-D1a allele and the Pinb-D1a allele) resulting in soft-texture phenotype [11], [14]. The causative role of Pin genes in kernel hardness has been fully established by transgenic lines of rice, wheat and maize expressing Pina and/or Pinb [15], [16], [17], [18], [19].
Durum wheat (Triticum turgidum L. ssp. durum) accounting for about 5% of wheat grown in the world is mainly used for pasta and couscous and also used for leavened and flat breads in Mediterranean and the Middle East countries [2]. Besides the primary importance of breeding durum wheat cultivars with superior pastamaking quality, there is increasing interest in using durum for breadmaking. However, the absence of the D-genome is considered partly responsible for the relative poor breadmaking quality of durum wheat [20]. Furthermore, the extreme hardness of durum wheat grains, due to the absence of Pin genes on the D-genome, increases the energy consumption during milling and starch damage levels, exerting detrimental effects on breadmaking quality of durum wheat [21]. Studies on improvement of breadmaking quality of durum wheat also suggest that it may be necessary to achieve a balance of gluten strength and extensibility with increased overall dough strength [22].
We have reported that overexpression of HMW-GS subunit 1Ax1 in several durum wheat cultivars resulted in increased dough strength [7]. More recently, we introduced wild-type Puroindoline a gene (the Pina-D1a allele) into a durum wheat cultivar Luna [23]. In this study, we used conventional crossing of these transgenic lines to develop lines combining both the 1Ax1 and Pina transgenes which determines dough strength and grain hardness, respectively. These transgenic isolines that expressed transgenic 1Ax1, Pina, or 1Ax1 and Pina allow us to study the separate and combined effects of 1Ax1 and PINA on grain hardness and dough mixing properties, and to gain new insight into influences of combinations of dough strength and grain hardness on the end-use quality of wheat.
Materials and Methods
Plant Materials
Both transgenic line expressing Pina (the Pina-D1a allele) and 1Ax1, respectively, were produced by transformation of durum wheat cv. Luna, which expresses only HMW-GS pair 1Bx7+1By8 [7], [23]. Pina-expressing line was generated by particle bombardment with the plasmid pUbi-pinA, which contains the Pina-D1a gene driven by the constitutive maize ubiquitin promoter, in combination with the plasmid pCa-neo, which contains the neomycine phosphotransferase II (nptII) gene under the control of cauliflower mosaic virus 35S (CaMV 35S) promoter. 1Ax1-expressing line was transformed with the plasmid p1Ax1 [24] and pAHC25 [25]. The plasmid pAHC25 contains the bar gene, conferring the resistance to the herbicide BASTA, and the uidA gene, encoding for β-glucuronidase (GUS), both under the control of the constitutive maize ubiquitin promoter. The plasmid p1Ax1 contains a 7.0 kb EcoRI genomic fragment including the coding sequence of the Glu-A1-1a (1Ax1) gene flanked by 2.2 kb and 2.1 kb of 5′ and 3′ sequence, respectively.
Crosses of the two transgenic lines were carried out in 2007. F1 plants were screened by PCR amplification of Pina gene as described by Luo et al. [23], and F2 seeds harvested in growth chamber were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in order to screen cross progenies containing the transgenic 1Ax1, Pina or 1Ax1+Pina, respectively. Selection of homozygous progeny with stable expression of 1Ax1, PINA or 1Ax1+PINA was carried out in the following three years (2008 and 2010) by SDS-PAGE analysis of total storage proteins and Triton X-114 soluble proteins (see below). In addition, the presence of bar, uidA and nptII genes were determined by PCR in the F3, F4 and F5 generations (see below). We selected two homozygous F5 lines coexpressing 1Ax1 and Pina (named HP-19 and HP-245), two lines expressing transgenic 1Ax1 (named H-182 and H-293), two lines expressing transgenic Pina (named P-121 and P-149) and one null segregant line named N-1. Durum wheat cv. Luna was used as non-transformed control line in this study. These homozygous lines and non-transformed control were then self-pollinated and analyzed in 2011 under field conditions.
DNA Extraction and PCR Amplification
DNA was extracted from leaves by the CTAB method. PCR screening of Pina was as described by Luo et al. [23]. Determination for the presence of marker genes (bar, uidA and nptII) were carried out using primers specific for them (Table S1).
Field Trials
Field trials were performed in the experimental field of the Chinese National Center of Plant Gene Research (Wuhan) HUST Part (Wuhan, Hubei Province, China), under irrigation, using a randomized complete block design with two replicates. Each plot consisted of four rows, 2.5-m long, with 50 seeds per row. The space between rows was 30 cm, and the separation between plots was 50 cm. 1,000-seed weight was determined on 500 seeds from each plot per line with three replicates each (six measurements). Test weight expressed in grams per liter was determined by weighing 100 ml of seeds with three replicates per line and per plot (six measurements).
Seed Storage Protein Characterization
For determining grain protein contents (GPC) and flour protein contents (FPC) of each line, seeds harvested from two plots were blended together. GPC and FPC were measured on grains and flours, respectively, by near-infrared reflectance spectroscopy (NIRS) method using an Infratec TM1241 Grain Analyzer (Foss North America, Silver Spring, MD) and adjusted to a 14% moisture basis (standard methods of the International Association for Cereal Science and Technology, ICC, no. 159 and no. 202).
Total storage proteins from endosperm were extracted from single kernels of transgenic and control lines according to Liu et al. [26].
To characterize storage proteins from each line, gliadins, glutenins and other proteins were sequentially extracted from 100 mg flour from each sample according to DuPont et al. [27]. The albumin/globulin, gliadin, and glutenin fractions were sequentially extracted from the same flour sample with 0.3 M NaI in 7.5% 1-propanol followed by 2% SDS, 25 mM DTT in 25 mM TRIS, pH 8.0, and precipitation of the solubilized proteins with ammonium acetate/methanol followed by acetone. The gliadin, glutenin and albumin/globulin fractions from 15 flour samples per line were separated by SDS-PAGE as described previously [26] and quantified by densitometry method using a Bio-Rad Quantity One 1-D software version 4.6.2 (Bio-Rad, Hercules, CA). Densitometry method was selected because its higher reproducibility in characterization of storage proteins than that of HPLC [28].
Grain Hardness Measurement
Grain hardness, kernel weight and kernel diameter were measured using the Perten Single Kernel Characterization System (SKCS) 4100 (Perten Instrument North American Inc., Springfield, IL, USA) on samples of 300 seeds harvested from each plot in 2011 according to the AACC approved method 55-31 [29].
Scanning Electron Microscopy (SEM)
Dry mature wheat kernels were cut into halves with stainless steel uncoated single-edge industrial blades at room temperature and placed on aluminum specimen stub by double-sided tape. The kernel sections were gold coated at 10 mA for 6 min to get an approximately 15–20 nm thick coating (Technics Hummer V Sputter Coater, Technics, San Jose, California, USA).Then wheat kernel sections were examined using a Hitachi SEM-600 (Hitachi High-Technologies Corp., Tokyo, Japan) at 1.5 K magnification (15 kV).
Extraction of Triton X-114 Soluble Protein, Friabilin and Western Blotting
In order to detect the total puroindoline, Triton X-114 soluble protein was extracted from single crushed kernel using Triton X-114 (TX-114) detergent according to Giroux and Morris [14]. The TX-114 soluble proteins were electrophoretically separated by SDS-PAGE using standard method with 15% separating gels and stained overnight with Commassie blue R-250.
To determine the amounts of starch bound puroindoline, starch granule surface proteins were isolated from 100 mg of wholemeal flour as described previously [11], separated by SDS-PAGE using 13.5% T, 2.6% C gels and stained with Commassie blue R-250. For the SDS-PAGE and Western blotting analysis (see below) of total and starch-bound PINA, proteins extracted from bread wheat cv. Chinese Spring (CS, contains the Pina-D1a allele) and durum wheat cv. Luna (lacks Pina gene) were used as positive and negative controls, respectively.
To identify the total and starch-bound PINA and estimate their amounts, TX-114 soluble proteins and starch granule surface proteins, both of which were extracted from 100 mg of flour per line, were used in Western blotting using the same loading volume for each sample. Western blotting was performed using a rabbit anti-PINA polyclonal antibody raised from the recombinant PINA proteins expressed in E. coli. The heterologous expression of PINA protein and its purification were reported previously by Miao et al. [30]. After protein electrophoresis, gels were blotted onto nitrocellulose membranes. Membranes were blocked overnight at 4°C in 1× TBST (Tris buffered saline plus 0.1% Tween-20) containing 5% NFDM (non-fat dry milk). Anti-PINA polyclonal antibody was used at a dilution of 1∶10000 in TBST/5% BSA and incubated for 2.5 h at room temperature. Membranes were then washed with TBST for five times and incubated with 1∶4000 dilution of alkaline phosphatase conjugated goat anti-rabbit secondary antibody for 1 h at room temperature, then detected according to the manufacturer instructions. The amounts of total and starch-bound PINA were determined by densitometry analysis of Western blotting results in three biological replications using a Bio-Rad Quantity One 1-D software version 4.6.2 (Bio-Rad, Hercules, CA).
Mixograph
Seeds from six transgenic lines, one null segregant line and one non-transformed control line harvested in 2011 were used for analysis of dough mixing properties. Prior to milling, kernel moisture was adjusted to 16% by incubation overnight at room temperature. One hundred grams of seeds per line were milled to flour with a Brabender Quandrumat Junior Mill (C. W. Brabender Instruments, Inc., South Hackensack, NJ) following AACC method 26–50 [29]. Samples were mixed to optimum water absorption and the dough mixing properties for each line were determined using a 10 g Mixograph (National Manufacturing Co., Lincoln NE) with two replicates according to the approved AACC method 54-40A [29].
Mixograph parameters were obtained from Mixsmart software version 3.8 (AEW Consulting, Lincoln, NE, commercially available through National Manufacturing Division of TMCO, Lincoln NE, USA). They include four parameters describing the heights of Mixogram curve (midline left value, MLV; midline peak value, MPV; midline right value, MRV and midline value at 8 min, MTxV) and four describing the widths of curve (midline left width, MLW; midline peak width, MPW; midline right width, MRW; and midline width at 8 min, MTxW). Other parameters were midline peak time (MPT) and the area under midline for 8 min (MTxI). Weakening slope (WS) expressing dough weakening was computed by the difference of MPV and MTxV. These mixing parameters were used for they maintain a good representation of dough properties with a minimum of information redundancy [31].
Statistics Analysis
Data were analysed using the SPSS version 11.0 statistical software package (SPSS Inc., Chicago, Illinois, USA). The general analysis of variance and the least significant difference pairwise comparisons of means were used to determine significant difference. The statistical significance for mixing parameters from lines expressing 1Ax1 and/or Pina was determined using Student’s t test.
Ethics Statement
The described filed studies were approved according to the document ‘The Biosafety Permit of Transgenic Plant Research: The Permit for Field Trial of Transgenic Wheat (No. 033)’, authorized by the Ministry of Agriculture of the People’s Republic of China.
Results
We have previously reported lines of durum wheat expressing transgenic 1Ax1 with increased dough strength [7] and transgenic lines with expression of PINA [23], both of which were generated in the same durum wheat cv. Luna. To study the combined effects of 1Ax1 and PINA on dough mixing properties, these lines were used as parents to obtain hybrid lines expressing the combination of transgenic 1Ax1 and Pina by conventional crossing. It is the first report on the characterization of transgenic lines coexpressing 1Ax1 and Pina in durum wheat and on studying the combined impacts of 1Ax1 and PINA on dough mixing properties. After selection for three consecutive years, two transgenic lines coexpressing 1Ax1 and Pina (containing bar, uidA and nptII genes) were designated HP-19 and HP-245; two lines expressing 1Ax1 (containing bar and uidA genes) were designated H-182 and H-293; two lines expressing only Pina (containing nptII gene) were designated P-121 and P-149; one null segregant line selected from crossing progeny with the absence of both target genes and marker genes were designated N-1 (Figure S1). These lines, together with a non-transformed control (cv. Luna), were used to determine the separate and combined effects of 1Ax1 and PINA on the dough mixing properties.
Analysis of Grain Hardness
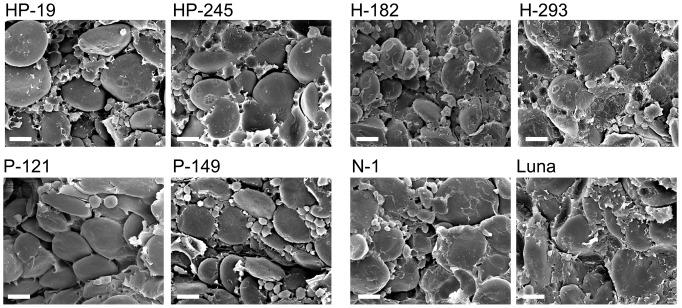
As Pina was demonstrated to be a major determinant gene for grain hardness in bread wheat [18], we therefore analyzed the grain hardness of these transgenic lines, as well as kernel weight and kernel diameter, by SKCS (Table 1). Grain hardness was significantly decreased in Pina-expressing lines, including lines coexpressing 1Ax1 and Pina; while 1Ax1-expressing lines showed similar grain hardness values with control lines. Grain hardness values of Pina-expressing lines were decreased to about 50, whereas lines without PINA expression had values of ca. 75. Analysis of variance (ANOVA) results did not find any significant difference in kernel weights and kernel diameters among these lines (Table 1). To further confirm the differences in kernel hardness among these lines, the endosperm structures of transgenic and control lines were imaged by using SEM and are shown in Figure 1. Endosperm structures of soft and hard wheat differ by the amount of protein matrix adhering to starch granule surface [16]. The starch granules in soft wheat appears to be round and smooth due to their weak bonding with protein matrix, while more adhesions of protein to the starch granules are observed in hard wheat [32]. As shown in Figure 1, all Pina-expressing lines (HP-19, HP-245, P-121 and P-149) had smoother starch granules and less adhering proteins than lines without expression of PINA, where layers of protein matrix were observed to be covered on the surfaces of starch granules. Therefore, differences in endosperm structures among these lines are in accord with their grain hardness, demonstrating that expression of PINA in durum wheat decrease grain hardness.
Table 1. Kernel characteristics, protein contents and Mixograph parameters of the transgenic and control lines.
| Parameters | Line | |||||||
| HP-19 | HP-245 | H-182 | H-293 | P-121 | P-149 | N-1 | Luna | |
| Transgene | 1Ax1+Pina-D1a | 1Ax1+Pina-D1a | 1Ax1 | 1Ax1 | Pina-D1a | Pina-D1a | None | None |
| Kernel characteristics | ||||||||
| Grain hardness | 52.2 c | 50.8 c | 76.7 a | 76.9 a | 51.6 c | 46.0 d | 73.6 b | 75.3 ab |
| Kernel weight (mg) | 31.38 | 33.22 | 34.95 | 35.46 | 34.52 | 36.78 | 35.1 | 37.1 |
| Kernel diameter (mm) | 1.89 | 1.91 | 2.09 | 2.12 | 1.99 | 2.06 | 2.06 | 2.33 |
| 1,000-seed weight (g) | 30.2 g | 31.7 e | 32.1 d | 32.7 c | 33.3 b | 34.3 a | 32.8 c | 31.1 f |
| Test weight (g/l) | 709 cd | 727 b | 713 c | 729 b | 738 a | 738 a | 699 e | 705 d |
| Protein content | ||||||||
| Grain protein content (%) | 15.5 b | 16.2 a | 13.9 cd | 13.8 d | 13.2 e | 14.0 cd | 13.6 de | 14.3 c |
| Flour protein content (%) | 13.8 b | 14.4 a | 12.5 c | 12.4 cd | 11.7 e | 12.1d | 11.4 e | 12.2 cd |
| Mixograph | ||||||||
| Midline left value (% Torque) | 37.25 ab | 38.49 a | 35.48 ab | 34.94 b | 29.89 c | 29.72 c | 27.26 cd | 25.81 d |
| Midline left width (% Torque) | 18.92 bcd | 21.03 ab | 20.05 bc | 17.17 bcd | 15.27 cd | 14.27 d | 24.34 a | 24.86 a |
| Midline peak value (% Torque) | 38.11 ab | 40.37 a | 36 bc | 35.22 cd | 30.23 e | 30.17 e | 33.15 d | 33.29 d |
| Midline peak width (% Torque) | 20.28 c | 19.56 cd | 16.14 e | 17.47 de | 13.61 f | 11.49 f | 25.4 b | 29.85 a |
| Midline peak time (min) | 5.14 bc | 4.5 cd | 6.03 ab | 6.25 a | 4.37 cd | 4.12 d | 2.19 e | 2.04 e |
| Midline right value (% Torque) | 36.94 ab | 38.77 a | 34.92 bc | 34.13 c | 29.56 de | 28.94 e | 30.72 de | 31.14 d |
| Midline right width (% Torque) | 16.69 ab | 18.93 a | 15.67 abc | 13.85 bc | 12.18 c | 12.21 c | 15.92 abc | 18.96 a |
| Midline value at 8 min (% Torque) | 36.6 ab | 37.59 a | 34.98 bc | 34.38 c | 28.13 de | 27.81 e | 28.97 de | 29.54 d |
| Midline width at 8 min (% Torque) | 15.46 ab | 16.52 a | 16.54 a | 14.91 abc | 11.36 cd | 9.96 d | 13.65 abcd | 12.43 bcd |
| Midline integral at 8 min (% Torque* min) | 275.79 b | 289.43 a | 258.17 c | 252.42 c | 228.33 d | 228.44 d | 229.31 d | 236.17 d |
| Weakening slope (% Torque) | 1.51 cde | 2.77 bc | 1.02 de | 0.84 e | 2.11 cde | 2.36 cd | 4.17 a | 3.76 ab |
Values within the same parameter followed by the same letter are not significantly different at 0.05 probability level.
Protein contents determined by near-infrared reflectance spectroscopy (NIRS) method were adjusted to a 14% moisture basis.
Figure 1. Scanning electron microscopy analyses of endosperms from transgenic and control lines.
Seeds from two lines expressing 1Ax1 and Pina (lines HP-19, HP-245), two lines expressing 1Ax1 (lines H-182, H-293), two lines expressing Pina (lines P-121, P-149), one null segregant line (lines N-1) and non-transformed control cv. Luna were subjected to scanning electron microscope analysis to reveal the structure differences of endosperm. Scale bar = 10 µm.
The 1,000-seed weight varied from 30.2 g for line HP-19 to 34.3 g for line P-149; while the test weight ranged from a maximum of 738 g/l for line P-149 to a minimum of 699 g/l for the null segregant line. Although significant difference was detected in 1,000-seed weight and test weight among lines, no clear tendency was found in these traits.
Analysis of Total and Starch-bound PINA
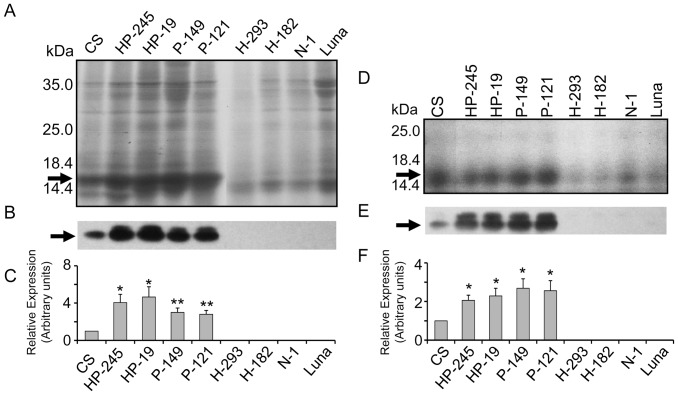
To investigate the expression levels of the Pina-D1a transgene among transgenic and control lines, total TX-114-soluble proteins were extracted and fractionated by SDS-PAGE, followed by Western blotting using a polyclonal antibody raised against the PINA peptide (Figure 2A, B and C). Soft bread wheat variety Chinese Spring (CS) has functional PINA and PINB proteins (encoded by the Pina-D1a and Pinb-D1a alleles), yielding a visible band approximately 15 kDa. Non-transformed control Luna does not express both PINs due to the lacking of the D-genome and therefore no bands corresponding to PINs was observed (Figure 2A). Four Pina-expressing lines showed clearly increase in PINA levels compared to that of CS, while PINA was not detected in Triton X-114 extracts from lines H-182, H-292, N-1 and Luna. Similar results were given by Western blotting analysis, with expression levels of PINA from lines HP-19, HP-245, P-121 and P-149 increasing from about 2-fold to 4-fold compared with that from CS (Figure 2C).
Figure 2. SDS-PAGE and Western blotting analyses of total and starch-bound PINA in transgenic and control lines.
A. SDS-PAGE of TX-114-soluble proteins isolated from flours of transgenic and control lines. B. Western blotting results of total PINA. C. Densitometry quantification of western blotting results of total PINA. D. SDS-PAGE of starch bound puroindolines isolated from flours of transgenic and control lines. E. Western blotting results of starch-bound PINA. F. Densitometry quantification of western blotting results of starch bound PINA. PINA protein is indicated by arrow on both stained SDS-PAGE and Western blots. Data are given as mean ± SEM. *and ** indicates the significant differences with the PINA levels of control variety Chinese Spring at 0.05 or 0.01 probability level, respectively.
It has been demonstrated that the amount of PINs bound on the surface of water-washed starch granules is well associated with grain hardness and can be used to measure the proportion of PINs that are present in functional form [18]. Thus, the starch-bound PINA levels in the seeds of transgenic and control lines were investigated. Increased starch-bound PINA levels were observed, with all four Pina-expressing lines showing a 2- to 3-fold of starch-bound PINA levels in comparison with that of CS (Figure 2D, E and F). Briefly, similar amounts of starch-bound PINA between transgenic lines were not totally consistent with the Triton X-114 results (Figure 2), suggesting that it appears to be a limitation on the binding of PINA to starch granules.
Unexpectedly, an additional band about 18 kDa was detected in the Western blotting results of starch-bound PINA. In addition, a faint band with similar relative molecular weight was observed in the Western blotting results of total PINA. Specific detection of PINA in Pina-expressing lines and positive control CS but not in negative control Luna demonstrated the specificity of this polyclonal antibody. The co-presence of PINA and the 18-kDa band implied that the additional band was specifically detected by the PINA antibody. Moreover, due to the presence of reducing agents (SDS and dithiothreitol) and the heating process before loading protein samples, we eliminate the possibility of heterodimerization between PINA and some unknown proteins. For all the above reasons, we speculate that this additional 18-kDa band may be attributed to posttranslational modification of PINA protein.
Storage Protein Characterization
The grain protein contents and flour protein contents of transgenic and control lines were compared and are shown in Table 1. The grain protein contents ranged from 13.2% to 16.2% for line P-121 and line HP-245, while the flour protein contents varied from 11.4% to 14.4% for the null segregant line and line HP-245. All transgenic lines expressing 1Ax1 had higher flour protein contents than the lines without transgenic 1Ax1 but only the protein contents of line HP-19 and HP-245 were significant higher than the others (Table 1).
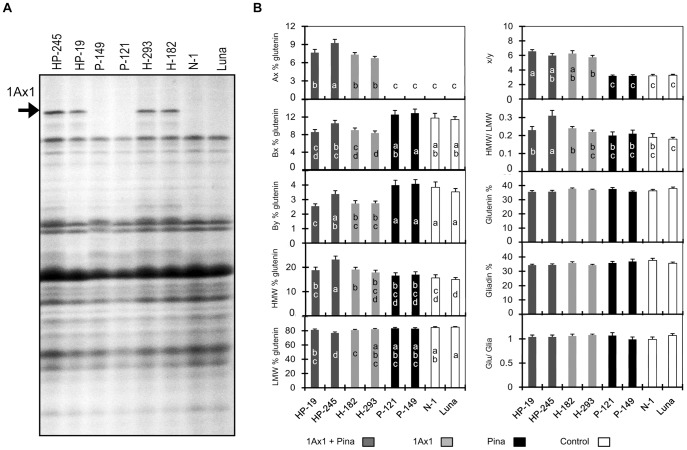
Further, SDS-PAGE results of total storage protein extracts from transgenic and control lines showed that expression patterns of major storage proteins did not appear to be affected by neither 1Ax1 gene nor Pina gene (Figure 3A). Moreover, to compare the protein compositions of flour samples from these lines we separated, recovered and quantified glutenins and gliadins according to DuPont et al. [27]. As shown in Figure 3B, expression of PINA did not influence the amounts and proportions of storage proteins. In contrast, the transgenic subunit 1Ax1, accounting for approximately 8% of total glutenins in four 1Ax1-expressing lines, resulted in significant changes in the proportions of endogenous storage proteins. The increase of 1Ax1 was significantly associated with decrease in endogenous HMW-GS in three 1Ax1-expressing lines, except for line HP-245 (Figure 3B). The endogenous Bx subunit decreased from ca. 12% in lines without 1Ax1 to ca. 8% in 1Ax1-expressing lines, while By subunit decreased from ca. 4% to ca. 2.5%. These changes in the amounts of transgenic and endogenous HMW-GS increased the ratio of x-type/y-type HMW-GS in transgenic lines expressing 1Ax1. Therefore, the ratios of x-type/y-type HMW-GS in the 1Ax1-expressing transgenic lines were increased to about 6 as a consequence of the addition of foreign 1Ax1 gene. Although the expression of transgenic 1Ax1 was compensated by endogenous HMW-GS, the amounts of total HMW-GS were still increased, albeit nonsignificantly. Furthermore, increase of total HMW-GS also appeared to be compensated by LMW-GS, with the amounts of LMW-GS in four 1Ax1-expressing lines being slightly lower than those in the other lines (Figure 3B). Particularly, the increase of HMW-GS and compensation of LMW-GS were significant in line HP-245. Finally, changes in the proportions of glutenins did not affect the amounts of total glutenins and gliadins. No significant differences in the ratio of glutenins and gliadins were observed in all cases.
Figure 3. Characterization of storage proteins in transgenic and control lines.
A. SDS-PAGE of seed protein extracts from transgenic lines, null segregant line and non-transformed control line. Transgenic 1Ax1 is indicated by arrow on the left side of the gel. B. Characterization of storage proteins from the transgenic and control lines. HMW % glutenin and LMW % glutenin means quantities of HMW-GS and LMW-GS, respectively, expressed related to total quantity of the glutenins (and the same for Ax, Bx and By). x/y: ratio of the x- and y- type HMW-GS. HMW/LMW: ratio of the high and low molecular weight glutenin subunits. Glutenin %: quantity of the glutenins expressed related to total proteins extracted by the sequential extraction methods (and the same for Gliadin %) [27]. Glu/Glia: ratio of the glutenins and gliadins. 1Ax1+Pina = transgenic lines coexpressing 1Ax1 and Pina genes (dark grey); 1Ax1 = transgenic lines expressing only 1Ax1 (light grey); Pina = transgenic lines expressing only Pina (black). Control = both null segregant and non-transformed control lines (white). Data are given as mean ± SEM. Values within the same characteristics of storage proteins followed by the same letter are not significantly different (P<0.05).
Dough Mixing Properties
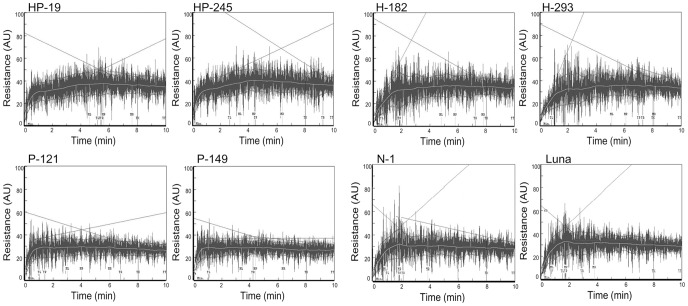
The rheological properties of transgenic and control lines were determined using a 10 g Mixograph (Figure 4). The Mixograph is widely used in cereal research as it measures a variety of rheological parameters that relate to the dough behavior in breadmaking and other food processing systems [31]. Briefly, Mixograms of lines expressing 1Ax1 were higher and wider than lines without transgenic 1Ax1 (including both Pina-expressing and control lines). Lines expressing Pina showed narrower Mixogram curves and different shapes of curve was observed in the first two minutes of mixing in comparison with those of 1Ax1-expressing and control lines, indicating that the hydration and blended stages of dough mixing might be affected by expression of PINA [33]. We further compared the mixing parameters between lines expressing 1Ax1, Pina and 1Ax1+Pina (Figure 5). No significant differences were found in the mixing parameters between null segregant line (N-1) and non-transformed control (cv. Luna), suggesting that genetic transformation of wheat does not affect dough mixing properties (Figure S2). Transgenic lines expressing only 1Ax1 (H-182 and H-293) showed significant increases in parameters relating to the heights of curves (MLV, MPV, MRV and MTxV) in comparison with the non-transformed control (Figure 5). The heights of the curve after peak resistance remained stable at about 35% torque in lines H-182 and H-293, whereas those in the Luna control decreased to 29% torque. Moreover, 1Ax1-expressing lines showed significant decreases in MLW and MPW, while a non-significant increase of MTxW was observed in lines H-182 and H-293. MPT and MTxI values for lines H-182 and H-293 were significantly higher than those of control. Expression of 1Ax1 also reduced the weakening slope. Unlike 1Ax1-expressing lines, mixing parameters from lines P-121 and P-149 revealed marked differences in dough behaviors between 1Ax1-expressing and control lines. The expression of PINA in lines P-121 and P-149 was associated with decreased curve widths with respect to non-transformed control, with MLW, MPW and MRW being significantly reduced. MPT was significantly higher for lines P-121 and P-149 than that for the control. But weakening slope was lower for these lines with respect to Luna. No significant difference was observed in the MTxI from Pina-expressing and control lines.
Figure 4. Mixograph curves of dough prepared from transgenic and control lines.
Flour samples from two lines coexpressing 1Ax1 and Pina (lines HP-19, HP-245), two lines expressing 1Ax1 (lines H-182, H-293), two lines expressing Pina (lines P-121, P-149), one null segregant line (lines N-1) and non-transformed control cv. Luna were subjected to Mixograph analysis to reveal differences in dough mixing properties.
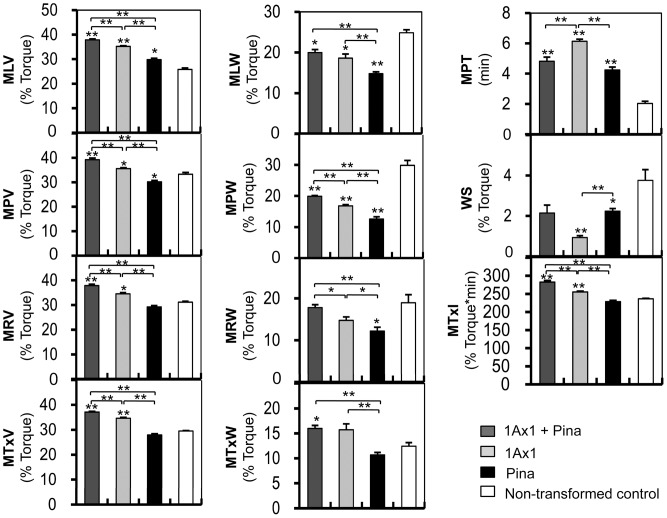
Figure 5. Combined effects of 1Ax1 and PINA on dough mixing properties.
Eleven mixing parameters were compared by Student’s t test between lines coexpressing 1Ax1and Pina (lines HP-19 and HP-245, represented by dark grey bar), lines expressing only 1Ax1 (lines H-182 and H-293, represented by light grey bar), lines expressing only Pina (lines P-121 and P-149, represented by black bar) and non-transformed control line (cv. Luna, represented by white bar). Data are given as mean ± SEM. *and ** indicates the significant differences with mixing parameters of non-transformed control Luna at 0.05 or 0.01 probability level, respectively.
Differences in mixing behaviors between lines expressing 1Ax1 and/or Pina revealed the combined effects of 1Ax1 and PINA on dough properties. As shown in Figure 5, both the heights and widths of the curve were significantly higher for lines HP-19 and HP-245 as compared with those for lines expressing either 1Ax1 or Pina, with the exception of a non-significant increase of MRW observed in lines HP-19 and HP-245. Moreover, MPT was significantly increased for lines HP-19 and HP-245 with respect to the Luna control, but was similar to those for the Pina-expressing lines (P-121 and P-149). Non-significant reduction in WS was seen in lines HP-19 and HP-245 compared with that of Luna, the decreased WSs in HP-19 and HP-245, however, were similar to those in lines P-121 and P-149. These results demonstrated that lines expressing the combination of 1Ax1 and Pina did not differ in MPT and WS in comparison with lines expressing only Pina. More importantly, the MTxI for lines HP-19 and HP-245 were significantly increased compared with all the other transgenic lines and the Luna control, suggesting stronger doughs from lines HP-19 and HP-245 than those from the other lines.
Discussion
Dough mixing is a critical process in the production of flour-based food and greatly influences the end-use quality of wheat that mainly derives from two traits: grain hardness and gluten protein [2]. On one hand, extensive studies have demonstrated that the HMW-GS plays a determinant role in dough properties and breadmaking quality of wheat [34]. In wheat, small-scale and large-scale rheological tests of transgenic wheat with expression of 1Ax1, 1Dx5 or 1Dy10 have confirmed that these three subunits can improve dough strength and are associated with good breadmaking quality [3], [35], [36], [37]. On the other hand, a few studies showed that grain hardness primarily controlled by Pina and Pinb genes had influences on milling and baking qualities [38], [39]. In addition, overexpression of Pina and Pinb in transgenic lines of bread wheat negatively affected crumb grain score and loaf volume [40]. Therefore, both HMW-GS and Pin genes should be taken into consideration in developing breeding strategy for improvement of wheat end-use quality. In this study, transgenic lines of durum wheat expressing 1Ax1 and/or Pina were screened out and allowed the separate and combined effects of 1Ax1 and PINA on dough mixing properties to be determined. A null segregant line (line N-2) and the non-transformed cv. Luna were used as controls. Both control lines were null background for the target genes (1Ax1 and Pina) and marker genes (nptII, uidA and bar), whereas marker genes were present in transgenic lines expressing 1Ax1 and/or Pina (Figure S1). Previous studies on transgenic lines expressing HMW-GS genes have demonstrated that no differences were found in agronomic performance and rheological properties between lines constitutively expressing the marker genes from those which only expressed the HMW-GS genes [37], [41]. Furthermore, numerous studies supported that the use of selectable marker genes (nptII, uidA and bar) in transformation posed no safety concerns [42], and few studies showed these marker genes were related to phenotypic variations in transgenic lines of wheat. Therefore, it is very unlikely that the presence or expression of bar, uidA and nptII genes has effects on the grain hardness, dough properties, as well as agronomic performance of transgenic lines.
Overexpression of PINA in Durum Wheat Leads to Medium Hard Grain Texture
Previous data indicated that friabilin is as a marker protein for grain hardness as it is abundant on the water-washed starch granules from soft wheat and scarce on hard wheat starch [12]. Then friabilin was found to be composed of PINA and PINB proteins which are encoded by two closely linked Pin genes at the Ha locus [11], [13]. Furthermore, the results that complementation of the mutant Pina-D1b or Pinb-D1b allele with their wild-type alleles restored a soft-texture phenotype demonstrate that Pina and Pinb genes are the major causal genes for grain hardness [16], [17], [18]. In addition, transgenic plants with expression of Pina and/or Pinb in rice and maize which are null background for the Ha locus had decreased grain hardness and lowered levels of damaged starch [15], [19]. However, unlike transgenic lines of wheat, transgenic rice and maize did not show apparent difference in grain hardness as large as those observed in wheat, indicating that the mechanisms for grain hardness in rice and maize may be different from that in wheat. In our work, overexpression of Pina in durum wheat resulted in similar phenotype (medium hard texture of grain) as observed in bread wheat [17]. Therefore, the results of transgenic lines presented here, together with results from durum wheat cv. Langdon and its substitution line Langdon 5D [15] prove that grain hardness can be modulated by PINA and PINB proteins in both durum wheat and bread wheat in a similar way. Previous data implicate that PINA and PINB proteins may determine grain hardness of wheat in some type of synergism. Expression of the wild-type Pina-D1a allele in a background with mutant Pinb led to medium-hard phenotype of grain texture [17]. Moreover, decrease in grain hardness for the addition of wild-type Pina (the Pina-D1a allele) to a Pina-mutant background was not as dramatic as the addition of wild-type Pinb (the Pinb-D1a allele) to a Pinb-mutant background [43], [44]. The Pina-expressing lines herein showed increased total and starch-bound PINA, as well as decreased grain hardness (Table 1 and Figure 2). Interestingly, regardless of larger variations in total PINA levels among Pina-expressing lines, similar levels of starch-bound PINA were observed these lines which are null background for functional PINB protein (Figure 2) indicates that PINB may be a limiting factor in PINA binding to starch and in reduction of grain hardness.
Expression of Transgenic 1Ax1 is Compensated by other Storage Proteins
Expression patterns of endogenous storage proteins in transgenic lines of durum and bread wheat with overexpression of HMW-GS subunits have been extensively investigated in previous studies. In a field-grown transgenic line overexpressing 1Dx5, expression levels of most endogenous HMW-GS subunits uniformly dropped about 30%, with an increase in the overall LMW-GS level and a decrease in gliadin level being observed [36]. Another group reported that increases in the transgenic 1Dx5 and/or 1Dy10 expression were compensated by the decrease in HMW-GS Ax-, Bx- and By- subunits and dropped LMW-GS levels were also detected [45]. Moreover, besides significant decreases in endogenous HWM-GS subunits reported in most transgenic events overexpressing HMW-GS, nonsignificant decreases in LMW-GS were also reported in recent studies on transgenic lines expressing 1Ax1, 1Dx5 or 1Dy10 under field conditions [3], [37]. In the present work, we found that expression of transgenic 1Ax1 was compensated by the significant decrease in endogenous Bx- and By- subunits, with the overall LMW-GS levels being non-significant lower (Figure 3). Our results along with previous data indicate that additional expression of transgenic HMW-GS is strongly related to the decrease in endogenous HMW-GS and tend to reduce the overall LMW-GS expression levels. However, mechanisms underlying this compensation effects is largely unknown. One possibility is that the compensatory phenomenon may reflect the competition for amino acids for protein synthesis [46]. This hypothesis is supported by the “in silico” amino acid composition analysis of gluten proteins for transgenic wheat lines with down-regulation of γ-gliadins [47]. On the other hand, there are results against this hypothesis in rice and barley, where the available sulfur-containing amino acids may be key regulator in controlling the amino acid homeostasis [48], [49]. Nevertheless, the mechanism for storage protein compensation in wheat is worthy of in-depth investigation in future.
Effects of 1Ax1 and/or PINA on Dough Mixing Properties
The dough mixing characteristics of transgenic and control lines were shown in Table 1. Relationships between Mixograph parameters and dough visco-elasticity have been explained in details [31] and associations of these parameters with other wheat quality traits were discussed previously [50]. Generally, MPW and MRW are positively correlated with dough resistance to extension [51], whereas WS and MTxW are, respectively, negatively and positively correlated with the over-mixing tolerance [31], [47]. MPV and MTxI are positively correlated with dough strength [3]. Weak dough has higher WS, shorter MPT and lower MPV and MTxI than those of strong dough. In our study, no differences were found in mixing properties between the null segregant line (N-1) and non-transformed control (Luna) was consistent with the identical protein patterns storage between these two control lines (Figure S2 and Figure 3), demonstrating that possible somaclonal variations in the transformation did not led to significant variations in storage proteins.
In transgenic lines exclusively expressing 1Ax1 (lines H-182 and H-293), significant increase of all parameters relating to the curve heights with respect to Luna demonstrated that dough elasticity was increased by expression of 1Ax1. Furthermore, increased MPT and MTxI for lines H-182 and H-293 indicated the enhanced dough strength in comparison with those for Luna. Lower WS values and non-significantly higher MTxW values for lines H-182 and H-293 revealed that the mixing tolerance was also improved by 1Ax1 expression. In previous studies, although subunit 1Ax1 was generally reported to have positive effects on dough strength in bread wheat, durum wheat and Tritordeum [5], [6], [7], [8], differential effects of 1Ax1 on dough functional properties were also revealed by rheological tests in transgenic lines with different wheat varietal backgrounds. In transgenic lines of cv. Bobwhite and Anza, both dough strength and mixing tolerance were increased by expression of transgenic 1Ax1 [3], [52]. Overexpression of 1Ax1 improved both dough strength and extensibility in transgenic lines of L88-31 [35]. Interestingly, a study on a series of transgenic lines of three commercial wheat cultivars (Imp, Canon and Cadenza) with expression of 1Ax1 showed that high expression levels of 1Ax1 conferred “over-strong” dough properties and had negative effects on breadmaking performance; whereas lower expression levels of 1Ax1 increased dough strength and stability [37]. In the cases of our study, expression of 1Ax1 led to improved dough strength and mixing tolerance, and decreased dough resistance to extension. Regarding the effects of 1Ax1 reported in this work as well as those reported previously, it should be born in mind that the effects of transgenic 1Ax1 on dough functionality may vary depending on the expression level of transgene and on the composition of endogenous HMW-GS, although usually overexpression of 1Ax1 improve dough strength.
In transgenic lines expressing only Pina, significant decreased curve-width-related mixing parameters (MPW and MRW) demonstrated that dough resistance to extension was reduced by expression of PINA. Moreover, slight but significant decrease of MPV and non-significant decrease of MTxI in Pina-expressing lines indicated that dough strength was slightly decreased by PINA expression. In addition, lower WS values for linesP-121 and P-149 with respect to non-transformed control indicated increased mixing tolerance. Previous data in transgenic lines of bread wheat expressing Pina and/or Pinb showed that transgenic addition of PINs detrimentally affected loaf volume and crumb grain score [40]. In soft-textured lines of durum wheat developed by chromosome engineering [53], Alveograph analysis revealed that reduction in grain hardness caused by the presence of the Ha locus, was related with drastic decrease in dough resistance to extension, and increased dough extensibility and over-mixing tolerance, with dough strength not significantly affected [4]. Partly agreed with Gazza et al. [4], our results confirmed the detrimental effect of PINA on dough resistance to extension and minor effect on dough strength. It is noteworthy that PINA and PINB interact with each other in vivo [54] and, in planta, control grain hardness in some type of synergism [43], [44]. Unlike the data reported by Gazza et al. [4], the results herein revealed the individual effects of transgenic PINA on dough mixing properties in a null background of durum wheat without any interaction with PINB.
In contrast with lines expressing either 1Ax1 or Pina, transformation with the combination of 1Ax1 and Pina-D1a resulted in better dough mixing properties. Significantly higher values of both curve-height-related mixing parameters (MLV, MPV, MRV and MTxV) and curve-width-related parameters (MLW, MPW, MTxW) from lines HP-19 and HP-245 than those from lines expressing either 1Ax1 or Pina demonstrated that coexpression of 1Ax1 and PINA improved dough strength and resistance to extension. Negative effects of PINA on dough properties were not found as detected in the Pina-expressing lines (P-121 and P-149); however, combined enhancing effects of 1Ax1 and PINA on dough strength resistance to extension were observed. The significantly higher MTxI for lines HP-19 and HP-245 with respect to lines expressing 1Ax1 or Pina further supports an enhancement of coexpressed 1Ax1 and PINA on dough strength. No significant difference was observed in WS among lines expressing 1Ax1 and/or Pina, suggesting that the over-mixing tolerance was not affected by the coexpressiong of 1Ax1 and PINA.
Interestingly, the finding of dough properties from these lines raises the question of why there were combined effects of 1Ax1 and PINA on dough mixing properties and how could coexpression of 1Ax1 and PINA lead to stronger dough than those from lines expressing either 1Ax1 or Pina. A remarkable difference between Mixograms from lines coexpressing 1Ax1 and Pina and those expressing only 1Ax1 is the shape of curves in the first two minutes of dough formation (Fig. 4). The beginning stages of dough development are the hydration stage and the blending stage. As described by Stauffer [33], protein network is gradually softened by water penetration in the hydration stage, with damage starch granules being absorbing water. As the protein network is agitated by mixing, all the ingredients of dough are being blended into a homogenous dough mass, with starch granules being less firmly associated with the protein fibers and lipids being uniformly distributed in the protein network. It has been documented that the hydration of dough formation depends on dough water absorption and starch damage level [55], [56]. Quantitative trait locus (QTL) identification analysis revealed that the Ha locus on chromosome 5D, consisting of Pina and Pinb genes, is a QTL for hydration traits including dough water absorption and damaged starch [57]. Further, it was demonstrated that expression of PINA and/or PINB led to enhanced grain softness and reduced starch damage levels in both transgenic rice and wheat [15], [16]. More recently, Pin genes’ association with damaged starch and water absorption were confirmed by Farinograph in soft-textured F8 lines of durum wheat containing the complete Ha locus [4]. Study of the hydration of wheat dough by tandem use of rheological tests and nuclear magnetic resonance (NMR) spectroscopy also supports the correlation between grain hardness and water absorption, finding that hard wheat required a higher water level to achieve a dough of satisfactory consistency [58]. Moreover, it was reported that PINA prevent absorption of lipids to air-water interfaces in dough and make lipids embedded within the protein-starch matrix, leading to a homogeneous size distribution of gas cells in wheat dough [59]. All the above leads up to an explanation for the difference in Mixogram shapes that decreased grain hardness caused by PINA expression could lead to lower levels of water absorption and damaged starch and hence result in faster hydration during mixing in comparison with those lines without transgenic Pina. Further, distribution of lipids in protein-starch matrix and lower levels of damaged starch in lines coexpressing 1Ax1 and Pina may help to form stronger dough than lines expressing 1Ax1. This inference matches the higher values of MPV, MRV, MTxV and MTxI for lines HP-19 and HP-245 with respect to H-182 and H-293 (Figure 5). In addition, expressiong of 1Ax1 appears to compensate the detrimental effect of PINA on dough resistance to extension for unknown reason. In particular, due to the differential effects of transgenic 1Ax1 on dough properties in different genetic backgrounds as is discussed above [35], [37], [52], we can reasonably infer that the enhancing effects of 1Ax1 and PINA on dough mixing properties may possibly vary in durum wheat varieties with different HMW-GS compositions. Possible changes in the combined effects of 1Ax1 and PINA on dough functionality in different varietal backgrounds may be related with complicated interactions between starch and protein matrix during dough development and need intensive studies in future.
In summary, we have demonstrated that coexpression of 1Ax1 and PINA in durum wheat have combined effects on dough mixing behaviors with a better dough strength and resistance to extension than those from lines expressing 1Ax1 or Pina. Moreover, detrimental effect of PINA on dough resistance to extension was observed in Pina-expressing lines with respect to non-transformed cv. Luna, whereas coexpression of 1Ax1 and PINA improved dough strength and resistance to extension in comparison with the 1Ax1-expressing lines. The results in our study showed that expression of HMW-GS could offset detrimental effects of PINA on dough mixing properties in durum wheat, and implicate that combinations of transgenic HMW-GS and PINs may lead to different dough properties of durum wheat, thus varied end-use qualities. Therefore, these results suggest that simultaneous modification of dough strength and grain hardness in durum wheat could significantly improve its breadmaking quality and may even not weaken its pastamaking potential. Furthermore, expression of PINs in durum wheat would likely decrease the energy consumption during milling due to the close relationship between grain hardness and milling energy [60]. For all these reasons, modification of both dough strength and grain hardness in durum wheat has practical implications for breeding durum wheat with dual functionality (for pasta and bread) and therefore may improve the economic values of durum wheat.
Supporting Information
PCR detection for the nptII , bar and uidA genes in transgenic and control lines. The presence or absence of nptII, bar and uidA genes in genomic DNA were determined by PCR lines HP-19 (lane 1), HP-245 (lane 2), H-182 (lane 3), H-293 (lane 4), P-121 (lane 5), P-149 (lane 6), N-1 (lane 7) and non-transformed cv. Luna (lane 8). Lane 9 and lane 10 represented the plasmid control and water negative control of PCR amplification.
(TIF)
No significant differences were found in dough mixing parameters between lines N-1 and Luna. Dough mixing parameters for null segregant line (N-1, indicated by grey bars) and non-transformed control cv. Luna (WT, indicated by white bars) were compared by Student’s t test. All the eleven mixing parameters for line N-1 used in this study were not significant different from those for the Luna control.
(TIF)
Primers designed for PCR amplification of bar , uidA and npt II genes.
(DOC)
Acknowledgments
The authors acknowledge Min Tu for technical assistance and helpful discussion.
Funding Statement
This work was supported by the National Science and Technology Major Project of China (2011ZX08002-004; 2011ZX08010-004) (http://www.nmp.gov.cn/), International S & T Cooperation Key Projects of MoST (2009DFB30340), the National Natural Science Foundation of China (No.30871524 and No.31071403) (http://www.nsfc.gov.cn/Portal0/default152.htm) the National Natural Science Foundation of Hubei, China (2010 CBD 02403) and Wuhan Municipal S & T research project (201120922286). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shewry PR (2009) Wheat. J Exp Bot 60: 1537–1553. [DOI] [PubMed] [Google Scholar]
- 2. Bushuk W (1998) Wheat breeding for end-product use. Euphytica 100: 137–145. [Google Scholar]
- 3. Leon E, Marin S, Gimenez MJ, Piston F, Rodriguez-Quijano M, et al. (2009) Mixing properties and dough functionality of transgenic lines of a commercial wheat cultivar expressing the 1Ax1, 1Dx5 and 1Dy10 HMW glutenin subunit genes. J Cereal Sci 49: 148–156. [Google Scholar]
- 4. Gazza L, Sgrulletta D, Cammerata A, Gazzelloni G, Perenzin M, et al. (2011) Pastamaking and breadmaking quality of soft-textured durum wheat lines. J Cereal Sci 54: 481–487. [Google Scholar]
- 5. Altpeter F, Vasil V, Srivastava V, Vasil IK (1996) Integration and expression of the high molecular weight glutenin subunit 1Ax1 gene into wheat. Nat Biotechnol 14: 1155–1159. [DOI] [PubMed] [Google Scholar]
- 6. Barro F, Rooke L, Bekes F, Gras P, Tatham AS, et al. (1997) Transformation of wheat with high-molecular-weight subunit genes results in improved functional properties. Nat Biotechnol 15: 1295–1299. [DOI] [PubMed] [Google Scholar]
- 7. He GY, Rooke L, Steele S, Bekes F, Gras P, et al. (1999) Transformation of pasta wheat (Triticum turgidum L. var. durum) with high-molecular-weight glutenin subunit genes and modification of dough functionality. Mol Breed 5: 377–386. [Google Scholar]
- 8. Rooke L, Barro F, Tatham AS, Fido R, Steele S, et al. (1999) Altered functional properties of tritordeum by transformation with HMW glutenin subunit genes. Theor Appl Genet 99: 851–858. [Google Scholar]
- 9.Mattern PJ, Morris R, Schmidt JW, Johnson VA (1973) Location of genes for kernel properties in the wheat cultivar ‘Cheyenne’ using chromosome substitution lines. In: Sears ER, Sears LMS. editors. Proc. 4th Int.Wheat Genetics Symp. University of Missouri, Columbia. 703–707.
- 10.Law CN, Young CF, Brown JWS, Snape JW, Worland JW (1978) The study of grain protein control in wheat using whole chromosome substitution lines. In: Seed protein improvement by nuclear techniques. Vienna: International Atomic Energy Agency. 483–502.
- 11. Giroux MJ, Morris CF (1997) A glycine to serine change in puroindoline b is associated with wheat grain hardness and low levels of starch-surface friabilin. Theor Appl Genet 95: 857–864. [Google Scholar]
- 12. Greenwell P, Schofield JD (1986) A starch granule protein associated with endosperm softness in wheat. Cereal Chem 63: 379–380. [Google Scholar]
- 13. Jolly CJ, Rahman S, Korrt AA, Higgins TJV (1993) Characterization of the wheat Mr 15,000 ‘grain softness protein’ and analysis of the relationship between its accumulation in the whole seed and grain softness. Theor Appl Genet 86: 589–597. [DOI] [PubMed] [Google Scholar]
- 14. Giroux MJ, Morris CF (1998) Wheat grain hardness results from highly conserved mutations in the friabilin components puroindoline a and b. Proc Natl Acad Sci USA. 95: 6262–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krishnamurthy K, Giroux MJ (2001) Expression of wheat puroindoline genes in transgenic rice enhances grain softness. Nat Biotechnol 19: 162–166. [DOI] [PubMed] [Google Scholar]
- 16. Beecher B, Bettge A, Smidansky E, Giroux MJ (2002) Expression of wild-type pinB sequence in transgenic wheat complements a hard phenotype. Theor Appl Genet 105: 870–877. [DOI] [PubMed] [Google Scholar]
- 17. Hogg AC, Sripo T, Beecher B, Martin JM, Giroux MJ (2004) Wheat puroindolines interact to form friabilin and control wheat grain hardness. Theor Appl Genet 108: 1089–1097. [DOI] [PubMed] [Google Scholar]
- 18. Martin JM, Meyer FD, Smidansky ED, Wanjugi H, Blechl AE, et al. (2006) Complementation of the pina (null) allele with the wild type Pina sequence restores a soft phenotype in transgenic wheat. Theor Appl Genet 113: 1563–1570. [DOI] [PubMed] [Google Scholar]
- 19. Zhang JR, Martin JM, Beecher B, Morris CF, Hannah LC, et al. (2009) Seed-specific expression of the wheat puroindoline genes improves maize wet milling yields. Plant Biotechnol J 7: 733–743. [DOI] [PubMed] [Google Scholar]
- 20. Joppa LR, Williams ND (1988) Langdon durum disomic substitution lines and aneuploid analysis in tetraploid wheat. Genome 30: 222–228. [Google Scholar]
- 21. Dexter JE, Preston KR, Martin DG, Gander EJ (1994) The effects of protein content and starch damage on the physical dough properties and breadmaking quality of Canadian durum wheat. J Cereal Sci 20: 139–151. [Google Scholar]
- 22. Rao BN, Pozniak CJ, Hucl PJ, Briggs C (2010) Baking quality of emmer-derived durum wheat breeding lines. J Cereal Sci 51: 299–304. [Google Scholar]
- 23. Luo LT, Zhang JR, Yang GX, Li Y, Li KX, et al. (2008) Expression of puroindoline a enhances leaf rust resistance in transgenic tetraploid wheat. Mol Biol Rep 35: 195–200. [DOI] [PubMed] [Google Scholar]
- 24. Halford NG, Field JM, Blair H, Urwin P, Moore K, et al. (1992) Analysis of HMW glutenin subunits encoded by chromosome 1A of bread wheat (Triticum aestivum L.) indicates quantitative effects on grain quality. Theor Appl Genet 83: 373–378. [DOI] [PubMed] [Google Scholar]
- 25. Christensen AH, Quail PH (1996) Ubiquitin promoterbased vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5: 213–218. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Xiong ZY, He YG, Shewry PR, He GY (2007) Genetic diversity of HMW glutenin subunit in Chinese common wheat (Triticum aestivum L.) landraces from Hubei province. Genet Resour Crop Evol 54: 865–874. [Google Scholar]
- 27. DuPont FM, Chan R, Lopez R, Vensel WH (2005) Sequential extraction and quantitative recovery of gliadins, glutenins, and other proteins from small samples of wheat flour. J Agric Food Chem 53: 1575–1584. [DOI] [PubMed] [Google Scholar]
- 28. Shewry PR, Powers S, Field JM, Fido RJ, Jones HD, et al. (2006) Comparative field performance over 3 years and two sites of transgenic wheat lines expressing HMW subunit transgenes. Theor Appl Genet 113: 128–136. [DOI] [PubMed] [Google Scholar]
- 29.American Association of Cereal Chemists (2000) Approved methods of the AACC, 10th edn. American Association of Cereal Chemistry, Minnesota.
- 30.Miao YJ, Chen L, Wang C, Wang YJ, Zheng Q, et al. (2012) Expression, purification and antimicrobial activity of puroindoline A protein and its mutants. Amino Acids DOI: 10.1007/s00726-012-1250-x (Epub ahead of print). [DOI] [PubMed]
- 31. Martinant JP, Nicolas Y, Bouguennec A, Popineau Y, Saulnier L, et al. (1998) Relationships between mixograph parameters and indices of wheat grain quality. J Cereal Sci 27: 179–189. [Google Scholar]
- 32. Chen MJ, Wilkinson M, Tosi P, He GY, Shewry PR (2005) Novel puroindoline and grain softness protein alleles in Aegilops species with the C, D, S, M and U genomes. Theor Appl Genet 111: 1159–1166. [DOI] [PubMed] [Google Scholar]
- 33.Stauffer CE (2007) Principles of dough formation. In: Cauvain SP, Young LS, editors. Technology of breadmaking, 1st edn, Springer. 299–332.
- 34. Shewry PR, Halford NG, Tatham AS, Popineau Y, Lafiandra D, et al. (2003) The high molecular weight subunits of wheat glutenin and their role in determining wheat processing properties. Adv Food Nutr Res 45: 219–302. [DOI] [PubMed] [Google Scholar]
- 35. Barro F, Barcelo P, Lazzeri PA, Shewry PR, Ballesteros J, et al. (2003) Functional properties of flours from field grown transgenic wheat lines expressing the HMW glutenin subunit 1Ax1 and 1Dx5 genes. Mol Breed 12: 223–229. [Google Scholar]
- 36. Rakszegi M, Bekes F, Lang L, Tamas L, Shewry PR, et al. (2005) Technological quality of transgenic wheat expressing an increased amount of a HMW glutenin subunit. J Cereal Sci 42: 15–23. [Google Scholar]
- 37. Rakszegi M, Pastori G, Jones HD, Bekes F, Butow B, et al. (2008) Technological quality of field grown transgenic lines of commercial wheat cultivars expressing the 1Ax1 HMW glutenin subunit gene. J Cereal Sci 47: 310–321. [Google Scholar]
- 38. Slaughter DC, Norris KH, Hruschka WR (1992) Quality and classification of hard red wheat. Cereal Chem 69: 428–432. [Google Scholar]
- 39. Ohm JB, Chung OK, Deyoe CW (1998) Single-kernel characteristics of hard winter wheats in relation to milling and baking quality. Cereal Chem 75: 156–161. [Google Scholar]
- 40. Hogg AC, Beecher B, Martin JM, Meyer F, Talbert L, et al. (2005) Hard wheat milling and bread baking traits affected by the seed-specific overexpression of puroindolines. Crop Sci 45: 871–878. [Google Scholar]
- 41. Barro F, Barcelo P, Lazzeri PA, Shewry PR, Martin A, et al. (2002) Field evaluation and agronomic performance of transgenic wheat. Theor Appl Genet 105: 980–984. [DOI] [PubMed] [Google Scholar]
- 42. Ramessar K, Peremarti A, Gomez-Galera S, Naqvi S, Moralejo M, et al. (2007) Biosafety and risk assessment framework for selectable marker genes in transgenic crop plants: a case of the science not supporting the politics. Transgenic Res 16: 261–280. [DOI] [PubMed] [Google Scholar]
- 43. Swan CG, Meyer FD, Hogg AC, Martin JM, Giroux MJ (2006) Puroindoline B limits binding of Puroindoline A to starch and grain softness. Crop Sci 46: 1656–1665. [Google Scholar]
- 44. Wanjugi HW, Hogg AC, Martin JM, Giroux MJ (2007) The role of Puroindoline A and B Individually and in combination on grain hardness and starch association. Crop Sci 47: 67–76. [Google Scholar]
- 45. Blechl A, Lin J, Nguyen S, Chan R, Anderson OD, et al. (2007) Transgenic wheats with elevated levels of Dx5 and/or Dy10 high-molecular-weight glutenin subunits yield doughs with increased mixing strength and tolerance. J Cereal Sci 45: 172–183. [Google Scholar]
- 46. Leon E, Aouni R, Piston F, Rodriguez-Quijano M, Shewry PR, et al. (2010) Stacking HMW-GS transgenes in bread wheat: Combining subunit 1Dy10 gives improved mixing properties and dough functionality. J Cereal Sci 51: 13–20. [Google Scholar]
- 47. Piston F, Gil-Humanes J, Rodriguez-Quijano M, Barro F (2011) Down-regulating γ-gliadins in bread wheat leads to non-specific increases in other gluten proteins and has no major effect on dough gluten strength. PLoS ONE 6(9): e24754 doi:10.1371/journal.pone.0024754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hansen M, Lange M, Friis C, Dionisio G, Holm PB, et al. (2007) Antisense-mediated suppression of C-hordein biosynthesis in the barley grain results in correlated changes in the transcriptome, protein profile, and amino acid composition. J Exp Bot 58: 3987–3995. [DOI] [PubMed] [Google Scholar]
- 49. Kawakatsu T, Hirose S, Yasuda H, Takaiwa F (2010) Reducing rice seed storage protein accumulation leads to changes in nutrient quality and storage organelle formation. Plant Physiol 154: 1842–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bordes J, Branlard G, Oury FX, Charmet G, Balfourier F (2008) Agronomic characteristics, grain quality and flour rheology of 372 bread wheats in a worldwide core collection. J Cereal Sci 48: 569–579. [Google Scholar]
- 51. Tosi P, Masci S, Giovangrossi A, D’Ovidio R, Bekes F, et al. (2005) Modification of the low molecular weight (LMW) glutenin composition of transgenic durum wheat: effects on glutenin polymer size and gluten functionality. Mol Breed 16: 113–126. [Google Scholar]
- 52. Vasil IK, Bean S, Zhao JM, McCluskey P, Lookhart G, et al. (2001) Evaluation of baking properties and gluten protein composition of field grown transgenic wheat lines expressing high molecular weight glutenin gene 1Ax1. J Plant Physiol 158: 521–528. [Google Scholar]
- 53.Gazza L, Zanella L, Pogna NE (2008) Development of durum wheat (Triticum turgidum ssp durum) lines with soft kernel texture by chromosome engineering. In: Proceedings of 11th International Wheat Genetics Symposium. Brisbane, QLD, Australia, vol. 2. pp: 339–441.
- 54. Ziemann M, Ramalingam A, Bhave M (2008) Evidence of physical interactions of puroindoline proteins using the yeast two-hybrid system. Plant Sci 175: 307–311. [Google Scholar]
- 55. Miller RA, Hoseney RC, Morris CF (1997) Effect of formula water content on the spread of sugar-snap cookies. Cereal Chem 74: 669–671. [Google Scholar]
- 56. Ohm JB, Chung OK (1999) Gluten, pasting, and mixograph parameters of hard winter wheat flours in relation to breadmaking. Cereal Chem 76: 606–613. [Google Scholar]
- 57. Campbell KG, Finney PL, Bergman CJ, Gualberto DG, Anderson JA, et al. (2001) Quantitative trait loci associated with milling and baking quality in a soft – hard wheat cross. Crop Sci 41: 1275–1285. [Google Scholar]
- 58. Lopes-Da-Silva JA, Santos DMJ, Freitas A, Brites C, Gil AM (2007) Rheological and nuclear magnetic resonance (NMR) study of the hydration and heating of undeveloped wheat doughs. J Agric Food Chem 55: 5636–5644. [DOI] [PubMed] [Google Scholar]
- 59. Dubreil L, Biswas SC, Marion D (2002) Localization of Puroindoline-a and lipids in bread dough using confocal scanning laser microscopy. J Agric Food Chem 50: 6078–6085. [DOI] [PubMed] [Google Scholar]
- 60.Pomeranz Y, Williams PC (1990) Wheat hardness: its genetic, structural, and biochemical background, measurement, and significance. In: Pomeranz Y, editor. Advances in Cereal Science and Technology, vol. 10. St. Paul: American Association of Cereal Chemists. 471–548.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR detection for the nptII , bar and uidA genes in transgenic and control lines. The presence or absence of nptII, bar and uidA genes in genomic DNA were determined by PCR lines HP-19 (lane 1), HP-245 (lane 2), H-182 (lane 3), H-293 (lane 4), P-121 (lane 5), P-149 (lane 6), N-1 (lane 7) and non-transformed cv. Luna (lane 8). Lane 9 and lane 10 represented the plasmid control and water negative control of PCR amplification.
(TIF)
No significant differences were found in dough mixing parameters between lines N-1 and Luna. Dough mixing parameters for null segregant line (N-1, indicated by grey bars) and non-transformed control cv. Luna (WT, indicated by white bars) were compared by Student’s t test. All the eleven mixing parameters for line N-1 used in this study were not significant different from those for the Luna control.
(TIF)
Primers designed for PCR amplification of bar , uidA and npt II genes.
(DOC)