Abstract
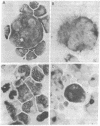
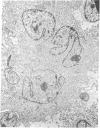
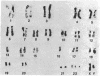
Long-term growth of lymphoblastoid T cells from tissue samples from six of six patients with cutaneous T-cell lymphoma (CTCL) and six of six patients with acute T-lymphoblastic leukemia (ALL) has been achieved by using partially purified mitogen-free human T-cell growth factor (pp-TCGF). One cell line, CTCL-2, is now independent of added growth factor; the others continue to show absolute dependency on its presence. All lines have been in continuous culture for at least 4 months and some for > 1 year. They are erythrocyte-rosette positive and are negative for Epstein-Barr virus nuclear antigen. Most of the lines are negative for Fc and complement receptors and for surface immunoglobulin except that CTCL-1 and CTCL-2 have some cells positive for these cell surface markers. Results of histochemical studies on these cell lines are similar to the known patterns for fresh cells from their disease of origin. Cell line CTCL-3 has an abnormal karyotype, but no detectable chromosomal abnormalities were found in the other lines, consistent with the karyologic features of their clinical sources. Because T cells from normal donors do not respond to pp-TCGF unless the cells are first "activated" by a lectin mitogen such as phytohemagglutinin or an antigen, the direct response to pp-TCGF of T cells from patients with T-cell neoplasias suggests that the cell lines represent a transformed neoplastic cell population. Although some of the cell lines may be normal T cells activated by the malignant cells, the morphologic and histochemical properties of the cell lines, the abnormal karyotype of CTCL-3, and the independent growth of CTCL-2 support the conclusion that most of these cell lines are of malignant origin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Classification of acute leukemia. Ann Intern Med. 1977 Dec;87(6):740–753. doi: 10.7326/0003-4819-87-6-740. [DOI] [PubMed] [Google Scholar]
- Frei E., 3rd, Sallan S. E. Acute lymphoblastic leukemia: treatment. Cancer. 1978 Aug;42(2 Suppl):828–838. doi: 10.1002/1097-0142(197808)42:2+<828::aid-cncr2820420704>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Russell E. K., Schechter G. P., Bunn P. A., Jr In vitro growth of cutaneous T-cell lymphomas. Cancer Treat Rep. 1979 Apr;63(4):587–590. [PubMed] [Google Scholar]
- Gillis S., Baker P. E., Ruscetti F. W., Smith K. A. Long-term culture of human antigen-specific cytotoxic T-cell lines. J Exp Med. 1978 Oct 1;148(4):1093–1098. doi: 10.1084/jem.148.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guccion J. G., Fischmann A. B., Bunn P. A., Jr, Schechter G. P., Patterson R. H., Matthews M. J. Ultrastructural appearance of cutaneous T cell lymphomas in skin, lymph nodes, and peripheral blood. Cancer Treat Rep. 1979 Apr;63(4):565–570. [PubMed] [Google Scholar]
- Gupta S., Good R. A. Markers of human lymphocyte subpopulations in primary immunodeficiency and lymphoproliferative disorders. Semin Hematol. 1980 Jan;17(1):1–29. [PubMed] [Google Scholar]
- Janckila A. J., Li C. Y., Lam K. W., Yam L. T. The cytochemistry of tartrate-resistant acid phosphatase. Technical considerations. Am J Clin Pathol. 1978 Jul;70(1):45–55. doi: 10.1093/ajcp/70.1.45. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. M., Miller L. D., Olson C., Gillette K. G. Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst. 1969 Dec;43(6):1297–1305. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Sarngadharan M. G., Sarin P. S., Reitz M. S., Gallo R. C. Reverse transcriptase activity of human acute leukaemic cells: purification of the enzyme, response to AMV 70S RNA, and characterization of the DNA product. Nat New Biol. 1972 Nov 15;240(98):67–72. doi: 10.1038/newbio240067a0. [DOI] [PubMed] [Google Scholar]
- Strausser J. L., Rosenberg S. A. In vitro growth of cytotoxic human lymphocytes. I. Growth of cells sensitized in vitro to alloantigens. J Immunol. 1978 Oct;121(4):1491–1495. [PubMed] [Google Scholar]
- Variakojis D., Vardiman J. W., Golomb H. M. Cytochemistry of hairy cells. Cancer. 1980 Jan 1;45(1):72–77. doi: 10.1002/1097-0142(19800101)45:1<72::aid-cncr2820450113>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Bunn P., Knutsen T., Schechter G. P., Gazdar A. F., Matthews M. J., Minna J. D. Cytogenetic abnormalities in patients with cutaneous T-cell lymphomas. Cancer Treat Rep. 1979 Apr;63(4):575–580. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]