Abstract
The wide spectrum of vitamin D activity has focused attention on its potential role in the elevated burden of disease in a northern Canadian First Nations (Dené) cohort. Vitamin D insufficiency, and gene polymorphisms in the vitamin D receptor (VDR) and vitamin D binding protein (VDBP) have been implicated in susceptibility to infectious and chronic diseases. The objectives of this study were to determine the contribution of vitamin D from food, and measure the serum concentrations of 25-hydroxyvitamin D3 (25-OHD3) and VDBP in Dené participants. Single nucleotide polymorphisms (SNPs) associated with the dysregulation of the innate immune response were typed and counted. Potential correlations between the SNPs and serum concentrations of 25-OHD3 and VDBP were evaluated. Venous blood was collected in summer and winter over a one-year period and analyzed for 25-OHD3 and VDBP concentrations (N = 46). A questionnaire was administered to determine the amount of dietary vitamin D consumed. Sixty-one percent and 30% of the participants had 25-OHD3 serum concentrations <75 nmol/L in the winter and summer respectively. Mean vitamin D binding protein concentrations were within the normal range in the winter but below normal in the summer. VDBP and VDR gene polymorphisms affect the bioavailability and regulation of 25-OHD3. The Dené had a high frequency of the VDBP D432E-G allele (71%) and the Gc1 genotype (90%), associated with high concentrations of VDBP and a high binding affinity to 25-OHD3. The Dené had a high frequency of VDR Fok1-f allele (82%), which has been associated with a down-regulated Th1 immune response. VDBP and VDR polymorphisms, and low winter 25-OHD3 serum concentrations may be risk factors for infectious diseases and chronic conditions related to the dysregulation of the vitamin D pathway.
Introduction
Vitamin D has a wide spectrum of activity including calcium and bone homeostasis, cardiovascular and immune system function, as well as skin, muscle and cell proliferation. The elevated burden of both infectious and non-infectious diseases borne by Canada’s Aboriginal (First Nations, Metis and Inuit) people has focused attention on the potential causal, preventive and/or therapeutic role, if any, of this vitamin [1]. Case reports of rickets, elevated fracture risk and low bone mineral density in First Nations and Inuit children and women suggest that vitamin D deficiency is not rare in these groups [2]–[4]. There are currently no published data on the gene-nutrient interaction with regards to vitamin D in Canadian northern First Nation populations.
Vitamin D is derived nutritionally from a limited number of foods. The primary source comes from the skin conversion of 7-dehydrocholesterol, induced by exposure to solar ultraviolet B (UVB) radiation. Vitamin D is converted in the liver to 25-hydroxyvitamin D3 (25-OHD3) and further hydroxylated in the kidney to 1,25-dihyroxyvitamin D3 (1,25(OH)2D3), the most active form of vitamin D3. Serum 25-OHD3 concentrations are used as the clinical measure of vitamin D status. In addition to the classical function of vitamin D on skeletal development, 1,25(OH)2D3 binds with VDRs found in many tissue types to regulate cell growth and maturation, stimulate insulin secretion, and modulate the function of activated T- and B-lymphocytes and macrophages [5].
Serum 25-OHD3 is transported to organs, tissues and cells by VDBP (also known as group-specific component, or Gc) which regulates the availability of serum vitamin D and its metabolites [6]. Circulating 25-OHD3 is bound to VDBP, enters macrophages, is converted to 1,25(OH)2D3 by mitochondrial CP27B, and then binds to the VDR in the cell. Once bound to VDR, 1,25(OH) 2D3 mediates the induction of human cathelicidin, an immunomodulatory peptide contributing to antimicrobial activity against pathogens including Mycobacterium tuberculosis [7], [8]. In a recent in vitro study a single dose of vitamin D (2.5 mg) enhanced anti-mycobacterial immunity among tuberculosis contacts [9]. In a longitudinal study that tested the effects of vitamin D supplementation on Mycobacterium tuberculosis-induced innate immune response in the Dené we found that vitamin D supplementation did not enhance the innate immune response to Mycobacterium tuberculosis lipoprotein (TLR2/IL) [10]. Dysregulation of the vitamin D pathway may be influenced by VDBP and VDR gene polymorphisms that regulate the upstream vitamin D bioavailability and downstream transcriptional activity in response intracellular pathogens.
The genes controlling VDBP and VDR are highly polymorphic and have been associated with the dysregulation of the innate immune response [11]–[16]. Single-nucleotide polymorphisms (SNPs) D432E (GenBank rs7041) and T436K (GenBank rs4588) result in changes in the VDBP protein structure that produce the three most common variants of VDBP; Gc1f, Gc1s and Gc2. These variants differentially influence the bioavailability of VDBP and its binding affinity to 25-OHD3 and the induction of cathelicidin in monocytes [6], [11], [17]. Five VDR SNPs (restriction fragment length polymorphisms identified using the enzymes Fok1, Bsm1 (GenBank rs1544410), Apa1 (GenBank rs7975232), Taqα1 (GenBank rs731236), and Cdx2 (GenBank rs11568820)) result in differential gene transcription and are associated with changes in bone mineral density, calcium absorption, vitamin D-related disease conditions, metabolic disorders, and susceptibility to infectious diseases [16], [18]–[21].
The purpose of this study was to; 1) determine the contribution of vitamin D from food sources and measure the serum concentrations of 25-OHD3 and VDBP; 2) detect the frequency of VDR and VDBP SNPs that are associated with dysregulation of the innate immune response, and 3) evaluate potential correlations between VDBP SNPs and VDBP serum concentrations. This analysis of the vitamin D pathway is part of a larger community participatory research partnership focused on the biologic and social determinants of health and illness, particularly infectious diseases such as tuberculosis, which is endemic and epidemic in the Dené community and in this region of Canada [22]–[24]. The investigation of the genetic and environmental conditions related to vitamin D and the innate immune response may contribute to a better understanding of the risk factors for infectious diseases.
Results
The total number of study participants was 46 (21 males/25 females). The mean age (41.6±15.6 years) and body mass index (BMI) (30.7±6.3) did not differ significantly by sex. There were no significant correlations between age, gender or BMI in either season (data not shown). The participants were generally healthy. The most common chronic illness was hypertension (N = 7 (15%)); other conditions included gastroesophageal reflux, asthma and eczema. The mean number of chronic diseases per participant (0.6±1.0) was calculated by averaging the number of diseases across all participants to assess health. No participant was taking a medication that affected vitamin D absorption. Eleven participants self-reported a diagnosis of latent tuberculosis, and nine others self-reported having had active tuberculosis that was treated in the past.
Dietary Vitamin D Assessment
The mean daily intake of vitamin D was 271.4 IU/day in winter and 298.3 IU/day in summer (Table 1). There was no significant difference in mean daily vitamin D intake within any of the sub-groups (season (winter/summer), sex (male/female), age (<40 years/≥40 years) or BMI (<25/≥25)). Fifty percent of the daily vitamin D came from milk (fluid and powder) in the winter and summer. Local fish provided 20% of the vitamin D in the winter and 28% in the summer (Figure S1). The mean vitamin D intake of participants who took vitamin D supplements was significantly higher than those who were not taking supplements (Table 1).
Table 1. Vitamin D intake, and serum concentrations of 25-OHD3 and VDBP for the study participants by demographic group and season (mean ± standard deviation).
| Vitamin D Intake (IU/day) | 25-OHD3 concentration (nmol/L) | VDBP concentration (ug/ml) | |||||||
| Variable (N = winter, summer) | Winter | Summer | Winter | Summer | Winter | Summer | |||
| Total (N = 45, 46) | 271.4±214.4 | 298.3±226.1 | 66.5±34.5 | 103.3±41.8∧ | 364.3±124.6 | 204.5±78.8∧ | |||
| Males (N = 20, 21) | 285.0±222.3 | 311.3±233.8 | 63.5±26.0 | 100.9±44.5∧ | 375.7±158.1 | 204.2±94.2∧ | |||
| Females (N = 25, 25) | 260.2±211.8 | 287.4±223.7 | 69.9±40.7 | 105.3±40.2∧ | 353.0±94.6 | 208.2±64.9∧ | |||
| <40 years old (N = 19, 20) | 216.9±170.2 | 281.5±228.1 | 46.5±18.4 | 77.7±32.8∧ | 434.8±104.5 | 227.7±82.4∧ | |||
| ≥40 years old (N = 26, 26) | 276.0±236.9 | 320.0±228.3 | 82.1±36.3* | 122.7±37.5*∧ | 310.7±114.9* | 190.2±73.1*∧ | |||
| BMI (Kg/m2) <25 (N = 10, 10) | 314.4±187.7 | 391.7±237.0 | 57.1±30.7 | 102.4±57.2 | 392.3±135.9 | 220.3±59.2∧ | |||
| BMI (Kg/m2) ≥25 (N = 34, 34) | 259.9±223.9 | 277.9±223.7 | 67.0±31.8 | 103.5±37.2∧ | 354.8±123.5 | 202.3±83.8∧ | |||
| No vitamin D supplements (N = 39, 38) | 231.0±198.2 | 257.0±216.1* | 60.8±30.1 | 96.5±42.2∧ | 365.3±129.2 | 209.3±82.6 | |||
| Vitamin D supplements (<400 IU/day) (N = 6, 8) | 534.1±135.3* | 494.3±188.1 | 104.9±40.0* | 133.9±25.1*∧ | 368.7±109.7 | 191.0±54.8 | |||
∧difference between seasons p<0.005 (ANOVA).
difference within sub-groups p<0.005 (ANOVA ).
Serum Concentrations of Vitamin D
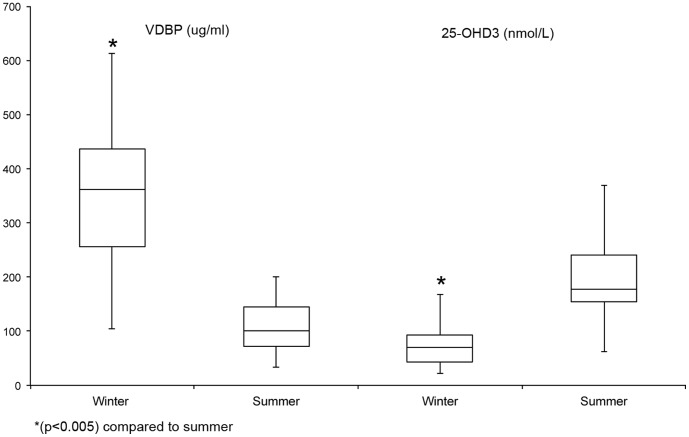
Mean serum concentrations of 25-OHD3 were significantly higher in the summer compared to the winter for the total study group and for each of the sub-groups (Table 1). Participants who were ≥40 years of age had higher mean serum concentrations than those <40 years of age in winter and summer.
Serum concentrations of 25-OHD3 were positively correlated with vitamin D intake in the winter (R = 0.4, p = 0.002) but not in the summer (R = 0.03, p = 0.25). Age also had a positive correlation with serum concentrations of 25-OHD3 in the winter (R = 0.75, p<0.005) and summer (R = 0.4, p<0.005). BMI was not correlated with serum concentrations of 25-OHD3 in either season. Overall, 61% and 30% of the study participants had serum concentrations <75 nmol/L in the winter and summer respectively. The mean concentrations of 25-OHD3 were significantly higher for those participants who took vitamin D supplements (≤400 IU/day) in both winter and summer compared to those who did not (Table 1).
The mean serum concentrations of VDBP were significantly lower in the summer compared to the winter for the total study group and for each of the sub-groups (Table 1). Participants who were ≥40 years of age had significantly lower mean serum concentrations of VDBP than those <40 years of age in both winter and summer (Table1).
There was no correlation between concentrations of VDBP and any of the traits in the sub-groups in the winter (sex (R = 0.09 p<0.5), age (R = 0.1 p<0.3) or BMI (R<0.006 p = 0.5)) or summer (sex (R = −0.04 p<0.7), age (R = 0.07 p<0.6) or BMI (R<0.03 p = 0.8)).
The mean 25-OHD3 concentrations were lower in the winter and higher in the summer and in contrast, VDBP concentrations were higher in the winter and lower in the summer for the study group (Figure 1). There were no correlations between serum concentrations of VDBP and 25-OHD3 in either season or in any demographic subgroup.
Figure 1. Seasonal comparison of the serum concentrations of VDBP (ug/ml) and 25-0HD3 (nmol/L).
The mean serum concentration of VDBP was significantly lower in the summer. The mean serum concentration of 25-OHD3 was significantly higher in the summer. The concentrations of VDBP and 25-OHD3 were inversely correlated.
VDBP and VDR Gene Polymorphisms
Genetic analysis of VDBP alleles was performed on all participants however the T436K SNP did not amplify in 6 samples. Allele and genotype frequencies for D432E and T436K were in Hardy-Weinberg Equilibrium (HWE). Table 2 summarizes the allele and genotype frequencies.
Table 2. Alleles and genotype frequencies for the vitamin D binding protein (D432E and T436K).
| VDBP SNPs | N | % | ||||||||
| D432E (rs7041) | ||||||||||
| Alleles | G | 65 | 71 | |||||||
| T | 27 | 29 | ||||||||
| Genotypes | G/G | 20 | 45 | |||||||
| G/T | 21 | 48 | ||||||||
| T/T | 3 | 7 | ||||||||
| T436K (rs4588) | ||||||||||
| Alleles | C | 77 | 91 | |||||||
| A | 8 | 9 | ||||||||
| Genotypes | C/C | 28 | 70 | |||||||
| C/A | 12 | 30 | ||||||||
| A/A | 0 | 0 | ||||||||
| D432E+T436K | ||||||||||
| Diplotypes | Gc1f (T+C) | 15 | ||||||||
| Gc1s (G+C) | 75 | |||||||||
| Gc2 (T+A) | 10 | |||||||||
| Haplotypes | Gc1f-1f | 0 | 0 | |||||||
| Gc1f-1s | 11 | 27 | ||||||||
| Gc1s-1s | 22 | 54 | ||||||||
| Gc1f-Gc2 | 1 | 2 | ||||||||
| Gc1s-Gc2 | 7 | 17 | ||||||||
| Gc2-Gc2 | 0 | 0 | ||||||||
One-way ANOVA was used to compare differences in VDBP concentrations by genotype. The D432E-G/G genotype had significantly higher VDBP concentrations in the winter compared to the G/T and T/T genotypes (p<0.005) but not in the summer (Table 3). No differences in VDBP concentrations were found between for the T436K genotypes (Table 3). No correlation was found between serum concentrations of 25-OHD3, and VDBP genotypes (data not shown).
Table 3. Vitamin D binding protein serum concentrations (ug/ml) stratified by vitamin D binding protein genotypes (mean ± standard deviation).
| Winter | Summer | |
| VDBP Genotypes | ||
| D432E (rs7041) | ||
| #G/G (N = 22) | 469.4±72.85 | 246.4±72.61 |
| T/G (N = 20) | 277.2±66.40* | 173.9±58.51 |
| T/T (N = 3) | 208.1±153.8* | 143.9±120.6 |
| T436K (rs4588) | ||
| #C/C (N = 32) | 381.3±131.4 | 217.1±80.50 |
| C/A (N = 8) | 290.8±63.6 | 165.0±57.2 |
| A/A (N = 0) | 0 | 0 |
| VDBP Haplotypes | ||
| D432E+T436K | ||
| #Gc1f-1f (N = 1) | 408.0±0 | 62.0±0 |
| Gc1f-1s (N = 9) | 380.6±170.8 | 193.9±75.0 |
| Gc1s-1s (N = 23) | 339.6±116.8 | 241.4±70.85 |
| Gc1f-Gc2 (N = 2) | 332.2±148.4 | 205.8±108.3 |
| Gc1s-Gc2 (N = 6) | 414.3±99.5 | 154.4±41.1 |
| Gc2-Gc2 (N = 0) | 0 | 0 |
#reference group.
within season comparison p<0.005 (ANOVA).
The three most common VDBP diplotypes (Gc1s, Gc1f and Gc2) were all present in the Dené study group. The Gc2 and Gc1f have aspartic acid (GAT) at position 416 compared to a glutamic acid (GAG) in the Gc1s allele. Gc2-A allele encodes for lysine amino acid (AAG) at position 420 compared with the threonine (ACG) in Gc1f and Gc1s. The combination of glutamic acid and lysine is rarely seen in humans so only the three variants are possible. The Dené had a greater frequency of the Gc1 (Gc1f and Gc1s) alleles compared to the Gc-2 (Table 2). The Gc1 diplotype is characterized by high and intermediate binding affinity for 25-OHD3, whereas the Gc2 diplotype is associated with a low binding affinity [6].
The mean serum concentrations of VDBP were significantly higher for the D432E – G/G genotype compared to the G/T and T/T genotypes (Table 3). The mean concentrations of VDBP were compared by haplotype and no significant differences were found (Table 3).
The VDR gene has more than 470 polymorphisms but only a small number have been demonstrated to have functional effect [16], [25]–[34]. In our study the Apa1 and Taqα1genotypes were in HWE (Table 4). The Fok1, Bsm1 and Cdx-2 sites were not in HWE likely due to the small sample size or as a result of selective pressure on the alleles. The Dené had a high frequency of the Fok1-f SNP (82%), which results in an alteration of the start codon. The Fok1-f SNP has moderately lower VDR transcriptional activity than the Fok1-F SNP [7], [11], [19]. The Bsm1-b allele and the b/b genotype at the Bsm1 restriction site occurred most frequently (b-96% and b/b-89%) in the Dené cohort compared to the major allele and genotypes. This pattern of allele and genotype frequency was also noted for the Taqα1 and Cdx2 restriction sites (Taqα1-T allele - 96% and Taqα1-T/T genotype–89%; Cdx2–G allele–96%; Cdx2-G/G genotype-91%).
Table 4. Allele and genotype frequencies for the vitamin D receptor gene polymorphisms (Fok1, Bsm1, Apa1, Taqα1 and Cdx2).
| VDR SNPs | N | % | ||||||
| Fok1 (rs10735810) | ||||||||
| Alleles | C (F) | 13 | 18 | |||||
| T (f) | 74 | 82 | ||||||
| Genotypes* | C/C (F/F) | 4 | 8 | |||||
| C/T (F/f) | 9 | 20 | ||||||
| T/T (f/f) | 33 | 72 | ||||||
| Bsm1 (rs1544410) | ||||||||
| Alleles | T (B) | 4 | 4 | |||||
| C (b) | 86 | 96 | ||||||
| Genotypes | T/T (B/B) | 1 | 2 | |||||
| T/C (B/b) | 4 | 9 | ||||||
| C/C (b/b) | 41 | 89 | ||||||
| Apa1 (rs7975232) | ||||||||
| Alleles | T (A) | 29 | 32 | |||||
| G (a) | 61 | 68 | ||||||
| Genotypes* | T/T (A/A) | 4 | 9 | |||||
| T/G (A/a) | 22 | 48 | ||||||
| G/G (a/a) | 20 | 43 | ||||||
| Taqα1 (rs731236) | ||||||||
| Alleles | T (T) | 86 | 96 | |||||
| C (t) | 4 | 4 | ||||||
| Genotypes | T/T (T/T) | 41 | 89 | |||||
| T/C (T/t) | 5 | 11 | ||||||
| C/C (t/t) | 0 | 0 | ||||||
| Cdx2 (rs11568820) | ||||||||
| Alleles | G | 86 | 96 | |||||
| A | 4 | 4 | ||||||
| Genotypes* | G/G | 41 | 91 | |||||
| G/A | 4 | 9 | ||||||
| A/A | 0 | 0 | ||||||
(*not in Hardy-Weinberg Equilibrium p<0.005).
No correlation was found between serum concentrations of 25-OHD3 or VDBP and the VDR gene polymorphisms (data not shown).
Discussion
The interaction between diet, environment, and the genes that influence the vitamin D pathway impacts the functioning of a broad range of human tissue and cell types. Vitamin D’s effect on calcium absorption is critical for skeletal health. However, many cells, including macrophages, have the capacity to synthesize 1,25-OHD3. This process is integral to cellular development and health. Inadequate vitamin D intake both from UVB radiation or dietary sources can impact innate and adaptive immunity, and has been implicated in autoimmune disorders, chronic conditions and infectious diseases.
Study Limitations
The use of culturally respectful research methodologies and knowledge translation processes in partnership with the people of this Dené First Nation, has yielded results that the community has indicated, hold legitimacy and meaning. However, the ability to generalize the study findings (external validity) to the general Dené population and to other First Nation communities is potentially limited due the small number of those recruited, and the method of recruitment. The sample size of the study group was small and the serum concentrations of 25-OHD3 and VDBP were highly variable. Other limitations include the possibility that the diet and sunlight exposure of those who chose to participate in the study differed from those who did not participate. In terms of the internal validity of the study, the use of a food questionnaire to calculate nutrient intake poses a challenge. Recognizing this, the questionnaire used in this study was field-tested and adapted to the community and their culture in an effort to maximize accuracy and reproducibility [24]. Future studies that include a larger sample will help to clarify the extent of seasonal and individual variability in the Dené population.
Dietary Assessment
Recognizing these limitations, the results of this study demonstrate that optimal vitamin D intake from diet (with or without taking ≤400 IU/day of vitamin D) was not achieved by the participants in this study; 89% did not meet the 2010 Institute of Medicine’s recommended daily dietary intake of 600 IU/day of vitamin D [37]. Vitamin D intake was higher among the older study participants. Similar results were found for rural Aboriginal women over 50 year olds. This group had a mean intake that was higher than 25–50 year olds in the same study [35]. Kuhnlein found that the traditional diet of the Dené people of Canada’s Northwest Territory is being replaced by market foods, particularly among the younger age groups [36]. Increasing reliance on expensive market foods is occurring in most northern aboriginal communities. The causes include the increasing expense associated with hunting and fishing, environmental degradation, climate change, and loss of traditional knowledge [37]–[39].
Serum Concentrations of 25-OHD3
Despite the consistently low dietary intake of vitamin D in winter and summer, the mean serum concentrations of 25-OHD3 varied between seasons. Taking low doses (≤400 IU/day) of vitamin D supplements significantly increased serum concentrations of 25-OHD3 compared to participants who did not take supplements. However, the majority of Dené participants (61%), including those taking vitamin supplementations (≤400 IU/day), did not meet current recommended serum concentration of 25-OHD3 (≥75 nmol/L) during the winter [40]–[42]. Summer concentrations of 25OHD3 were ≥75 nmol/L for 70% of the participants likely due to sunlight exposure.
Serum concentrations of 25-OHD3 and VDBP were correlated with age. Compared to the <40 year olds, the ≥40 year olds had lower mean concentrations of VDBP and higher mean concentrations of 25-OHD3. There was also a positive correlation between increasing age and serum concentrations of 25-OHD3. Most studies report decreased vitamin D concentrations in older individuals and attribute this to a more restricted diet, less physical activity and time spent outdoors [43], [44]. Higher serum concentrations of vitamin D in the older individuals in the Dené community might be attributed to the older adults spending more time outdoors than those participants who were <40 years olds or increased consumption of local fish which is high in vitamin D [45].
In support of the importance of UVB as a vitamin D source, the serum 25-OHD3 concentrations were correlated with dietary vitamin D in the winter but not in the summer. This finding is in contrast to other studies that found a significant correlation between dietary intake of vitamin D and serum concentrations of 25-OHD3 in winter and summer/fall [35], [46]–[49]. This discrepancy may be related to a number of factors, including possible flaws in the food frequency questionnaire, the small sample size or the relative unavailability of vitamin D containing foods. More likely it is related to fact that the major source of vitamin D is linked to an individual’s sunlight exposure making dietary intake a relatively minor source [50].
The findings of low winter and summer vitamin D dietary intake among participants in this study, and low winter serum concentrations of 25-OHD3 were in alignment with previous studies among circumpolar and Canadian indigenous populations [3], [4], [40], [41], [46]–[48], [51]. However, the serum concentrations of 25-OHD3 in this study were higher than reported for other Aboriginal groups (41.8±14.5 nmol/L) [35]. The continued consumption of traditional food sources (fish) and increased UVB exposure in the summer by participants in this study may explain the higher 25-OHD3 concentrations. While vitamin D deficiency, is clearly not an issue for the majority of study participants, in the summer, vitamin D insufficiency in the winter may play a role in dysregulation of the immune response to the current situation of endemic tuberculosis.
Serum Concentrations of VDBP
In this study serum concentrations of VDBP were within the normal range (300–600 µg/ml) in the winter but below normal levels in the summer [6]. Serum concentrations of VDBP are highly variable within and between studies, and cell and tissue trauma caused by liver diseases, nephrotic syndrome and malnutrition can result in low VDBP concentrations [6]. To our knowledge there are only a few studies that report seasonal serum VDBP concentrations. Seasonal differences were found in VDBP concentrations in breast-fed infants but not in their mothers [49]. Although summer is short in the north, the increased availability of 25-OHD3 (as evidenced by the higher serum concentrations in our study group) through UVB exposure may have an effect on the concentrations of VDBP. One of the many functions of VDBP is to bind with 25-OHD3 and 1,25-OHD3 and transport it to target cells. VDBP also binds and transports fatty acids, scavenges actin and has a role in macrophage activation [6], [17], [52]. VDBP is typically present in high concentrations (5×10−6 M) and has a short half-life (2.5 days) compared to 25-OHD3, which has a half-life of 12 days and plasma concentrations of 5×10−8M.
VDBP and VDR Gene Polymorphisms and their Functional Effects
VDBP gene polymorphisms have been shown to affect the serum concentrations of VDBP and its avidity to 25-OHD3. The D432E–G allele and the Gc1 diplotype are associated with higher concentrations of VDBP, intermediate to high binding affinity, and slower metabolism of 25-OHD3. For our study group, given the low UVB exposure in the winter there would be an advantage to having high concentrations of VDBP with high binding affinity to maximize the limited availability of vitamin D. The Dené had a high frequency of the VDBP D432E– G allele and the G/G genotype was associated with higher concentrations of VDBP in the winter but not in the summer. In contrast to other studies there was no association between VDBP genotypes and concentrations of 25-OHD3 [17].
Population genetic studies have shown an association between Gc1f, Gc1s and Gc2 variants of VDBP, geographic distribution of populations, and insulin resistance. Among the Dogrib Dené the Gc1f genotype was associated with lower measures of fasting insulin than those with the Gc2 genotype [6], [41], [53]. The Gc1f genotype has also been shown to be protective against Type II diabetes [54]. In another study the lower affinity Gc2 genotype was correlated with higher induction of the antibacterial peptide LL-37 by 25-OHD3 [10]. The homozygous Gc1f genotype is a significant risk factor for the development of chronic obstructive pulmonary disease (COPD) and for carriers of the Gc1f variant the age related decline of the Forced Expiratory Volume (FEV1) was significantly higher (6). It is possible that selection for the Gc2 genotype occurred in populations exposed to recurrent intra-cellular infections, which might account for its higher frequency among European decent groups [55]–[57].
The VDR genotypes found in high frequency among Dené participants have been associated with decreased macrophage response to pathogens, and a number of chronic, and infectious diseases, cancer, osteoporosis, autoimmune conditions, multiple sclerosis and tuberculosis [5], [16], [30]–[33], [58]–[61]. The Dené group in this study did not differ significantly from previously published results for a separate Dené cohort from the same community [23].
The VDR Fok1 restriction site defines a SNP in the first of two potential translation initiation start sites for the VDR mRNA. Two protein variants can exist corresponding to the two available start sites: the longer VDR, encoded by the alternate allele form (ATG) (designated f), is three amino acids longer and 1.7 times less efficient than the common allele form (ACG) (designated F) [27]. This has functional consequences for the intra-cellular activity whereby the amino acid structure created by the Fok1-f allele will reduce the transcriptional activity and the production of antimicrobial cathelicidin [58]. The Cdx2 SNP has an important role in the transcriptional activity of VDR in the small intestine, calcium absorption, and bone mineral density [27]. The VDR promoters that have the Cdx2-G allele have 70% less transcriptional activity than the A allele resulting in poorer calcium absorption.
The VDR restriction sites Bsm1, Apa1 and Taqα1 have not been demonstrated to have an effect on gene expression but they may be linked to functional SNPs [25], [26]. There are however studies that have found associations between these SNPs and infectious diseases [30], [59], [60]. In a recent study in India, individuals with Bsm1-b/b, Fok1-f/f, Taqα1-t/t genotypes were at higher risk of developing multi-drug resistant tuberculosis and/or smear positive disease than heterozygotes or homozygous dominant individuals [62]. The high frequency of the Fok1-f, Bsm1-b, Apa1-a and Taqα1-T alleles in the Dené population may increase their risk of tuberculosis infection or disease. The combination of 25-OHD3 deficiencies, the high frequency of the Fok1-f/f genotype, and the Taqα1-T/T or T/t genotype, were strongly associated with tuberculosis in an Asian population [30]. Children with the Fok1-f/f genotype were also at increased risk of acute lower respiratory infection and in another study, women with this genotype had an increased risk of breast cancer [25], [31], [32]. Although the Fok1-f allele is associated with a number of adverse outcomes, there may be selective pressures that favour this allele for functions related to tissue, bone and/or muscle. Despite the inconsistencies that occur between studies with regards to VDR SNPs and bone mineral density, there does appear to be an association between Bsm1, Fok1 and bone development. The Fok1-f allele was associated with muscle strength in homozygote females and the Bsm1-b allele with increased bone mineral density [63]. In other studies the Bsm1-b and Fok1-f alleles have both been associated with low bone mineral density [27], [28].
Summary
Among the Dené participants of this study, low winter serum levels of 25-OHD3 were likely related to dietary deficiency and limited exposure to sunlight-induced cutaneous production of vitamin D. In this context, the high frequency of VDBP genotypes associated with avid binding affinity, and VDR alleles associated with reduced transcriptional activity, may adversely affect the diverse functions of this vitamin, including innate immune responses to pathogens. The observed VDBP and VDR allele and genotype frequencies in the study group may reflect population based selective pressures for the so called “classic vitamin D pathways” (calcium absorption, bone mineralization and remodelling, anti-inflammatory regulation and rapid tissue repair). In northern First Nation communities where infectious diseases such as tuberculosis are endemic, and high quality nutritious foods are difficult to access, a vitamin D pathway that strongly down-regulates a Th1 immune response may have serious health implications [64], [65].
Identification of nutrient deficiencies, and detection of functional gene polymorphisms associated with infectious and chronic disease, may provide guidance for future prevention and treatment programs. To-date, studies on vitamin D and innate immune regulation have occurred only in non-aboriginal groups with one recent exception [10]. In vitro studies of vitamin D metabolism and innate immunity in aboriginal groups are crucial for clarifying the regulatory pathways and function of vitamin D in this population. This may pave the way for in vivo supplementation trials.
Materials and Methods
Ethics Statement
Canadian Aboriginal research principles of ownership, control, access and possession (OCAP) were followed (The First Nations Principles of OCAP. Ottawa: First Nations Governance Centre; 2010. Available at: http://www.fnigc.ca/node/2). The University of Manitoba Health Research Ethics Board and the community’s Chief and Council approved the study. Study participants were 18 years of age or older and provided informed written consent.
Study Participants
Northlands Denésuline First Nation (Dené) members were recruited at Lac Brochet, located at 58° latitude in northern Manitoba, Canada. The Dené community in this study is remote and accessible only by air and winter road. The Dené are part of the larger Na-Dené (Athapaskan) language family, which includes the Alaskan Gwich’in and the American Apache and Navajo. The Dené have a long history of reliance on migratory caribou and fish as primary food sources. However, their food security is currently challenged by environmental, social, economic, and political pressure [37]–[39].
This community is comprised of 145 census families which include 605 individuals (350 of whom are ≤19 years of age) who self-identified as Dené [66]. Community consultation determined that convenience sampling, rather than random sampling, was the only methodology considered acceptable. Participants were eligible for inclusion if they were 18 years of age or older, able and willing to give informed consent, self-identified as Dené, able to commit to participation for the duration of the study and committed to avoid taking vitamin D supplements >400 IU/day. Individuals taking a low dose of vitamin D (≤400 IU/day) were allowed to participate in the study. In this way it was hoped that the study cohort might more accurately reflect the general community population (Figure S2). Supplementary vitamin D intake was added to the calculation of dietary intake. Forty-six participants (representing approximately 14% of the adult population) completed the study.
Data collected in winter (February-March) and late summer/fall (September-October) of 2010, included age, sex, body mass index (BMI), medications, chronic health conditions and self-identified ethnicity. A food frequency questionnaire (FFQ) from Wu [67] was modified to include available vitamin D containing market foods (i.e. milk, margarine, etc.) and traditional foods (i.e. local fish caribou fat, meat and organs). The frequency of vitamin D containing foods and portion sizes were assessed using the vitamin D values from the Canadian Nutrient File [45]. The questionnaire was field-tested and administered at winter and summer time points. Participants were asked to recall food consumption patterns for the previous month.
Analysis of 25-OHD3 and VDBP Serum Concentrations by ELISA
Venous blood samples were collected at the winter and summer visits in 2010.
Serum concentrations of 25-OHD3 and VDBP were evaluated by ELISA (Immunodiagnostic Systems, Inc. Scottsdale, AZ, USA and R&D Systems, Minneapolis, MN, USA) [68]. For the 25-OHD3 ELISA intra- and inter-plate coefficients of variation were 2.2±2.9% and 5.7±1.4%. For the VDBP ELISA intra- and inter-plate coefficients of variation were 3.3±2.4% and 8.6±6.2%. Vitamin D sufficiency in this study was based on serum concentrations of 25-OHD3≥75 nmol/L [40]–[42].
SNP Analysis
Genomic DNA was manually extracted from buffy coats using the QIAamp DNA Blood Mini Kit (Qiagen Inc. Toronto, ON). The following PCR conditions were used for VDBP amplification in a 25 µl reaction: 3.5 µl extracted DNA; 2.5 µl 10X PCR Buffer; 1.0 µl 50 mM MgSO4; 0.2 µl 10 mM dNTP mix; 16.2 µl reagent grade water; 0.1 µl Platinum® Taq (Invitrogen Life Technologies Corp. Burlington, ON); 0.5 µl each of the forward primer and reverse primers [52]. PCR conditions were 95°C for 15 min followed by 35 cycles of 94°C for 20s, 58°C for 20s, and 72°C for 20s with a 1sec increment for each subsequent cycle, and one cycle at 72°C for 10 min [25]. PCR amplification of VDR SNPs Bsm1 (T/C), Apa1 (G/T), Taqα1 (C/T), Fok1(T/C), and Cdx-2 (G/A), was performed using published protocols and primers [25]–[27]. Analysis of the VDR SNPs at the restriction sites Bsm1 (B/b (T/C)), Apa1(A/a (T/G)), Taqα1(T/t (T/C)), Fok1 (F/f (C/T)), and Cdx-2 (G/A) and VDBP SNPs at D432E (G/T) and T436K (C/A) were detected using RFLP and were visualized with ethidium bromide staining and ultraviolet illumination [25]–[27]. Allele counting was conducted manually.
Statistical Analysis
Statistical analysis was preformed using AnalystSoft, StatPlus:mac - statistical analysis program for Mac OS. Version 2009. Comparison of mean concentrations of 25-OHD3 and VDBP were evaluated between demographic groups (sex, age, BMI) and seasons (winter/summer) using Pearson’s Correlation or Student’s-T Test on the transformed data.
The Shapiro-Wilk test was used to assess the null hypothesis that the sampled concentrations of vitamin D (25-OHD3) and VDBP were normally distributed population. The winter concentrations of 25-OHD3 were skewed and this data was log transformed for multiple regression analysis. Differences in mean concentrations of 25-OHD3 and VDBP were evaluated by season, age, sex, BMI, and vitamin D intake were evaluated for the main effects using ANOVA. Multiple regression was used to test correlations between the vitamin D intake, season, age, sex, BMI (independent variables), and concentrations of 25-OHD3 (dependent variable). VDBP concentrations were dependent variables in a multiple regression using season, age, sex, BMI were the independent variables.
VDBP and VDR allele and genotype frequencies were evaluated for deviations for Hardy-Weinberg Equilibrium (HWE) using a chi-square test. Associations between VDBP allele, genotypes, and serum concentrations of 25-OHD3 and VDBP, were analyzed using multiple regressions in separate analyses for winter and summer.
Seasonal 25-OHD3 and VDBP concentrations were evaluated separately against T436K and D432E alleles using multiple linear regressions controlling for BMI, age and sex. VDBP concentrations were used as the dependent variable and T436K and D432E alleles 1 and 2 were independent variables.
P values <0.05 were considered statistically significant unless otherwise stated.
Supporting Information
Relative (%) contribution of traditional and market foods to daily vitamin D intake (IU/day) by season. Fifty percent of the dietary intake of vitamin D came from milk (fluid and powdered). Local fish obtained from the lake provided 20% in the winter and 28% in the summer of the daily vitamin D. Margarine and eggs were also source of vitamin D. Caribou was not a major source of vitamin D however values for some animal parts that were consumed were unknown (i.e. fat, blood, liver).
(TIF)
Enrolment of study participants. One-hundred and five of 330 adults in the community were screened for the study [68]. Exclusion criteria included the use of vitamin D supplements >600 IU/day for three months prior to study on-set, clinical evidence of infection at the time of enrolment, first-degree kinship with an individual already enrolled, and immunosuppressive medical condition or use of immunosuppressive medication, including systemic steroids. Fifty-four of the 105 people screened met the study criteria and were enrolled. During the course of the one-year study 2 individuals moved away permanently from the community, 2 were absent from the community during one of the two test periods, 3 individuals withdrew for personal reasons, and 1 person developed a serious inter-current illness precluding further study participation.
(TIF)
Acknowledgments
The authors wish to thank the community of Northlands Denésuline First Nation and the local research assistants for the opportunity to engage with them in this research partnership.
Funding Statement
Funding for this research was provided by the Canadian Institutes of Health Research and the National Sanitarium Association (Canada). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Health Canada (1999) Tuberculosis in First Nations Communities. Available: http://www.hc-sc.gc.ca/fniah-spnia/diseases-maladies/tuberculos/index-eng.php. Accessed 2012 October 2.
- 2. Lebrun JB (1993) Vitamin D deficiency in a Manitoba community. Can J Public Health 84: 394. [PubMed] [Google Scholar]
- 3. Moffatt ME (1995) Current status of nutritional deficiencies in Canadian Aboriginal people. Can J Physiol Pharmacol 73: 754–758. [DOI] [PubMed] [Google Scholar]
- 4. El Hayek J, Egeland G, Weiler H (2010) Vitamin D status of Inuit preschoolers reflects season and vitamin D intake. J Nutr 140: 1839–1845 DOI: 10.3945/jn.110.124644 [DOI] [PubMed] [Google Scholar]
- 5. Bikle DD (2011) Vitamin D regulation of immune function. Vitam Horm 86: 1–21 DOI: 10.1016/B978-0-12-386960-9.00001-0 [DOI] [PubMed] [Google Scholar]
- 6. Speeckaert M, Huang G, Delanghe JR, Taes YE (2006) Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta 372: 33–42 DOI: 10.1016/j.cca.2006.03.011 [DOI] [PubMed] [Google Scholar]
- 7. Liu P, Stenger S, Li H, Wenzel B, Tan H, et al. (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773. [DOI] [PubMed] [Google Scholar]
- 8. Mookherjee N, Lippert DN, Hamill P, Falsafi R, Nijnik A, et al. (2009) Intracellular receptor for human host defense peptide LL-37 in monocytes. J Immunol 183: 2688–2696 DOI: 10.4049/jimmunol.0802586 [DOI] [PubMed] [Google Scholar]
- 9. Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, et al. (2007) A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med 176: 208–213. [DOI] [PubMed] [Google Scholar]
- 10.Larcombe L, Orr P, Turner-Brannen E, Slivinski C, Nickerson P, et al. (2012) Effect of vitamin D supplementation on Mycobacterium tuberculosis-induced innate immune responses in a Canadian Dené First Nations cohort. PLoS One 7. DOI: 10.1371/journal.pone.0040692. [DOI] [PMC free article] [PubMed]
- 11. Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, et al. (2010) Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 95: 3368–3376 DOI: 10.1210/jc.2010-0195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chishimba L, Thickett DR, Stockley RA, Wood AM (2010) The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax 65: 456–462 DOI: 10.1136/thx.2009.128793 [DOI] [PubMed] [Google Scholar]
- 13. Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, et al. (2010) Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 65: 215–220 DOI: 10.1136/thx.2009.120659 [DOI] [PubMed] [Google Scholar]
- 14. Adams JS, Liu PT, Chun R, Modlin RL, Hewison M (2007) Vitamin D in defense of the human immune response. Ann N Y Acad Sci 1117: 94–105 DOI: 10.1196/annals.1402.036 [DOI] [PubMed] [Google Scholar]
- 15. Kresfelder TL, Janssen R, Bont L, Pretorius M, Venter M (2011) Confirmation of an association between single nucleotide polymorphisms in the VDR gene with respiratory syncytial virus related disease in South African children. J Med Virol 83: 1834–1840 DOI: 10.1002/jmv.22179 [DOI] [PubMed] [Google Scholar]
- 16. Roth D, Jones A, Prosser C, Robinson J, Vohra S (2008) Vitamin d receptor polymorphisms and risk of acute lower respiratory tract infection in early childhood. Journal of Infectious Diseases 197: 676–680. [DOI] [PubMed] [Google Scholar]
- 17. Lauridsen AL, Vestergaard P, Nexo E (2001) Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin Chem 47: 753–756. [PubMed] [Google Scholar]
- 18. Lombard Z, Dalton DL, Venter PA, Williams RC, Bornman L (2006) Association of HLA-DR, -DQ, and vitamin D receptor alleles and haplotypes with tuberculosis in the Venda of South Africa. Hum Immunol 67: 643–654 DOI: 10.1016/j.humimm.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 19. Motsinger-Reif A, Antas PR, Oki N, Levy S, Holland S, et al. (2010) Polymorphisms in IL-1β, vitamin D receptor Fok1, and Toll-like receptor 2 are associated with extrapulmonary tuberculosis. BMC Med Genet 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Realegeno S, Modlin RL (2011) Shedding light on the vitamin D-tuberculosis-HIV connection. Proc Natl Acad Sci U S A 108: 18861–18862 DOI: 10.1073/pnas.1116513108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, et al. (2011) Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med 3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodge A, Orr P, Larcombe L, Denechezhe L, Oakley L, et al. Tuberculosis in a remote Canadian Dené community: the impact of virulence, genetic and environmental factors on epidemiology and control; 2006; Novosibersk, Siberia. Available: http://www.ict.nsc.ru/ws/show_abstract.dhtml?en1259191. Accessed 2011 October 1.
- 23. Larcombe L, Orr P, Lodge A, Brown J, Dembinski I, et al. (2008) Functional gene polymorphisms in Canadian Aboriginal populations with high rates of tuberculosis. Journal of Infectious Diseases 198: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 24. Larcombe L, Nickerson P, Singer M, Robson R, Dantouze J, et al. (2011) Housing conditions in 2 Canadian First Nations communities. Int J Circumpolar Health 70: 141–153. [DOI] [PubMed] [Google Scholar]
- 25. Sainz J, Van Tornout JM, Loro ML, Sayre J, Roe TF, et al. (1997) Vitamin D-receptor gene polymorphisms and bone density in prepubertal American girls of Mexican descent. New England Journal of Medicine 337: 77–82. [DOI] [PubMed] [Google Scholar]
- 26. Selvaraj Pea (2004) Regulatory role of vitamin D receptor gene variants of Bsm I, Apa I, Taq I, and Fok I polymorphisms on macrophage phagocytosis and lymphoproliferative response to mycobacterium tuberculosis antigen in pulmonary tuberculosis. J Clin Immunol 24: 523. [DOI] [PubMed] [Google Scholar]
- 27. Arai H, Miyamoto M, Yoshida M, Yamamoto H, Taketani U, et al. (2001) The polymorphism in the caudal-related homeodomain protein Cdx-2 binding element in the human vitamin D receptor gene. Journal of Bone Mineral Research 16: 1256–1264. [DOI] [PubMed] [Google Scholar]
- 28. Fischer PR, Thacher TD, Pettifor JM, Jorde LB, Eccleshall TR, et al. (2000) Vitamin D receptor polymorphisms and nutritional rickets in Nigerian children. Journal of Bone and Mineral Research 15: 2206–2210 DOI: 10.1359/jbmr.2000.15.11.2206 [DOI] [PubMed] [Google Scholar]
- 29.Babb C, van der Merwe L, Beyers N, Pheiffer C, Walzl G, et al. (2007) Vitamin D receptor gene polymorphisms and sputum conversion time in pulmonary tuberculosis patients. Tuberculosis (Edinb) 87: 295–302. DOI 10.1016/j.tube.2007.03.001. [DOI] [PubMed]
- 30. Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, et al. (2000) Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet 355: 618–621 DOI: 10.1016/S0140-6736(99)02301-6 [DOI] [PubMed] [Google Scholar]
- 31. Chen WY, Bertone-Johnson ER, Hunter DJ, Willett WC, Hankinson SE (2005) Associations between polymorphisms in the vitamin D receptor and breast cancer risk. Cancer Epidemiology, Biomarkers and Prevention 14: 2335–2339 DOI: 10.1158/1055-9965.EPI-05-0283 [DOI] [PubMed] [Google Scholar]
- 32. Sinotte M, Rousseau F, Ayotte P, Dewailly E, Diorio C, et al. (2008) Vitamin D receptor polymorphisms (FokI, BsmI) and breast cancer risk: association replication in two case-control studies within French Canadian population. Endocr Relat Cancer 15: 975–983 DOI: 10.1677/ERC-08-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jain R, von Hurst PR, Stonehouse W, Love DR, Higgins CM, et al. (2012) Association of vitamin D receptor gene polymorphisms with insulin resistance and response to vitamin D. Metabolism. 61: 293–301 DOI: 10.1016/j.metabol.2011.06.018 [DOI] [PubMed] [Google Scholar]
- 34. Smolders J, Thewissen M, Peelen E, Menheere P, Tervaert JW, et al. (2009) Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One 4: e6635 DOI: 10.1371/journal.pone.0006635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holick MF (2003) Vitamin D: A millenium perspective. J Cell Biochem 88: 296–307 DOI: 10.1002/jcb.10338 [DOI] [PubMed] [Google Scholar]
- 36. Kuhnlein HV, Receveur O, Soueida R, Egeland GM (2004) Arctic indigenous peoples experience the nutrition transition with changing dietary patterns and obesity. J Nutr 134: 1447–1453. [DOI] [PubMed] [Google Scholar]
- 37. Gordon BC (1981) Man-environment relationship in barrenland prehistory. Musk-ox 28: 1–19. [Google Scholar]
- 38. Receveur O, Boulay M, Kuhnlein HV (1997) Decreasing traditional food use affects diet quality for adult Dene/Metis in 16 communities of the Canadian Northwest Territories. American Society for Nutritional Sciences 127: 2179–2186. [DOI] [PubMed] [Google Scholar]
- 39.Helm J (2000) The People of Denendeh. Ethnohistory of the Indians of Canada’s Northwest Territories. Iowa City: University of Iowa Press.
- 40. Hanley DA, Cranney A, Jones G, Whiting SJ, Leslie WD, et al. (2010) Vitamin D in adult health and disease: a review and guideline statement from Osteoporosis Canada. Can Med Assoc J 182: E610–E618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Statistics Canada (2009) Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian health measures survey. Available: http://www.statcan.gc.ca/pub/82-625-x/2010001/article/11137-eng.htm. Accessed 2011 November 6.
- 42. Hewison M (2012) Vitamin D and immune function: an overview. Proceedings of the Nutrition Society 71: 50–61 DOI: 10.1017/S0029665111001650 [DOI] [PubMed] [Google Scholar]
- 43. Gloth FM, 3rd, Gundberg CM, Hollis BW, Haddad JG, Jr., Tobin JD (1995) Vitamin D deficiency in homebound elderly persons. Journal of the American Medical Association 274: 1683–1686. [DOI] [PubMed] [Google Scholar]
- 44. Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, et al. (2012) Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr 95: 91–100 DOI: 10.3945/ajcn.111.014779 [DOI] [PubMed] [Google Scholar]
- 45.Health Canada (2007) The Canadian Nutrient File. Available: http://www.hc-sc.gc.ca/fn-an/nutrition/fiche-nutri-data/cnf_aboutus-aproposdenous_fcen-eng.php. Accessed 2010 September 10.
- 46. Weiler HA, Leslie WD, Krahn J, Steiman PW, Metge CJ (2007) Canadian Aboriginal women have a higher prevalence of vitamin D deficiency than non-Aboriginal women despite similar dietary vitamin D intakes. J Nutr 137: 461–465. [DOI] [PubMed] [Google Scholar]
- 47. Gozdzik A, Zhu J, Wong BY, Fu L, Cole DE, et al. (2011) Association of vitamin D binding protein (VDBP) polymorphisms and serum 25(OH)D concentrations in a sample of young Canadian adults of different ancestry. J Steroid Biochem Mol Biol 127 405–412. 10.1016/j.jsbmb.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 48. Rejnmark L, Jorgensen ME, Pedersen MB, Hansen JC, Heickendorff L, et al. (2004) Vitamin D insufficiency in Greenlanders on a westernized fare: ethnic differences in calcitropic hormones between Greenlanders and Danes. Calcif Tissue Int 74: 255–263 DOI: 10.1007/s00223-003-0110-9 [DOI] [PubMed] [Google Scholar]
- 49. Specker BL, Tsang RC, Ho M, Buckley D (1986) Seasonal differences in serum vitamin D binding protein in exclusively breast-fed infants: negative relationship to sunshine exposure and 25-hydroxyvitamin D. J Pediatr Gastroenterol Nutr. 5: 290–294. [PubMed] [Google Scholar]
- 50. Holick MF (2003) Evolution and function of vitamin D. Recent Results Cancer Res. 164: 3–28. [DOI] [PubMed] [Google Scholar]
- 51. Lebrun JB, Moffatt ME, Mundy RJ, Sangster RK, Postl BD, et al. (1993) Vitamin D deficiency in a Manitoba community. Canadian Journal of Public Health 84: 394–396. [PubMed] [Google Scholar]
- 52. Fu L, Yun F, Oczak M, Wong BY, Vieth R, et al. (2009) Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem 42: 1174–1177 DOI: 10.1016/j.clinbiochem.2009.03.008 [DOI] [PubMed] [Google Scholar]
- 53. Szathmary EJ, Ritenbaugh C, Goodby CS (1987) Dietary change and plasma glucose levels in an Amerindian population undergoing cultural transition. Soc Sci Med 24: 791–804. [DOI] [PubMed] [Google Scholar]
- 54. Szathmary EJ (1987) The effect of Gc genotype on fasting insulin level in Dogrib Indians. Hum Genet 75: 368–372. [DOI] [PubMed] [Google Scholar]
- 55. Hewison M (2011) Antibacterial effects of vitamin D. Nat Rev Endocrinol 7: 337–345. 10.1038/nrendo.2010.226 [DOI] [PubMed] [Google Scholar]
- 56. Eaton S, Nelson D (1991) Calcium in evolutionary perspective. American Journal of Clinical Nutrition 54: 2815–2875. [DOI] [PubMed] [Google Scholar]
- 57. Gozdzik A, Zhu J, Wong BY, Fu L, Cole DE, et al. (2011) Association of vitamin D binding protein (VDBP) polymorphisms and serum 25(OH)D concentrations in a sample of young Canadian adults of different ancestry. Journal of Steroid Biochemistry and Molecular Miology 127: 405–412 DOI: 10.1016/j.jsbmb.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 58. Smolders J, Peelen E, Thewissen M, Menheere P, Tervaert JW, et al. (2009) The relevance of vitamin D receptor gene polymorphisms for vitamin D research in multiple sclerosis. Autoimmun Rev 8: 621–626 DOI: 10.1016/j.autrev.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 59. Andraos C, Koorsen G, Knight JC, Bornman L (2010) Vitamin D receptor gene methylation is associated with ethnicity, tuberculosis, and TaqI polymorphism. Hum Immunol 72: 262–268 DOI: 10.1016/j.humimm.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Etten E, Verlinden L, Giulietti A, Ramos-Lopez E, Branisteanu DD, et al. (2007) The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. Eur J Immunol 37: 395–405 DOI: 10.1002/eji.200636043 [DOI] [PubMed] [Google Scholar]
- 61. Bellamy R (2003) Susceptibility to mycobacterial infections: the importance of host genetics. Genes Immun 4: 4–11 DOI: 10.1038/sj.gene.6363915 [DOI] [PubMed] [Google Scholar]
- 62. Sharma PR, Singh S, Jena M, Mishra G, Prakash R, et al. (2011) Coding and non-coding polymorphisms in VDR gene and susceptibility to pulmonary tuberculosis in tribes, castes and Muslims of Central India. Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases 11: 1456–1461 DOI: 10.1016/j.meegid.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 63. Windelinckx A, De Mars G, Beunen G, Aerssens J, Delecluse C, et al. (2007) Polymorphisms in the vitamin D receptor gene are associated with muscle strength in men and women. Osteoporosis International 18: 1235–1242 DOI: 10.1007/s00198-007-0374-4 [DOI] [PubMed] [Google Scholar]
- 64. Rook GA (2012) Hygiene hypothesis and autoimmune diseases. Clinical Reviews in Allergy and Immunology 42: 5–15 DOI: 10.1007/s12016-011-8285-8 [DOI] [PubMed] [Google Scholar]
- 65. Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA Jr, et al. (2009) Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clinical Infectious Diseases 48: 418–424 DOI: 10.1086/596314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Statistics Canada (2008) 2006 Census: Aboriginal Peoples in Canada in 2006: Inuit, Métis and First Nations, 2006 Census: Findings. Available: http://www12.statcan.ca/census-recensement/2006/as-sa/97-558/index-eng.cfm. Accessed 2010 July 12.
- 67. Wu H, Gozdzik A, Barta JL, Wagner D, Cole DE, et al. (2009) The development and evaluation of a food frequency questionnaire used in assessing vitamin D intake in a sample of healthy young Canadian adults of diverse ancestry. Nutr Res 29: 255–261 DOI: 10.1016/j.nutres.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 68. Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, et al. (2009) Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med 7: 28 DOI: 10.1186/1479-5876-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative (%) contribution of traditional and market foods to daily vitamin D intake (IU/day) by season. Fifty percent of the dietary intake of vitamin D came from milk (fluid and powdered). Local fish obtained from the lake provided 20% in the winter and 28% in the summer of the daily vitamin D. Margarine and eggs were also source of vitamin D. Caribou was not a major source of vitamin D however values for some animal parts that were consumed were unknown (i.e. fat, blood, liver).
(TIF)
Enrolment of study participants. One-hundred and five of 330 adults in the community were screened for the study [68]. Exclusion criteria included the use of vitamin D supplements >600 IU/day for three months prior to study on-set, clinical evidence of infection at the time of enrolment, first-degree kinship with an individual already enrolled, and immunosuppressive medical condition or use of immunosuppressive medication, including systemic steroids. Fifty-four of the 105 people screened met the study criteria and were enrolled. During the course of the one-year study 2 individuals moved away permanently from the community, 2 were absent from the community during one of the two test periods, 3 individuals withdrew for personal reasons, and 1 person developed a serious inter-current illness precluding further study participation.
(TIF)