Abstract
Background
Trichinella spiralis expresses paramyosin (Ts-PMY) not only as a structural protein but also as an immunomodulatory protein to protect the worm from being attacked by host complement components. In this study, the functions of PMY in the viability and the growth development of T. spiralis were confirmed at the first time by silencing the gene function with RNA interference technique.
Methods and Findings
To understand its functions in the viability of the worm, we used RNA interference to silence the expression of Ts-pmy mRNA and protein in the parasite. Significant silencing of Ts-pmy mRNA expression in larval and adult T. spiralis was achieved by siRNA and dsRNA through soaking and electroporation. Electroporation of T. spiralis larvae with 8 µM siRNA1743 or 100 ng/µl dsRNA-PF3 resulted in 66.3% and 60.4% decrease in Ts-pmy transcript and 52.0% and 64.7% decrease in Ts-PMY protein expression, respectively, compared with larvae treated with irrelevant control siRNA or dsRNA. Larvae treated with siRNA1743 displayed significant reduction in molting (40.8%) and serious surface damage as detected with SYTOX fluorescent staining. Infection of mice with larvae electroporated with Ts-pmy siRNA1743 resulted in 37.6% decrease in adult worm burden and 23.2% decrease in muscle larvae burden compared with mice infected with control siRNA-treated larvae. In addition, adult worms recovered from mice infected with siRNA-treated larvae released 24.8% less newborn larvae.
Conclusion
It is the first time RNAi was used on T. spiralis to demonstrate that silencing PMY expression in T. spiralis significantly reduces the parasite’s viability and infectivity, further confirming that Ts-PMY plays an important role in the survival of T. spiralis and therefore is a promising target for vaccine development.
Introduction
Trichinellosis, a widespread foodborne zoonosis, is acquired through the ingestion of undercooked meat containing encapsulated larvae of Trichinella spiralis (T. spiralis) [1]. The Trichinella parasite is an intestinal nematode that infects more than 100 mammalian species, including humans [2]–[3]. Human trichinellosis outbreaks occur in many parts of the world, and it has been regarded as a reemerging disease [4]–[6]. Trichinellosis is not only a public health hazard that affects approximately 11 million people worldwide [4] but also represents an economic problem in porcine animal production and food safety [2]. It is difficult to prevent and control Trichinella infection because of the wide distribution of domestic and wild animal reservoirs and the lack of specific clinical symptoms or signs for diagnosis [6]–[8]. These concerns have prompted interest in identifying molecules that play important roles in the establishment of infection in domestic animals and humans as targets for developing therapeutic and preventive vaccines [9]–[10].
In an effort to identify protective antigens for vaccine development against Trichinella infection, we have identified paramyosin of T. spiralis (Ts-PMY) as not only a structural protein of the worm [11]–[12] but also an immunomodulatory protein expressed on the surface of larvae and adult worms that plays an important role in the immune escape from host complement components [13]–[14]. In our previous reports, a full-length cDNA encoding Ts-PMY was cloned by immunoscreening a T. spiralis cDNA library with immune sera, and the recombinant Ts-PMY was found to elicit partial protective immunity in BALB/c mice against T. spiralis larval challenge [15]–[16]. Additionally, Zhang et al demonstrated that PMY was constitutively expressed in all of the developmental stages of T. spiralis and bound to human complement C8 and C9 to inhibit complement-mediated killing of the parasite [17]. The protective epitope of Ts-PMY has been identified at the N-terminus of the protein, and it elicited even better protection in a vaccine trial compared with the full-length protein [18]. Despite its promise as a vaccine against T. spiralis infection, the exact function of PMY in the establishment of parasitism in the host is still not well understood, except for its binding activities to human complement C8 and C9. We therefore employed RNA interference (RNAi)-based gene silencing to explore the biological functions of PMY in T. spiralis through a loss-of-function approach.
In this study we examined the feasibility of using RNAi in the larvae and adult worms of T. spiralis for the first time and demonstrated that the silencing of Ts-pmy in Trichinella induced by small interfering RNA (siRNA) or double-stranded RNA (dsRNA) resulted in significant reduction in Ts-PMY expression at both RNA transcript and protein levels and the impaired viability of the parasite in both in vitro culture and in vivo infection.
Materials and Methods
Ethics Statement
All experimental animals were purchased from Laboratory Animal Services Center of Capital Medical University (Beijing, China). All experimental procedures were reviewed and approved by the Capital Medical University Animal Care and Use Committee and were consistent with the NIH Guidelines for the Care and Use of Laboratory Animals.
Parasites
T. spiralis (ISS 533) parasites used in this study were maintained in ICR mice. Each mouse was orally infected with 400 T. spiralis larvae. Adult worms were collected from the intestines of mice [19], and muscle larvae were recovered from the muscle of infected mice using a standard pepsin–hydrochloric acid digestion method [20]. Crude somatic extracts of the different stages of T. spiralis were prepared by conventional methods [21], and the protein concentration was determined by the BCA assay (Pierce, US).
Preparation of siRNA
The full-length cDNA encoding Ts-PMY (GenBank accession# EF429310) was used to design for the siRNA sequences with BLOCK-iT RNAi Designer (http://www.invitrogen.com/rnaidesigner). The Ts-pmy-specific 25-mer siRNA oligos (Stealth ™ RNAi duplexes) were chemically synthesized by Invitrogen (Life Technologies, US). The sequences of the three specific siRNA oligos and corresponding Stealth control siRNA used in this study are shown in Table 1. The same control siRNA labeled with FITC (Life Technologies, US) was used to monitor the transfection efficiency.
Table 1. The siRNA oligos used in this study.
| siRNA oligo | sense | anti-sense | position |
| siRNA371 | 5'-CGCCAAUCGAAAGCGUGAAUCCGAA-3' | 5'-UUCGGAUUCACGCUUUCGAUUGGCG-3' | 371–395 |
| siRNA643 | 5'-AAGCGCAUGCCAGAGAGCUUCAGAA-3' | 5'-UUCUGAAGCUCUCUGGCAUGCGCUU-3' | 643–667 |
| siRNA1743 | 5'-CAGGCGGAAAUUGCCGAACUGGAAA-3' | 5'-UUUCCAGUUCGGCAAUUUCCGCCUG-3' | 1,743–1,767 |
| control siRNA | 5'-AUCGGCUACCAAGUCAUACACAGUC-3' | 5'-GACUGUGUAUGACUUGGUAGCCGAU-3' |
Synthesis of dsRNAs
Three DNA fragments of Ts-pmy, based on the sequence regions 75–974 (PF1), 924–1,874 (PF2) and 1,825–2,668 (PF3), were generated with PCR using gene-specific primers flanked by T7 RNA polymerase promoter sequences. In addition, a DNA fragment (13–1,056) of Ts87, a T. spiralis-specific surface antigen [22], was generated with PCR for use as a parallel control.
The PCR fragments were used to generate dsRNAs through in vitro transcription using the MEGAscript™RNAi kit (Life Technologies, US). To confirm integrity, single-stranded RNA and dsRNA were visualized on a 1% agarose gel. The concentration of each dsRNA was determined using a NanoDrop 2000 spectrophotometer (Thermo, UK). A 500-bp irrelevant dsRNA from the MEGAscript™RNAi Kit (Life Technologies, US) served as a negative control.
SiRNA or dsRNA Delivery to T. spiralis Worms
T. spiralis larvae and adult worms were recovered from the infected ICR mice and washed three times in 0.9% saline solution. For soaking incubation, 2,000 larvae or 500 adult worms were suspended in a final volume of 500 µl culture medium RPMI 1640 (Gibco, HyClone) supplemented with 20% heat-inactivated fetal bovine serum (FBS, Sigma), 100 units/ml penicillin and 100 µg/ml streptomycin. Specific or control siRNA or dsRNA were incubated with 2 µl Lipofectin Reagent (Life Technologies, US) and 0.8 unit RNaseOUT™ (Life Technologies, US) for 10 min before being added to the larvae or adult worms to a final concentration of 4µM for siRNA and 50 ng/µl for dsRNA. The incubation was continued at 37°C and 5% CO2 for up to 6 days.
For electroporation, 2,000 larvae or 500 adult worms were suspended in 100 µl of electroporation buffer (Life Technologies, US) containing the same amount of siRNA or dsRNA as used for soaking incubation. The worm suspension was electroporated at 125 V for 20 ms by using a Gene Pulser II System (Bio-Rad, US), then added with culture medium up to 500 µl and incubated at 37°C and 5% CO2 for up to 10 days. FITC-labeled control siRNA was used as for visualizing the uptake of siRNA.
Conditions for incubation or electroporation (125 V, 20 ms) have been confirmed to be safe for keeping the worms alive up to 10 days when control siRNA or dsRNA were added.
Quantitative Real-time PCR (qRT-PCR)
Total RNA was extracted from siRNA- or dsRNA-treated larvae or adult worms using an RNAsimple Total RNA Kit (Tiangen, China) according to the manufacturer’s instructions. The extracted RNA was treated with 200 U DNAseI (Tiangen, China) to remove any genomic DNA contamination. The quality of the RNA was visualized with 1.2% denatured agarose gel electrophoresis using the One-Stop RNA Electrophoresis kit (TIANZE, China). The concentration of RNA was measured with the NanoDrop2000.
For qRT-PCR, 50 ng of total RNA from treated worms was reverse transcribed to first-strand cDNA using the Sensiscript® reverse transcription kit (Qiagen, US) and oligo dT15 primer. The following primers were designed for qRT-PCR: Ts-pmy (forward: CAG TCG GAA GTT GAA GTT TTG; reverse: CTC TGA GTT CTC G TT GTT GCG TAG); Ts87 (forward: GCA ACA GTA CCC GCT TTC TAT GAT TCA AC; reverse: TCA AAG TCG CTA CGC CAT CAC CAG); and GAPDH, which served as the endogenous control (forward: TGC TTC TTG CAC TAC CAA TGG CTT AG; reverse: ACC AGA TGG ACC ATC GAC TGT CTT TT). The qRT-PCR was conducted in triplicate using the Brilliant® II SYBR® Green QPCR Master Mix kit (Stratagene, US) and the Chromo4™ real-time PCR Detector (Bio-Rad, US) to evaluate target gene expression. Reactions were performed with 40 cycles of 1 min at 95°C, 20 s at 60°C and 20 s at 72°C. The levels of Ts-pmy transcripts in siRNA- or dsRNA-treated worms were calculated as the percentage relative to the level of untreated worms by using double standard curves calibrated with plasmid DNA containing Ts-pmy and endogenous control DAPDH gene, according to manufacturer’s protocol. Standard curves had a correlation coefficiency R2>0.99. QPCR data were analyzed with Bio-Rad Opticon Monitor™ software.
Evaluation of Protein Expression
SiRNA- or dsRNA-treated worms were harvested 6 days post-soaking incubation or electroporation. The harvested worms were homogenized to prepare the crude somatic extracts. The total protein concentrations were determined with a BCA assay (Pierce, US). An equal amount of protein from each treated worm group was separated through SDS-PAGE and subsequently transferred onto nitrocellulose membranes. The membranes were blocked with 5% (w/v) nonfat dry milk in Tris-buffered saline with 0.05% Tween 20 (TBS-T) and incubated with mouse antisera against Ts-PMY (1∶10,000) or Ts87 (1∶100) [17]. In addition, rabbit anti-GAPDH (1∶1,000, Cell Signaling Technology, US) was used to detect GAPDH expression as a quantitative protein control. IRDye 800CW-conjugated goat anti-mouse or rabbit IgG (H+L) (LI-COR, US) was used as secondary antibodies, and the reactions were detected using the Odyssey detection system (LI-COR, US).
Viability of siRNA-treated T. spiralis Worms
To observe the effect of RNAi on the molting and viability of treated larvae, the larvae were electroporated with siRNA1743 or control siRNA. Since the larva immediately invades intestinal mucosa and begins to molt 8–14 hours post tissue invasion during the natural infection [23], we started to count the molting larvae 18 hours after being electroporated with siRNA1743. Only those with apparent cuticle sheath at one or two ends of larva were counted as molting larvae. To measure the viability of the siRNA-treated larvae, 2 µM SYTOX green dye was added to the treated larvae. Larvae with low viability or surface damage were stained with fluorescence [24]–[26]. After incubation for 15 min, the larvae were examined under a fluorescence dissecting microscope to determine the percentage of larvae that incorporated the fluorescence. The fluorescence density of siRNA-treated larvae was measured with a Fluoroskan Ascent fluorometer (Thermo Labsystems, US) at 485 nm excitation and 530 nm emission. The fluorescent ratios to the worms treated with control siRNA were calculated as the average of triplicate values.
To examine the infectivity of siRNA-treated larvae, 60 mice were equally divided into 3 groups. Each group was orally infected with 500 T. spiralis larvae electroporated with siRNA1743, control siRNA or medium only within 3 hours. Ten mice from each group were sacrificed 4 days post infection, and the adult worms were collected from the intestine of sacrificed mice and counted. The fecundity of recovered female worms was observed after being incubated individually in each well of 24-well tissue culture plate with culture medium for 3 days, and the number of newborn larvae produced by each female worm was counted. The muscle larvae were collected from the remaining 10 mice of each group 35 days post infection using a routine digestion method described previously [20]. The worm reduction was calculated based on the mean number of adult worms or muscle larvae collected from the group treated with siRNA1743 compared with the control siRNA-treated group.
Statistics
Data were expressed as the mean ± standard error (S.E.) and evaluated by post-hoc test in ANOVA. p<0.05 was regarded as statistically significant. For all experiments, the gene expression level was set to 100% in untreated group as standard. The statistical difference in treated group was obtained by comparing with the group treated with control siRNA or dsRNA.
Results
SiRNA-mediated Suppression of Ts-pmy mRNA Expression
Eighteen hours after being electroporated with FITC-labeled control siRNA, more than 75% of the treated worms (larvae and adults) displayed fluorescence staining in different tissues under fluorescent microscopy (Fig. 1A). After being soaked with FITC-labeled siRNA for 60 hours, more than 70% of the worms (larvae and adults) also showed significant uptake of fluorescence (data not shown), indicating that siRNA can be efficiently delivered into T. spiralis worms through electroporation or soaking.
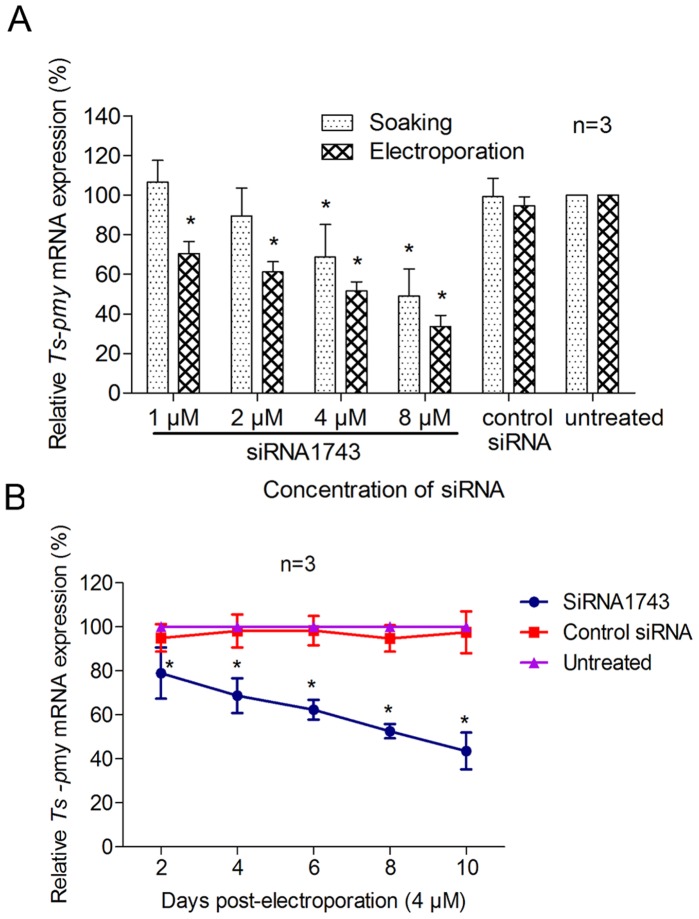
Figure 1. The silencing of Ts-pmy mRNA mediated by siRNA.
The adult and larval T. spiralis were transfected with FITC-labeled control siRNA by electroporation (A). Uptake of FITC-labeled siRNA into adult worms (a) and larvae (b) 18 hours after electroporation under a fluorescent microscope. No fluorescence was observed in the untreated group (c). The relative levels of Ts-pmy mRNA in parasites (larvae and adults) 6 days after being electroporated with different siRNAs, as determined with qRT-PCR (B). All of the assays were performed in triplicate, and the data are presented as the mean ± SE. *p<0.05 compared with control siRNA.
After being electroporated with 4 µM of siRNA643 or siRNA1743 for 6 days, the T. spiralis larvae or adult worms expressed significantly reduced level of Ts-pmy mRNA compared with worms treated with control siRNA or untreated worms (Fig. 1B). The relative levels of Ts-pmy mRNA expression in adult worms treated with siRNA643 or siRNA1743 detected by qRT-PCR were 65.3% and 54.6% of the level in control siRNA-treated worms, respectively (p<0.05). SiRNA371 did not elicit a significant reduction in the expression of Ts-pmy mRNA. There was no significant difference in silencing efficiency between larvae and adult worms. The control siRNA had no inhibitory effect on Ts-pmy mRNA expression.
Because siRNA1743 produced the highest silencing of Ts-pmy mRNA expression, it was used to optimize the working concentration and delivery approach in larva transfection. The efficacy of siRNA silencing was dose dependent, and the most effective silencing was observed at 8 µM which reduced the expression of Ts-pmy mRNA in treated larvae to 33.7% of the level in control siRNA-treated worms (66.3% reduction) when delivered with electroporation for 6 days (Fig. 2A). Even though the silencing efficiency induced by electroporation looked better than that induced by soaking, the difference was not statistically significant.
Figure 2. The silencing of Ts-pmy mRNA in larvae treated with siRNA1743.
The relative level of Ts-pmy mRNA in larvae following electroporation or soaking with various concentrations of siRNA1743 for 6 days, as determined with qRT-PCR (A). The better siRNA suppressive effect in treated larvae was observed when a longer incubation was applied after electroporation (B). All of the assays were performed in triplicate, and the data are presented as the mean ± SE. *p<0.05 compared with control siRNA.
A longer incubation of worms after electroporation with siRNA1743 led to better silencing efficiency of Ts-pmy mRNA expression (Fig. 2B). Ten days after 4 µM of siRNA1743 was delivered with electroporation, the expression of Ts-pmy mRNA was 43.5% of the levels in control siRNA-treated larvae.
DsRNA-mediated Suppression of Ts-pmy mRNA Expression
Similar to siRNA, the best Ts-pmy mRNA silencing was obtained in larvae transfected with dsRNAs derived from the C-terminus of Ts-pmy (dsRNA-PF3). The relative levels of Ts-pmy mRNA expression in parasites soaked with dsRNA-PF2 and dsRNA-PF3 for 6 days were only 64.7% and 51.2% of levels in the control dsRNA-treated group (Fig. 3A). There was no significant decrease in the level of Ts-pmy mRNA expression in the larvae treated with dsRNA-PF1. The control dsRNA had no inhibitory effect on Ts-pmy mRNA expression.
Figure 3. The silencing of Ts-pmy mRNA mediated by dsRNA.

The relative level of Ts-pmy mRNA expression in larvae soaked with different dsRNAs (50 ng/µl) for 6 days (A). The relative level of Ts-pmy mRNA in larvae following electroporation or soaking with various concentrations of dsRNA-PF3 for 6 days, as measured with qRT-PCR (B). Specific suppression of Ts-pmy and Ts87 mRNA expression in larvae 6 days after being soaked with 50 ng/µl dsRNA-PF3 or Ts87 dsRNA (C). All of the assays were performed in triplicate, and the data are presented as the mean ± SE. *p<0.05 compared with the control dsRNA-treated group.
The dsRNA-mediated inhibition of Ts-pmy mRNA expression occurred in a dose-dependent manner for dsRNA-PF3 (Fig. 3B). The best Ts-pmy silencing was achieved 6 days after T. spiralis larvae were electroporated with 100 ng/µl of dsRNA-PF3 (60.4% reduction). Electroporation had a higher efficiency than soaking in inhibiting the expression of the target gene, but the difference was not statistically significant, as observed for siRNA. The similar silencing efficiency for Ts-pmy mRNA transcription was observed in worms induced by dsRNA-PF3 and siRNA1743 (60.4% and 66.3% reduction, respectively).
The dsRNA-mediated silencing of Ts-pmy mRNA expression was gene specific. Ts-pmy dsRNA-PF3 only silenced Ts-pmy mRNA expression, whereas the Ts87 dsRNA knocked down the expression of Ts87 mRNA (Fig. 3C).
Reduction of Ts-PMY Protein Expression in Parasites Treated with Ts-pmy siRNA or dsRNA
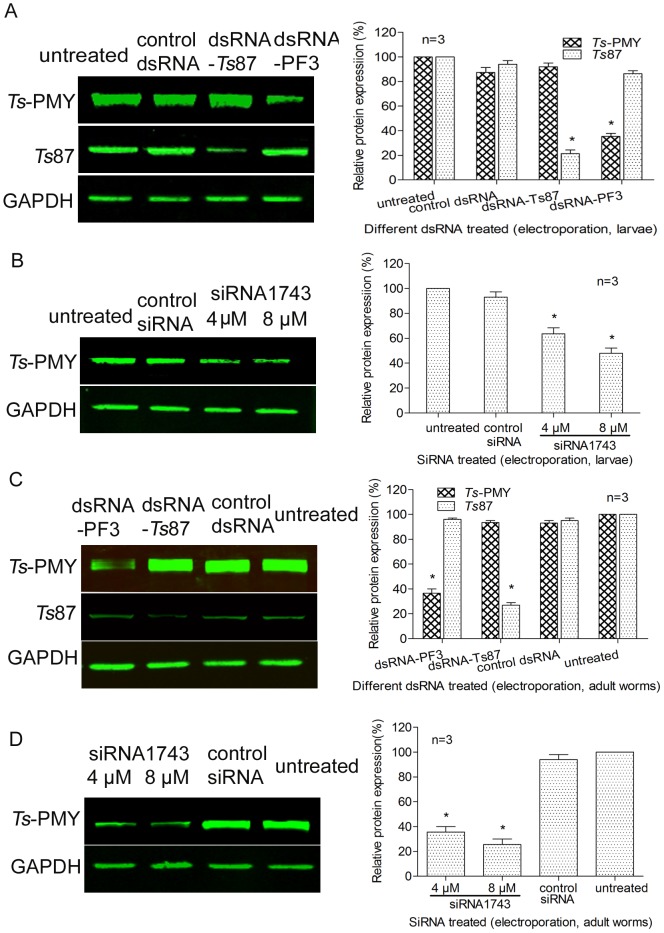
To determine whether the silencing of Ts-pmy mRNA mediated by the siRNA or dsRNA reduces the level of Ts-PMY protein expression, Western blotting with specific antibodies was performed on the extracts of treated adult worms and larvae. The expression of the Ts-PMY protein was significantly decreased in both adult worms and larvae treated with either siRNA1743 or dsRNA-PF3 compared with worms treated with control siRNA or dsRNA (Fig. 4). The expression of the Ts-PMY protein was inhibited 52.0% and 74.5% when larvae and adult worms were electroporated with 8 µM of siRNA1743, respectively, compared with control siRNA treated worms (Fig. 4B and 4D). The Ts-PMY protein expression level reduced 64.7% and 63.5% in larvae and adult worms, respectively, after being eletroporated with 100 ng/µl of dsRNA-PF3 compared to the control dsRNA group (Fig. 4A and 4C). In control worms treated with Ts87 dsRNA, only the expression of the Ts87 protein was suppressed (Fig. 4A and 4C). The housekeeping protein GAPDH was detected in all of the worm extracts at similar levels and was not affected by any Ts-pmy or Ts87 siRNA or dsRNA treatment.
Figure 4. The silencing of Ts-PMY protein expression mediated by siRNA or dsRNA.
Western blot with specific antibodies showing the specific inhibition of Ts-PMY protein expression in extracts of T. spiralis larvae (A, B) and adult worms (C, D) induced by dsRNA-PF3 (A, C) or siRNA1743 (B, D). Graphs on the right show the relative protein levels measured by densitometry from three independent experiments. *p<0.05 compared with the control dsRNA/siRNA-treated group.
Suppression of Ts-pmy mRNA Impaired the Molting and Viability of T. spiralis Larvae
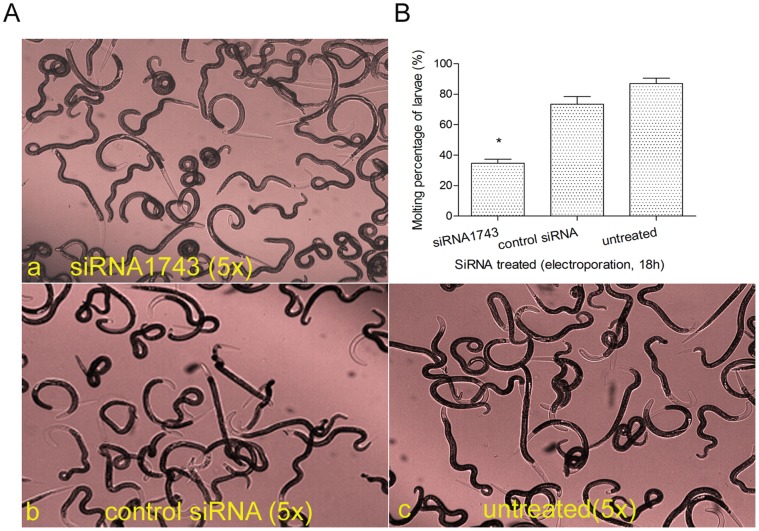
Knockdown of Ts-pmy mRNA by RNAi resulted in defect in T. spiralis larval molting. The molting rate was only 32.7% for larvae eletroporated with 8 µM siRNA1743 for 18 hours while 73.5% and 87.1% of larvae treated with control siRNA and untreated occurred molting (Fig. 5). The difference of molting rate between siRNA1743-treated group and control siRNA-treated group was statistically significant (40.8% reduction), indicating the reduction in larval molting rate was caused by specific inhibition of Ts-pmy mRNA expression. There was no significant reduction of molting rate in larvae electroporated with control siRNA and untreated larvae, suggesting the electroporation did not significantly cause the defect in larval molting. Our results suggested that PMY may play a role in regulating molting during larval development. Some of the normal larvae (untreated or treated with control siRNA) did not show the molting probably because the culture condition in vitro may not completely reflect the condition in vivo.
Figure 5. Suppression of Ts-pmy mRNA expression by siRNA1743 caused defects in larval molting.
A. The larvae were electroporated with 8 µM siRNA1743 (a) or control siRNA (b) and incubated for 18 hours. The untreated larvae (c) were used as a negative control. The larvae with cuticle sheath at the end(s) were counted as molting ones. B. The molting percentage for each treatment was calculated. All of the assays were performed in triplicate. *p<0.05 compared with the control siRNA-treated group.
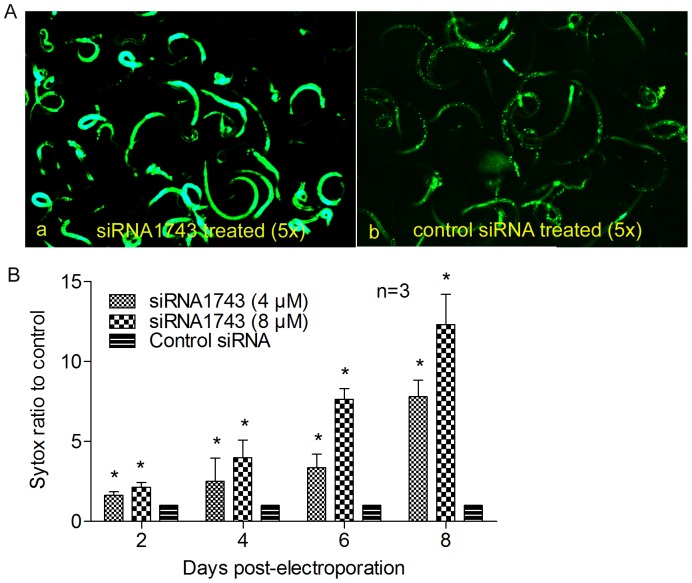
The suppression of Ts-pmy mRNA expression also resulted in larval surface damage and defect in larval viability. The larvae electroporated with siRNA1743 for 6 days showed more larvae stretched out from coiled form and less motile compared with those treated with control siRNA under microscopic observation (Data not shown). The siRNA1743 treated larvae were also incorporated with more fluorescence after being stained with SYTOX compared with those treated with control siRNA (Fig. 6A), indicating the surface damage and the defected viability in larvae treated with siRNA1743. Higher concentrations of siRNA1743 or longer the treatment time caused more serious surface damage and defected viability in treated larvae, indicating the damage is correlated to the suppressive level of Ts-pmy mRNA expression as showed in Fig. 2A and 2B. The larvae treated with 8 µM siRNA1743 for 8 days had incorporated more than 10 times higher fluorescence than those treated with control siRNA, as measured with a fluorescence microplate reader (Fig. 6B). The larvae treated with control siRNA showed much less fluorescent binding, suggesting the larval damage and the viability defect was siRNA1743-specific and not much related with electroporation.
Figure 6. Suppression of Ts-pmy mRNA expression by siRNA1743 caused surface damage and defected viability of treated larvae.
The larvae treated with siRNA1743 for 6 day were stained with SYTOX for 15 min and then observed under fluorescence microscopy (A) or measured with a fluorescence microplate reader at 485 nm excitation and 530 nm emission (B). All of the assays were performed in triplicate. *p<0.05 compared with the control siRNA-treated group.
Suppression of Ts-pmy mRNA in T. spiralis Larvae Affects Parasite Survival in vivo
Mice infected with T. spiralis larvae transfected with siRNA1743 by electroporation displayed a 37.6% reduction in adult worm burden and 23.2% reduction in muscle larval burden compared with the groups infected with control siRNA-treated larvae, and these differences were statistically significant (Table 2) (p<0.05).
Table 2. Recovery of adult worms, muscle larvae and newborn larvae of T. spiralis from mice infected with larvae transfected with siRNA1743 by electroporation.
| Group | Adult worms recovered (mean± S.E) | Reduction (%) | Muscle Larvae recovered (mean± S.E) | Reduction (%) | Newborn larvae/female (mean± S.E) | Reduction (%) |
| siRNA1743 | 123.6±27.3 | 37.6* | 34,885±3262 | 23.2* | 75.8±18.8 | 24.8* |
| control siRNA | 197.9±17.1 | 45,420±5173 | 100.6±24.5 | |||
| untreated | 201.9±32.0 | 46,675±5270 | 104.5±20.8 |
p<0.05 compared with the control siRNA-treated group.
Suppression of Ts-pmy mRNA Reduced Female Parasite Fecundity
The female adult worms collected from mice infected with siRNA1743-treated larvae demonstrated a significant reduction (24.8%) in producing newborn larvae compared with control siRNA-treated groups after being cultured in vitro for 72 hours (Table 2), but there was no significant difference in the motility of the newborn larvae compared with the control group (data not shown).
Discussion
Here we report the successful silencing of Ts-PMY in the larvae and adult worms of T. spiralis at either mRNA transcription or protein expression levels induced by specific siRNA and dsRNA. The knockdown of Ts-PMY expression in T. spiralis larvae resulted in impaired molting and viability in vitro and significant lower infectivity in mice, indicating the Ts-PMY plays an important role in the viability and survival of T. spiralis in the host. Specific gene silencing by RNA interference (RNAi) was first established in Caenorhabditis elegans and developed in this organism for high-throughput functional genomics analysis [27]–[29]. With the ease and reproducibility of RNAi in C. elegans and the conservation of RNAi mechanisms in a wide variety of other organisms [27], [30], this method has swept through all fields of eukaryotic biology, from yeasts and plants to animals, especially when organisms are not amenable to classical genetic approaches, with the aim to directly assess gene functions [31]–[34]. In parasitic nematodes, Nippostrongylus brasiliensis was the first animal parasitic nematode in which RNAi was successfully performed [35]. Subsequently, RNAi has been widely used in several parasitic nematodes, including Brugia malayi [36]–[37], Onchocerca volvulus [38], Ascaris suum [39], Trichostrongylus colubriformis [40], Litomosolides sigmodontis [41], Haemonchus contortus [42]–[45] and Heligmosomoides polygyrus [46], with a reduction in gene expression and visible suppressed phenotypes although they appear variable. There is no published report on using RNAi to study gene function in T. spiralis up to date although it has become a fascinating model organism for immunologists and biochemists [10]. Furthermore, the entire genomic sequencing of Trichinella has been completed, but the functions of many genes are unclear [47]. The development of RNAi technology in T. spiralis would allow us to exploit the data through manipulation of the Trichinella genome and provide a more direct approach for identifying essential genes and their products as targets for vaccine and drug development against trichinellosis. In this paper, for the first time, we report the significantly silenced transcription of Ts-pmy mRNA and expression of Ts-PMY protein in larval and adult T. spiralis through both siRNA and dsRNA. Both soaking and electroporation effectively delivered the siRNA or dsRNA into the worms. Using fluorescence-labeled siRNA as a control, we observed fluorescence distributed in different parts and organs of the treated worms (Fig. 1A), indicating that siRNA could penetrate the parasite through not only the mouth or excretory openings but also the surface cuticle or hypodermis of larvae and adult worms. It also could be that siRNA is entering through a single point source e.g. gut, and then spreading to these locations through mechanisms involving SID/RSD homologues [48]–[50].
Different delivery methods of RNAi can achieve various silencing results and phenotypes [46], [49]. In this study, siRNA or dsRNA delivered by electroporation had better silencing efficiency for Ts-pmy expression than those delivered by soaking in both larvae and adult worms, possibly because the electroporation shock may temporarily open channels on the surface of worms (cuticle or hypodermis) or mouth/excretory opening or mediate direct transmembrane transit of more siRNA or dsRNA to enter the worm tissues, therefore is not only dependent on SID-like uptake transporters [40], [51]–[52]. However, the combination of electroporation and soaking did not increase Ts-pmy silencing level induced by siRNA (data not shown), as shown by Pierson et al in Moniezia expansa [53], possibly because of the quick degradation of siRNA in the medium. Many results have confirmed that more effective gene suppression can be achieved when dsRNA is used in nematodes [27], [51], [54]. In our study, both dsRNA and siRNA had similar silencing effects on the expression of Ts-pmy mRNA or protein (Figs. 1, 3, 4).
The impaired viability of T. spiralis larvae with inhibited Ts-pmy mRNA and protein expression induced by RNAi was observed through in vitro culture and in vivo infection in mice. Silencing Ts-pmy mRNA and Ts-PMY protein expression caused deficit in T. spiralis larval molting, the necessary process for larval development, indicating the PMY may be involved in the development of T. spiralis larva. It was also observed that T. spiralis larvae stretched out from their coiled form and were less motile after being treated with Ts-pmy siRNA compared with the group treated with irrelevant control siRNA. Fluorescent SYTOX staining showed that Ts-pmy siRNA-treated larvae were stained with more fluorescence, indicating more surface damage and lower viability. This is the first time for SYTOX green being used to determine the surface damage and viability of T. spiralis larvae although it has been used for other nematodes, such as H. glycines and C. elegans [25]–[26], [55]. These impaired viability observations are partially consistent with the report of RNAi induced PMY suppression in C. elegans, in which some phenotypes such as uncoordinated, paralyzed worms and egg laying defects were observed in the RNAi treated free-living nematode [24]. The in vivo infection of mice with larvae transfected with Ts-pmy siRNA demonstrated a 37.6% reduction in adult burden and 23.2% reduction in muscle larvae burden compared with mice infected with control siRNA-treated larvae. In addition, adult worms recovered from mice infected with siRNA-treated larvae released 24.8% less newborn larvae.
These impaired viabilities are totally related to the specific silencing of Ts-pmy mRNA or protein expression because the control siRNA did not exhibit significant reduction in larval viability compared to untreated larvae. The condition for electroporation (125 V, 20 ms) has been confirmed not to impair the viability of treated larvae or adult worms up to 10 days. All of the results demonstrated that silencing PMY expression in T. spiralis significantly reduced parasite viability and infectivity, further indicating that Ts-PMY plays important roles in the life cycle of T. spiralis and survival in host. The expression of PMY in T. spiralis is necessary, at least for the development of larvae and fecundity of female worms, in addition to its function as an immunomodulatory protein against host complement attack [17]–[18], therefore confirming its promising target for vaccine development.
Acknowledgments
We thank Xiaodi Yang, Xi Zhao, Yunyun Wang, Jingjing Huang, Fengyun Wang and Jin Pan for their technical assistance.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (81171598), the National Science and Technology Major Project (2012ZX10004220-012), and the PhD Programs Foundation of the Municipal Education Commission of Beijing (20111002503). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Dupouy-Camet J (2000) Trichinellosis: a worldwide zoonosis. Vet Parasitol 93: 191–200. [DOI] [PubMed] [Google Scholar]
- 2. Pozio E (2007) World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol 149: 3–21. [DOI] [PubMed] [Google Scholar]
- 3. Ribicich M, Gamble HR, Rosa A, Sommerfelt I, Marquez A, et al. (2007) Clinical, haematological, biochemical and economic impacts of Trichinella spiralis infection in pigs. Vet Parasitol 147: 265–270. [DOI] [PubMed] [Google Scholar]
- 4. Pozio E, Gomez MM, Dupouy-Camet J (2003) Clinical aspects, diagnosis and treatment of trichinellosis. Expert Rev Anti Infect Ther 1: 471–482. [DOI] [PubMed] [Google Scholar]
- 5. Cui J, Wang ZQ, Xu BL (2011) The epidemiology of human trichinellosis in China during 2004–2009. Acta Trop 118: 1–5. [DOI] [PubMed] [Google Scholar]
- 6. Gottstein B, Pozio E, Nockler K (2009) Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev 22: 127–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hill DE, Forbes L, Zarlenga DS, Urban JJ, Gajadhar AA, et al. (2009) Survival of North American genotypes of Trichinella in frozen pork. J Food Prot 72: 2565–2570. [DOI] [PubMed] [Google Scholar]
- 8. Gajadhar AA, Forbes LB (2010) A 10-year wildlife survey of 15 species of Canadian carnivores identifies new hosts or geographic locations for Trichinella genotypes T2, T4, T5, and T6. Vet Parasitol 168: 78–83. [DOI] [PubMed] [Google Scholar]
- 9. Yang Y, Zhang Z, Yang J, Chen X, Cui S, et al. (2010) Oral vaccination with Ts87 DNA vaccine delivered by attenuated Salmonella typhimurium elicits a protective immune response against Trichinella spiralis larval challenge. Vaccine 28: 2735–42. [DOI] [PubMed] [Google Scholar]
- 10. Dupouy-Camet J (2009) Presidential address of ICT12 Conference: “Trichinella and trichinellosis–a never ending story”. Vet Parasitol 159: 194–196. [DOI] [PubMed] [Google Scholar]
- 11. Epstein HF, Miller DR, Ortiz I, Berliner GC (1985) Myosin and paramyosin are organized about a newly identified core structure. J Cell Biol 100: 904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gobert GN, McManus DP (2005) Update on paramyosin in parasitic worms. Parasitol Int 54: 101–107. [DOI] [PubMed] [Google Scholar]
- 13. Zhao QP, Moon SU, Na BK, Kim SH, Cho SH, et al. (2007) Paragonimus westermani: biochemical and immunological characterizations of paramyosin. Exp Parasitol 115: 9–18. [DOI] [PubMed] [Google Scholar]
- 14. Deng J, Gold D, LoVerde PT, Fishelson Z (2007) Mapping of the complement C9 binding domain in paramyosin of the blood fluke Schistosoma mansoni . Int J Parasitol 37: 67–75. [DOI] [PubMed] [Google Scholar]
- 15. Yang J, Gu Y, Yang Y, Wei J, Wang S, et al. (2010) Trichinella spiralis: Immune response and protective immunity elicited by recombinant paramyosin formulated with different adjuvants. Exp Parasitol 124: 403–408. [DOI] [PubMed] [Google Scholar]
- 16. Yang J, Yang Y, Gu Y, Li Q, Wei J, et al. (2008) Identification and characterization of a full-length cDNA encoding paramyosin of Trichinella spiralis . Biochem Biophys Res Commun 365: 528–533. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Z, Yang J, Wei J, Yang Y, Chen X, et al. (2011) Trichinella spiralis Paramyosin Binds to C8 and C9 and Protects the Tissue-Dwelling Nematode from Being Attacked by Host Complement. PLoS Negl Trop Dis 5: e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei J, Gu Y, Yang J, Yang Y, Wang S, et al. (2011) Identification and characterization of protective epitope of Trichinella spiralis paramyosin. Vaccine 29: 3162–3168. [DOI] [PubMed] [Google Scholar]
- 19. Martinez-Gomez F, Santiago-Rosales R, Ramon BC (2009) Effect of Lactobacillus casei Shirota strain intraperitoneal administration in CD1 mice on the establishment of Trichinella spiralis adult worms and on IgA anti-T. spiralis production. Vet Parasitol 162: 171–175. [DOI] [PubMed] [Google Scholar]
- 20. Wang S, Zhu X, Yang Y, Yang J, Gu Y, et al. (2009) Molecular cloning and characterization of heat shock protein 70 from Trichinella spiralis . Acta Trop 110: 46–51. [DOI] [PubMed] [Google Scholar]
- 21. Rees-Roberts D, Mullen LM, Gounaris K, Selkirk ME (2010) Inactivation of the complement anaphylatoxin C5a by secreted products of parasitic nematodes. Int J Parasitol 40: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jing Y, XinPing Z, YaPing Y, LiPing L (2003) Immunological characteristics and protective immunity of the Trichinella spiralis Ts87 antigen. Chin J Zool 38: 52–55. [Google Scholar]
- 23. Kozek WJ (1971) The molting pattern in Trichinella spiralis. I. A light microscope study. J Parasitol 57: 1015–1028. [PubMed] [Google Scholar]
- 24. Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, et al. (2000) Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408: 325–330. [DOI] [PubMed] [Google Scholar]
- 25. Alkharouf NW, Klink VP, Matthews BF (2007) Identification of Heterodera glycines (soybean cyst nematode [SCN]) cDNA sequences with high identity to those of Caenorhabditis elegans having lethal mutant or RNAi phenotypes. Exp Parasitol 115: 247–258. [DOI] [PubMed] [Google Scholar]
- 26. Roeder T, Stanisak M, Gelhaus C, Bruchhaus I, Grotzinger J, et al. (2010) Caenopores are antimicrobial peptides in the nematode Caenorhabditis elegans instrumental in nutrition and immunity. Dev Comp Immunol 34: 203–209. [DOI] [PubMed] [Google Scholar]
- 27. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, et al. (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans . Nature 391: 806–811. [DOI] [PubMed] [Google Scholar]
- 28. Maeda I, Kohara Y, Yamamoto M, Sugimoto A (2001) Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr Biol 11: 171–176. [DOI] [PubMed] [Google Scholar]
- 29. Struwe WB, Warren CE (2010) High-throughput RNAi screening for N-glycosylation dependent loci in Caenorhabditis elegans . Methods Enzymol 480: 477–493. [DOI] [PubMed] [Google Scholar]
- 30. Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, et al. (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. [DOI] [PubMed] [Google Scholar]
- 31. Chuang CF, Meyerowitz EM (2000) Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana . Proc Natl Acad Sci U S A 97: 4985–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fagard M, Boutet S, Morel JB, Bellini C, Vaucheret H (2000) AGO1, QDE-2, and RDE-1 are related proteins required for post-transcriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proc Natl Acad Sci U S A 97: 11650–11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wianny F, Zernicka-Goetz M (2000) Specific interference with gene function by double-stranded RNA in early mouse development. Nat Cell Biol 2: 70–75. [DOI] [PubMed] [Google Scholar]
- 34. Dalzell JJ, McMaster S, Fleming CC, Maule AG (2010) Short interfering RNA-mediated gene silencing in Globodera pallida and Meloidogyne incognita infective stage juveniles. Int J Parasitol 40: 91–100. [DOI] [PubMed] [Google Scholar]
- 35. Hussein AS, Kichenin K, Selkirk ME (2002) Suppression of secreted acetylcholinesterase expression in Nippostrongylus brasiliensis by RNA interference. Mol Biochem Parasitol 122: 91–94. [DOI] [PubMed] [Google Scholar]
- 36. Aboobaker AA, Blaxter ML (2003) Use of RNA interference to investigate gene function in the human filarial nematode parasite Brugia malayi. . Mol Biochem Parasitol 129: 41–51. [DOI] [PubMed] [Google Scholar]
- 37. Kumar S, Chaudhary K, Foster JM, Novelli JF, Zhang Y, et al. (2007) Mining predicted essential genes of Brugia malayi for nematode drug targets. PLoS One 2: e1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lustigman S, Zhang J, Liu J, Oksov Y, Hashmi S (2004) RNA interference targeting cathepsin L and Z-like cysteine proteases of Onchocerca volvulus confirmed their essential function during L3 molting. Mol Biochem Parasitol 138: 165–170. [DOI] [PubMed] [Google Scholar]
- 39. Islam MK, Miyoshi T, Yamada M, Tsuji N (2005) Pyrophosphatase of the roundworm Ascaris suum plays an essential role in the worm’s molting and development. Infect Immun 73: 1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Issa Z, Grant WN, Stasiuk S, Shoemaker CB (2005) Development of methods for RNA interference in the sheep gastrointestinal parasite, Trichostrongylus colubriformis . Int J Parasitol 35: 935–940. [DOI] [PubMed] [Google Scholar]
- 41. Pfarr K, Heider U, Hoerauf A (2006) RNAi mediated silencing of actin expression in adult Litomosoides sigmodontis is specific, persistent and results in a phenotype. Int J Parasitol 36: 661–669. [DOI] [PubMed] [Google Scholar]
- 42. Geldhof P, Murray L, Couthier A, Gilleard JS, McLauchlan G, et al. (2006) Testing the efficacy of RNA interference in Haemonchus contortus . Int J Parasitol 36: 801–810. [DOI] [PubMed] [Google Scholar]
- 43. Kotze AC, Bagnall NH (2006) RNA interference in Haemonchus contortus: suppression of beta-tubulin gene expression in L3, L4 and adult worms in vitro. Mol Biochem Parasitol 145: 101–110. [DOI] [PubMed] [Google Scholar]
- 44. Samarasinghe B, Knox DP, Britton C (2011) Factors affecting susceptibility to RNA interference in Haemonchus contortus and in vivo silencing of an H11 aminopeptidase gene. Int J Parasitol 41: 51–59. [DOI] [PubMed] [Google Scholar]
- 45.Zawadzki JL, Kotze AC, Fritz JA, Johnson NM, Hemsworth JE, et al. (2012) Silencing of essential genes by RNA interference in Haemonchus contortus. Parasitology: 1–17. [DOI] [PubMed]
- 46. Lendner M, Doligalska M, Lucius R, Hartmann S (2008) Attempts to establish RNA interference in the parasitic nematode Heligmosomoides polygyrus . Mol Biochem Parasitol 161: 21–31. [DOI] [PubMed] [Google Scholar]
- 47. Mitreva M, Jasmer DP, Zarlenga DS, Wang Z, Abubucker S, et al. (2011) The draft genome of the parasitic nematode Trichinella spiralis . Nat Genet 43: 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dalzell JJ, McVeigh P, Warnock ND, Mitreva M, Bird DM, et al. (2011) RNAi Effector Diversity in Nematodes. PLoS Negl Trop Dis 5: e1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Britton C, Samarasinghe B, Knox DP (2011) Ups and downs of RNA interference in parasitic nematodes. Exp Parasitol. doi:10.1016/j.exppara.2011.08.002. [DOI] [PubMed]
- 50. Habig JW, Aruscavage PJ, Bass BL (2008) In C. elegans, high levels of dsRNA allow RNAi in the absence of RDE-4. PLoS One 3: e4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Krautz-Peterson G, Radwanska M, Ndegwa D, Shoemaker CB, Skelly PJ (2007) Optimizing gene suppression in schistosomes using RNA interference. Mol Biochem Parasitol 153: 194–202. [DOI] [PubMed] [Google Scholar]
- 52. Shih JD, Hunter CP (2011) SID-1 is a dsRNA-selective dsRNA-gated channel. RNA 17: 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pierson L, Mousley A, Devine L, Marks NJ, Day TA, et al. (2010) RNA interference in a cestode reveals specific silencing of selected highly expressed gene transcripts. Int J Parasitol 40: 605–615. [DOI] [PubMed] [Google Scholar]
- 54. Grishok A (2005) RNAi mechanisms in Caenorhabditis elegans . FEBS Lett 579: 5932–5939. [DOI] [PubMed] [Google Scholar]
- 55. Gill MS, Olsen A, Sampayo JN, Lithgow GJ (2003) An automated high-throughput assay for survival of the nematode Caenorhabditis elegans . Free Radic Biol Med 35: 558–565. [DOI] [PubMed] [Google Scholar]