Abstract
The induction of the sucrose synthase (SuSy) gene (SuSy) by low O2, low temperature, and limiting carbohydrate supply suggested a role in carbohydrate metabolism under stress conditions. The isolation of a maize (Zea mays L.) line mutant for the two known SuSy genes but functionally normal showed that SuSy activity might not be required for aerobic growth and allowed the possibility of investigating its importance during anaerobic stress. As assessed by root elongation after return to air, hypoxic pretreatment improved anoxic tolerance, in correlation with the number of SuSy genes and the level of SuSy expression. Furthermore, root death in double-mutant seedlings during anoxic incubation could be attributed to the impaired utilization of sucrose (Suc). Collectively, these data provide unequivocal evidence that Suc is the principal C source and that SuSy is the main enzyme active in Suc breakdown in roots of maize seedlings deprived of O2. In this situation, SuSy plays a critical role in anoxic tolerance.
Well-aerated maize (Zea mays L.) primary roots do not survive more than 8 to 10 h of anoxia, but survival time can be increased to more than 72 h by HPT (Saglio et al., 1988; Johnson et al., 1989). Glc also has a positive effect. Studies of the effects of HPT on maize root tips led to the conclusion that several factors are involved in improved tolerance to anoxia: better energy status due to higher rates of glycolysis and ethanolic fermentation (Hole et al., 1992), adequate sugar supply to feed glycolysis, limitation of lactate accumulation (Xia and Saglio, 1992), and better regulation of cytoplasmic pH (Roberts et al., 1984, 1985). A developmental factor also influences root tolerance to anoxia. The germinating seedling retains the high tolerance of the embryo, but this tolerance is lost between 2 and 3 d postgermination (VanToai et al., 1995).
Among the enzymes induced during HPT, only HK has been shown to be limiting in activity during anoxic incubation of NHPT maize root tips (Bouny and Saglio, 1996). SuSy (UDP-Glc:d-Fru-glucosyl-transferase, EC 2.4.1.13), which catalyzes the reversible conversion of Suc and UDP to UDP-Glc and Fru in vitro, is another enzyme that is synthesized during O2 deprivation (Springer et al., 1986). However, posttranscriptional control results in activity and protein increases much below that of mRNA (Rowland et al., 1989; Taliercio and Chourey, 1989), leading to speculations on the physiological significance of induction. A SuSy gene is also induced in response to low O2 in wheat (Triticum aestivum L., Marana et al., 1990; Crespi et al., 1991) and in rice (Oryza sativa L., Ricard et al., 1991) and by other environmental stresses such as cold shock in wheat (Marana et al., 1990) and the limitation of carbohydrate supply in maize (Koch et al., 1992). As one of the two enzymes catalyzing Suc degradation in vivo, SuSy could play a crucial role in providing an adequate sugar supply during anoxic stress. During the anoxic germination of rice, for instance, Guglielminetti et al. (1995) reported that INV activities were depressed and that SuSy activities were enhanced. They suggested that SuSy was the enzyme mainly responsible for Suc breakdown under anoxia.
The isolation of a maize line mutant for both SuSy genes (Chourey et al., 1988) has permitted us to investigate the physiological significance of SuSy activity and induction in response to anaerobic stress. The double mutant exhibited the typical shrunken phenotype of mature kernels but was otherwise functionally normal (Chourey, 1988). Although these observations indicated that SuSy activity might not be required during aerobic growth, SuSy could still afford an advantage during stress situations affecting Suc metabolism. In this paper we present evidence that SuSy plays an essential role in anaerobic metabolism in maize seedlings.
MATERIALS AND METHODS
Plant Material and Stress Conditions
Three SuSy genotypes (Sh1Sus1, sh1Sus1, and sh1sus1) are represented in a lineage-related background of inbred maize (Zea mays L.) line W22. The first two genotypes share the line W22 inbred background with the sh1 mutation due to an insertion of a Dissociation (Ds)-transposable element, leading to deletion of the entire coding region of the Sh1 gene (sh1 bz-m4, Chourey, 1981). Isolation of the double mutant sh1sus1 from an initial identification of a mutant sus1 stock (ss2) was described previously (Chourey, 1988; Chourey et al., 1988; Chourey and Taliercio, 1994). The double mutant was extensively intercrossed with sh1Sus1(W22) to minimize background differences and to obtain lineage-related and near-isogenic genotypes.
Seeds were surface sterilized with undiluted commercial bleach, rinsed with distilled water, and allowed to germinate in the dark at 25°C for 3 to 4 d. Seedlings with radicals at least 40 mm long were placed overnight on floats with roots submerged in medium and the kernel was maintained in air. The medium consisted of a complete mineral solution without added sugars or antibiotics (Saglio and Pradet, 1980). For HPT, the medium was continuously sparged with 3% O2 in N2. For NHPT, the medium was continuously sparged with 50% O2 in N2. Since the critical O2 pressure for the respiration of submerged maize roots at 25°C is above the O2 concentration in air-saturated water (Saglio et al., 1984), such treatment was necessary to avoid any possibility that respiration could be limited by O2 levels. Anoxia was initiated by transferring the seedlings to floats over medium previously purged with N2 in airtight containers. A continuous flow of N2 was maintained throughout the anoxic treatment.
Root-tip viability was assessed by measuring the ability to elongate after return to air (Xia et al., 1995). Anoxically stressed or nonanoxically stressed HPT and NHPT seedlings were placed on moist filter paper at 25°C for 48 h. Elongation of the root tip was essentially an all-or-none phenomenon: viable root tips grew 2 to 6 cm after return to air for 48 h, whereas nonviable root tips showed no detectable elongation. Although loss of turgidity could often be correlated with loss of root-tip viability, this was more difficult to assess. Nonanoxically stressed seedlings, whether HPT or NHPT, were 100% viable, as shown by retention of elongation ability.
Enzyme Activities
Groups of 30 4-mm root tips were excised and homogenized in twice their fresh weight of 50 mm Hepes, pH 7.0, 10 mm MgCl2, 1 mm EDTA, 2.6 mm DTT, 0.02% Triton X-100, and 1% (w/v) BSA. The root brei was clarified by centrifugation for 5 min in an Eppendorf centrifuge. The resultant supernatant was then desalted on spin columns as described by Bouny and Saglio (1996). Control experiments verified that the addition of BSA to the extraction buffer allowed nearly complete and reproducible recovery of protein from the spin columns. Soluble proteins were quantitated on samples homogenized in 50 mm Hepes, pH 7.0, 10 mm MgCl2, and 1 mm EDTA, according to the method of Bradford (1976). Activities of neutral INV and SuSy were successively measured by spectrophotometry at pH 7.0, and acid INV was assayed at pH 4.8, essentially according to the method of Pelleschi et al. (1997). HK and FK activities were assayed by spectrophotometry at 25°C and pH 7.5, as described by Bouny and Saglio (1996).
In Vivo Labeling of Proteins and Two-Dimensional Gel Electrophoresis
Four to five seedlings were placed in a sterile polypropylene tube at 25°C with only the tips submerged in 1-mL of nutrient medium supplemented with 100 μg mL−1 ampicillin. The tubes were bubbled for 6 h with 3 or 50% O2 in N2. After 2 h, 500 μCi of Tran35S label (1150 Ci mmol−1, [35S]Met/Cys [70/30], ICN) was added. At the end of the 4-h labeling period, the seedlings were removed and rinsed with water. The apical 4 mm was excised and homogenized with a glass potter in 20 μL tip−1 of 10 mm Tris-HCl, pH 7.5, and 5 mm DTT. Bacteriological controls carried out on an aliquot of the root brei showed less than 104 colony-forming units tip−1.
After centrifugation of the brei for 15 min in an Eppendorf centrifuge, the supernatant was adjusted to contain 9 m urea, 2% (v/v) ampholines (comprising 1.6% pH range 5.0–7.0 and 0.4% pH range 3.0–10.0), 2% (v/v) β-mercaptoethanol, and 8% (w/v) Nonidet P-40 (Fluka, Buchs, Switzerland). Two-dimensional gel analysis was performed essentially as described by O'Farrell (1975), with IEF in the first dimension and a 15% SDS polyacrylamide gel in the second dimension.
Native Gel Analyses and Immunoblots
Root tips were excised and ground with a glass potter in 10 mm Tris-HCl, pH 7.5, and 5 mm DTT. The brei was centrifuged for 15 min in an Eppendorf centrifuge. Recovery experiments showed previously that very little enzyme activity is lost during homogenization and centrifugation using this technique. Aliquots of the crude extracts containing 20 to 25 μg of protein determined by the method of Bradford (1976) were analyzed immediately on native 8% polyacrylamide (Bouny and Saglio, 1996) or 12.5% SDS polyacrylamide gels. Native polyacrylamide gels were stained for activity as follows: HK or FK: 100 mm Tris-HCl, pH 8.0, 2 mm MgCl2, 0.75 mm NAD+, 1 mm ATP, 1 unit mL−1 Glu 6-P dehydrogenase, 0.1 mg mL−1 NBT, and 0.02 mg mL−1 PMS, with the addition of 1 mm Glc or 1 mm Fru and 4 units mL−1 PGI for HK or FK, respectively; ADH: 100 mm Tris-HCl, pH 8.5, 100 mm ethanol, 4 mm MgCl2, 20 mm NAD+, 0.1 mg mL−1 NBT, and 0.02 mg mL−1 PMS; and LDH: 150 mm Tris-HCl, pH 8.0, 20 mm lithium lactate, 4 mm MgCl2, 3.3 mm NAD+, 2 mm 4-methyl pyrazole, 0.1 mg mL−1 NBT, and 0.02 mg mL−1 PMS.
Proteins separated on SDS gels were transferred to nitrocellulose membranes and the blots were probed with a polyclonal antiserum that recognized both SuSy subunits (Chourey et al., 1986). Immunodetection was carried out by coupling the SuSy antiserum with peroxidase-labeled anti-rabbit IgG and visualization using 4-chloronaphthol.
Ethanol Accumulation and Soluble Sugar Determination
Groups of 20 root tips excised 4 mm from the apex were placed under anoxia in sealed syringes containing 10 mL of nutrient medium supplemented with 100 mm Glc. Samples were removed at the indicated times of imposed anoxia and the concentration of ethanol was determined enzymatically as previously described (Saglio et al., 1980).
Groups of five root tips incubated for the indicated times under anoxia were extracted successively for 15 min with 1 mL of hot ethanol:water (80:20, 50:50, and 0:100 [v/v] at 80°C). The extracts were dried under a vacuum and solubilized in 500 μL of water. Four-hundred microliters of extract was then purified on tandem ion-exchange resins consisting of 0.4 mEq of AG1-X8 (Bio-Rad) in the carbonate form and 0.5 mEq of Dowex 50W (Sigma) in the H+ form. The purified soluble sugars were analyzed by HPLC (Aminex HPX-87C column, Bio-Rad) with water as the eluant at 3.6 mL h−1 at 75°C and were then detected by refractometry.
RESULTS
Effect of HPT on Anoxic Tolerance of W22 Maize Lines of Different SuSy Genotypes
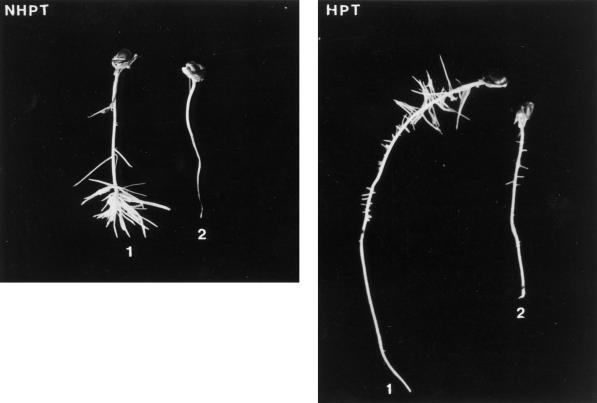
HPT has been shown to extend the survival of maize primary roots exposed to anoxia from 8 h to more than 72 h (Saglio et al., 1988; Johnson et al., 1989), whether survival is assessed by ATP content or viability. Table I contains data that confirm that HPT improved the anoxic tolerance of the inbred W22 maize line of the Sh1Sus1 genotype (henceforth referred to as homozygous wild type), as measured by ability of the root tip to elongate after return to air. HPT had an intermediate effect on the single mutant sh1Sus1 (62 compared with 77%) and the least effect on the double-mutant sh1sus1 (11%). In terms of the effects on individual seedlings, the loss of tolerance was most readily visualized in greatly reduced numbers of secondary roots as well as the length of primary roots of the sh1sus1 double mutant compared with the homozygous wild type (Fig. 1).
Table I.
Effect of HPT on root tip viability of anoxically stressed maize seedlings
| Genotype and Treatment | Elongated Seedlings | Elongation |
|---|---|---|
| % | ||
| Sh1Sus1 | ||
| NHPT | 0:4, 1:4, 0:4, 0:9, 0:7, 0:7 | 3 |
| HPT | 5:5, 3:3, 4:5, 6:9, 2:4 | 77 |
| sh1Sus1 | ||
| NHPT | 0:4, 0:3, 0:6 | 0 |
| HPT | 0:3, 5:5, 3:5 | 62 |
| sh1sus1 | ||
| NHPT | 0:5, 0:5, 0:5, 0:6, 0:5, 0:7 | 0 |
| HPT | 0:7, 1:6, 1:4, 0:5, 1:5, 0:5 | 11 |
Three- to 4-d-old seedlings were placed overnight on floats over medium bubbled with 3% O2 in N2 for HPT or with 50% O2 in N2 for NHPT. HPT or NHPT seedlings were then subjected to 24 h of anoxic stress. After return to air for 48 h, root-tip viability was quantitated from the percentage of seedlings that had elongated. Each ratio is from an independent experiment and represents the number of seedlings that had elongated with respect to the total number of seedlings.
Figure 1.
Effect of HPT on roots from inbred W22 line of the Sh1Sus1 homozygous wild-type (1) and the sh1sus1 double-mutant (2) genotype. Intact seedlings were placed overnight on floats over nutrient medium bubbled with 3% O2 in N2 (HPT) or with 50% O2 in N2 (NHPT). After a subsequent 24-h anoxic treatment, the seedlings were removed and placed between two layers of moist filter paper for 48 h.
In Vitro Catalytic Activities in NHPT and HPT Homozygous Wild-Type and Mutant Roots
The results shown in Table I show a correlation between the number of SuSy genes and the efficiency of HPT on root-tip viability. To determine whether this reflected differences in SuSy enzyme levels, immunoblot analyses were performed with 20 μg of soluble protein extracts from roots of the homozygous wild type, the sh1Sus1 single mutant, and the sh1sus1 double mutant. The results shown in Figure 2 indicate that SuSy protein was highest in the homozygous wild type, significantly lower in the single mutant, and nondetectable in the double mutant. To confirm these results and to assess the possibility of compensatory effects of other enzymes, we measured the activities of enzymes known to be involved in Suc breakdown in plants (Table II). As described in “Materials and Methods,” 3- to 4-d-old seedlings with radicals 40 mm in length were transferred to floaters over medium bubbled with 50 or 3% O2 in N2. The former treatment was routinely used to ensure that respiration was not limited by O2 levels and that roots were not hypoxic. After such treatment (NHPT), the double mutant had no detectable SuSy activity. However, activity was clearly present after HPT. Since the sh1 bz-m4 mutation is the result of deletion of the entire coding sequence, detection of SuSy activity in the double mutant must be due to hypoxic induction of the sus1 allele. Although not a null mutation, the sus1 mutation (ss2) results in a 17-fold decrease in SuSy activity compared with the homozygous wild type. Table II also shows that HPT depressed INV but induced HK activities. HPT had little effect on FK activity.
Figure 2.
SuSy protein levels in roots from the Sh1Sus1 homozygous wild type, the sh1Sus1 single mutant, and the sh1sus1 double mutant. Root-tip extracts containing 20 μg of protein were analyzed by SDS-PAGE. Detection of SuSy protein on immunoblots was carried out using a polyclonal antiserum that detected both SuSy subunits. The molecular mass (92 kD) of the SS1 subunit is indicated to the right of the digitized image of the original gels.
Table II.
In vitro enzyme activities in root extracts from NHPT and HPT seedlings of the Sh1Sus1 and the sh1sus1 genotype
| Genotype and Treatment | Enzyme Activity
|
Protein | ||||
|---|---|---|---|---|---|---|
| SuSy | INV
|
HK | FK | |||
| Neutral | Acid | |||||
| nmol h−1 tip−1 | μg tip−1 | |||||
| Sh1Sus1 | ||||||
| NHPT | 155 ± 27 | 162 ± 13 | 1046 ± 68 | 34 ± 3 | 68 ± 14 | 61 ± 1 |
| HPT | 326 ± 10 | 86 ± 14 | 202 ± 19 | 106 ± 14 | 82 ± 14 | 64 ± 1 |
| sh1sus1 | ||||||
| NHPT | nda | 123 ± 8 | 459 ± 25 | 23 ± 2 | 49 ± 2 | 40 ± 3 |
| HPT | 19 ± 6 | 67 ± 2 | 100 ± 6 | 77 ± 4 | 48 ± 8 | 38 ± 2 |
Groups of 30 excised root tips from NHPT or HPT seedlings were homogenized for measurement of enzyme activities as described in Methods. Data are means ± sd of three independent repetitions.
nd, Not detectable.
ANP Induction in Double Mutants
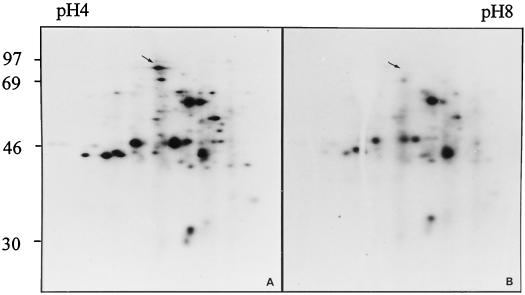
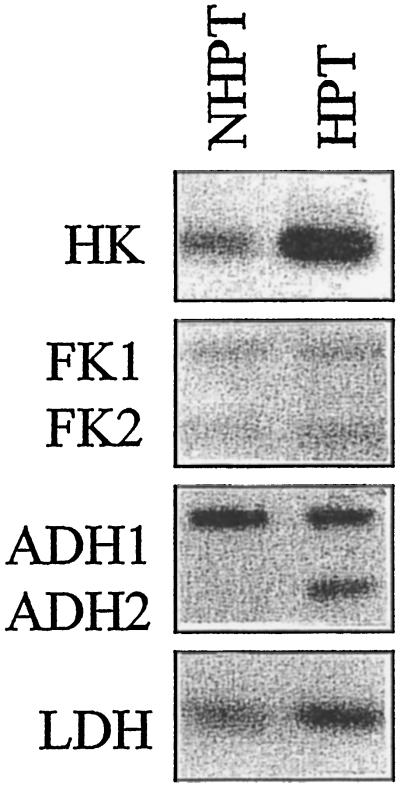
Although the in vitro activities of HK and SuSy were increased by HPT in double mutants, no conclusion could be drawn about the other ANPs. To determine whether other ANPs are induced in double mutants, proteins synthesized in vivo during HPT were labeled and analyzed by two-dimensional electrophoresis. Most of the major proteins synthesized during HPT of the homozygous wild type were also intensely labeled in the double mutant (in Fig. 3 compare A with B). One notable exception was a peptide of approximately 97 kD tentatively identified as SuSy. To substantiate these results, native gel analyses of root extracts containing 25 μg of protein were stained for HK, FK, LDH, and ADH activities (Fig. 4). As has previously been described for hybrid maize (cv DEA; Bouny and Saglio, 1996), the activities of several ANPs (ADH, LDH, and HK but not FK) increased after HPT. Similar results were obtained with the homozygous wild type (results not shown). Together with the two-dimensional analyses, these results indicated that HPT induced ANP expression in SuSy double mutants.
Figure 3.
Proteins synthesized in line W22 roots of the Sh1Sus1 (A) and the sh1sus1 (B) genotype during HPT. Proteins synthesized during HPT were analyzed by two-dimensional IEF/SDS-PAGE. The numbers to the left indicate the position of the molecular mass markers (in kilodaltons). The arrows indicate the position of SuSy.
Figure 4.
Native gel analysis of HK, FK, ADH, and LDH in root extracts from NHPT and HPT double mutants. Extracts containing 20 μg of protein were analyzed on nondenaturing polyacrylamide gels and stained for activity, as described in Methods. The figure is a digitized image of the original gels.
Fermentative Capacity of Double Mutants
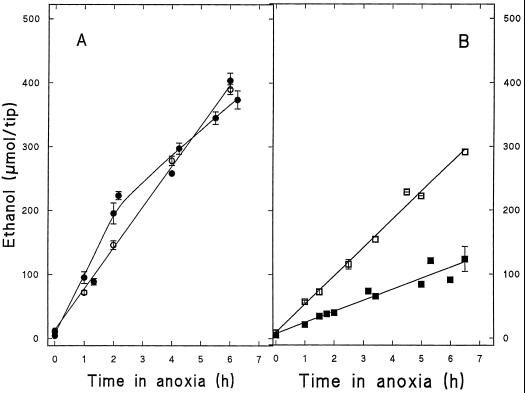
In the literature ANP induction during anoxia has often been presumed to play a role in maintaining high fermentation rates. Moreover, a correlation has been drawn between improved anoxic tolerance and fermentative capacity in maize (Hole et al., 1992) and wheat (Waters et al., 1991). However, the only enzyme with induction shown to be important for the expression of anoxic tolerance is HK in maize (Bouny and Saglio, 1996). It was therefore of interest to determine the fermentative capacity of the double mutant, which was able to induce the ANPs, including SuSy and HK, but was unable to elongate after anoxia, even when HPT. Since the major catabolic pathway under anoxia is ethanolic fermentation, we determined the rate of synthesis of ethanol under anoxia in excised root tips of the sh1sus1 double mutant and the homozygous wild type. Figure 5 shows that HPT induces a similarly high and sustained ethanolic fermentation in both genotypes for at least 6 h of anoxic incubation.
Figure 5.
Time course of ethanol produced by excised root tips from the Sh1Sus1 homozygous wild type (A) and the sh1sus1 double mutant (B) during anoxic incubation in nutrient medium supplemented with 100 mm Glc. Open symbols indicate root tips from HPT seedlings; closed symbols indicate root tips from NHPT seedlings. Each point is the mean ± sd of three independent determinations.
In root tips from NHPT seedlings the kinetics of ethanol production are different in the absence of SuSy activity (compare Fig. 5, A with B). This is particularly clear when the data shown in Figure 5 are expressed with the amount of ethanol synthesized by HPT root tips arbitrarily set at 100 (Table III). Thus, in the homozygous wild type, the amount of ethanol produced by NHPT roots is similar to that of HPT roots during the first 2 h of anoxic incubation but is reduced to 42% during the next 4 h. In the double mutant, the amount of ethanol produced is only 37% of that produced in HPT roots throughout the course of anoxic incubation.
Table III.
Ethanol synthesis in excised root tips from NHPT and HPT seedlings of the Sh1Sus1 and the sh1sus1 genotype
| Genotype and Treatment | Ethanol Synthesis
|
|
|---|---|---|
| 0 to 2 h of Anoxia | 2 to 6 h of Anoxia | |
| Sh1Sus1 | ||
| NHPT | 116 | 42 |
| HPT | 100 | 100 |
| sh1sus1 | ||
| NHPT | 37 | 37 |
| HPT | 100 | 100 |
The data shown in Figure 5 have been presented with the amount of ethanol synthesized by root tips from HPT seedlings arbitrarily set at 100.
Changes in Soluble Sugar Content during Anoxic Incubation
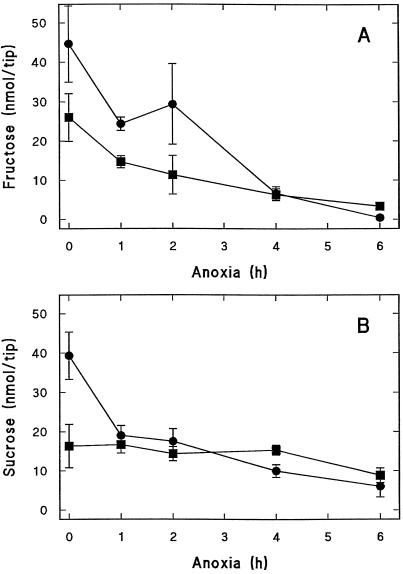
Differences in the rate of ethanol production in NHPT homozygous wild-type and double-mutant root tips could be due to impaired Suc utilization in the latter genotype. Soluble sugars were therefore quantitated in excised NHPT root tips during the course of anoxic incubation. Figure 6A shows that Fru initially present in excised root tips decreased to similarly low levels in both the homozygous wild type and double mutants, confirming that HKs are equally active. However, Suc levels remained stable in double-mutant root tips (Fig. 6B) in contrast to homozygous wild-type tips, in which Suc decreased about 50% within 1 h of anoxic incubation and was exhausted after 4 h (Fig. 6A). The most probable explanation for stable Suc levels is the drastic reduction of SuSy activity in the double mutant. No significant differences were found in levels of acid or neutral INV activities in the double mutant compared with the homozygous wild-type genotype (Table II). In addition, INV activity levels are reduced by O2 deficit (Table II; Guglieminetti et al., 1996) and, in the case of the alkaline and neutral INVs, would be expected to be further inhibited by the anoxic intracellular environment (lower pH). Stable Suc levels in the absence (or near absence) of SuSy activity are consistent with the inactivation of the INV pathway under anoxia.
Figure 6.
Changes in Fru (A) and Suc (B) content in excised root tips from NHPT seedlings of the Sh1Sus1 homozygous wild-type (•) and the sh1sus1 double-mutant (▪) genotype during anoxic incubation in nutrient medium supplemented with 100 mm Glc. Each point is the mean ± sd of three independent determinations.
Root-Tip Death Can Be Alleviated by Feeding with Glc
The preceding experiments using excised root tips identify two factors involved in root tolerance to anoxia. The first is the capacity (induced by hypoxia) to phosphorylate hexoses, which was previously described by Bouny and Saglio (1996). The second is the ability to use Suc to fuel glycolysis. Roots from NHPT seedlings, whatever the SuSy genotype, are limited in hexose phosphorylation due to low levels of HK (Bouny and Saglio, 1996; Table II), which are further inhibited by an unfavorable anoxic intracellular environment (lower pH, lower ATP levels). HPT induces HK activities in roots, even in the SuSy-deficient double mutant (Table II), as expected for lineage-related lines. Increased HK activities allow excised roots from HPT double-mutant seedlings to more than double the amount of ethanol produced if Glc is supplied (Fig. 5B).
Feeding with Glc was therefore predicted to restore the viability of intact roots from HPT double-mutant seedlings. HPT or NHPT seedlings of the Sh1Sus1 and sh1sus1 genotypes were incubated under anoxic conditions for 5 or 24 h in the presence of 100 mm Glc and 250 μg mL−1 cefoxtamine. In the absence of Glc, HPT root tips of the Sh1Sus1 genotype survived 24 h of anoxic incubation, as shown by the high percentage of radicals that elongated after return to air (77%, Table I). Glc improved viability (100%, Table IV). HPT double-mutant root tips were unable to survive anoxic stress in the absence of Glc (11%, Table I), but viability was similar to that of the homozygous wild type when Glc was present (88%, Table IV). Glc had little effect on the viability of NHPT seedlings.
Table IV.
Effect of Glc on root tip viability of HPT and NHPT maize seedlings
| Genotype and Treatment | Elongated Seedlings
|
Elongation | |
|---|---|---|---|
| 5 h of Anoxia | 24 h of Anoxia | ||
| % | |||
| Sh1Sus1 | |||
| NHPT | 1:7 | 14 | |
| HPT | 4:4 | 4:4, 8:8 | 100 |
| sh1sus1 | |||
| NHPT | 0:4 | 0:4, 0:9 | 0 |
| HPT | 4:4 | 3:4, 8:9 | 88 |
HPT and NHPT seedlings of the indicated genotypes were subjected to anoxia in the presence of 100 mm Glc and 250 μg mL−1 cefotaxime for 5 or 24 h. Each ratio is from an independent experiment and represents the number of seedlings that had elongated with respect to the total number of seedlings. The percentage is calculated from the sum of the seedlings that had elongated and the total number of seedlings.
DISCUSSION
Three genotypes, Sh1Sus1, sh1Sus1, and sh1sus1, in the maize line W22 inbred background were studied. The sh1 mutation (sh1 bz-m4) is a deletion of the entire coding region of the SS1 protein. Plants with this mutation lack both SS1 mRNA and protein. The sus1 mutation (ss2) produces a truncated transcript (Chourey and Taliercio, 1994). Small but significant amounts of SuSy activity (6% of the level seen in the homozygous wild type) can be detected in the roots of the double mutant after HPT (Table II) and is no doubt due to SS2 protein. Thus, the sus1 mutation is not a null. SuSy activity has also been reported in the endosperm of double mutants (<0.5% of the wild-type level) and has been attributed to the leakiness of the mutant sus1 locus (P. Chourey, unpublished data).
Roots of Maize Seedlings Deficient in SuSy Activity Are Less Tolerant to O2 Deficit
NHPT maize seedlings, whatever their SuSy genotype, lose root tip viability when subjected to 24 h of anoxia (Table I). There is nevertheless a distinct difference between the root axis of the homozygous wild type, which retains a white, fleshy appearance as well as the capacity to reinitiate lateral roots when returned to air, and the root axis of the sh1sus1 double mutant, which is nearly as inviable as the apical meristem (Fig. 1). That the presence of SuSy activity is associated with improved anoxic tolerance in maize seedlings is also shown from the fact that 3- to 4-d-old HPT seedlings tolerate 24 h of anoxic stress in direct correlation with the number of SuSy genes, enzyme activity, and protein (Tables I and II; Figs. 1 and 2).
HK Activity Limits Ethanol Fermentation under Anoxia
The excised root tips from NHPT double mutants are capable of ethanol production during anoxic incubation when supplied with exogenous Glc (Fig. 5B). This basal level of ethanol production can be attributed to the use of Glc by HKs, since endogenous Suc levels remain unchanged (Fig. 6B). However, the amount of ethanol produced is more than 2-fold lower than that produced by root tips from HPT seedlings and is below the level required for survival (Xia et al., 1995). In spite of ample supplies of Glc, low HK activity effectively limits fermentation. In excised root tips from the NHPT homozygous wild type, a higher rate of ethanol production (higher than that of HPT roots) was limited to the first 2 h of anoxic incubation (Fig. 5A) and was strictly correlated with the depletion of endogenous Suc (Fig. 6B). This transient increase can be attributed to the use of Suc by SuSy.
Suc Is the Principal C Source and SuSy Is the Main Enzyme Active during Suc Breakdown in Roots of Maize Seedlings Deprived of O2
Suc exported from the phloem can be unloaded (symplastically and/or apoplastically) and taken into sink cells either as intact Suc or after hydrolysis into Fru and Glc (Stitt, 1996). Symplastic unloading of Suc from the phloem in maize root tissue is generally inferred from studies of plasmodesmatal frequencies (Warmbrodt, 1985) and the retention of the asymmetry of translocated Suc labeled on the Fru moiety (Giaquinta et al., 1983). Although factors other than simple diffusion could contribute to the physical movement of Suc to the root tip from the point at which the phloem terminates (Bret-Harte and Silk, 1995), their existence would not invalidate a mainly symplastic mode of phloem unloading. Our results are consistent with symplastic but not with apoplastic unloading. If Suc were unloaded in the apoplast, INV present in the cell wall would be expected to hydrolyze Suc and allow the import of hexoses into the root cells. HPT double mutants would then be tolerant to anoxia, as shown by the fact that the addition of Glc effectively alleviates meristem death (Table IV). On the contrary, HPT double mutants remain much more sensitive to anoxic stress (Table I). Suc, not hexose, must therefore be the principal C source in maize root tissues.
Suc transported into root cells can be degraded by two enzymes: INVs produce Fru and Glc, and SuSy activity results in Fru and UDP-Glc. Figure 6B shows that excised root tips from sh1sus1 double mutants are unable to use Suc under anoxic conditions, principally because of the lack of SuSy activity. INVs must therefore be inactive. An HPT increases HK activities and also SuSy activity to detectable (but still 17-fold lower) levels in double-mutant compared with homozygous wild-type root tips. The decrease in cytoplasmic pH has also been shown to be more limited in HPT root tips (Xia et al., 1995). These factors probably account for the survival of a significant percentage (11%) of double-mutant root tips after hypoxic acclimation (Table I). Viability is affected only under situations and in tissues in which Suc is the principal C source and SuSy is the main enzyme active in the utilization of Suc. Our results show that such must be the case in maize roots during conditions of O2 deficit. During aerobic metabolism, INVs are able to compensate for SuSy, as shown by the functionally normal phenotype of the SuSy double mutant (Chourey, 1988).
Impaired Suc utilization would explain the greater anoxic sensitivity of HPT SuSy-deficient double mutants but does not account for that of the apical meristem compared with the root axis of the homozygous wild type. Other factors must be involved and could include more active metabolism, reduced Suc reserves, and/or energy levels inadequate for phloem unloading in the apical meristem.
Hypoxic Acclimation Improves Anoxic Tolerance via HK and SuSy Induction
The hypoxic induction of HK has previously been shown to be an important factor in accounting for higher glycolytic flux during anoxic incubation (Bouny and Saglio, 1996). These results were obtained in excised root tips supplied with exogenous Glc. Our results show that, in addition to HK induction, SuSy induction may be equally important in root tissue of intact maize seedlings. Hypoxic acclimation (Table I) improved root tip viability in correlation with the number of SuSy genes and the level of SuSy expression. Ethanol production was induced by hypoxic acclimation to similar extents in both the homozygous wild type and the double mutant (Fig. 5), in correlation with a 4-fold increase in HK activity (Table II). However, SuSy activity was also induced in double mutants because of the leakiness of the sus-1 gene and would be expected to contribute to higher ethanol production. A limited improvement of energy metabolism might then permit a certain percentage of root meristems (including the apical meristem) to survive. This interpretation is supported by the fact that the presence of Glc in the anoxic incubation medium significantly alleviated meristem death (Table IV), indicating that an increase in glycolytic flux alone was sufficient to restore root tip viability. Glc allows the doubling of the glycolytic flux in excised root tips of HPT double-mutant seedlings (Fig. 5B) to levels known to be compatible with survival (Fig. 5A; Xia et al., 1995).
Taken together, our results show that SuSy is of critical importance for the anoxic tolerance of maize roots, in which INV is inactive and Suc is the major source of C.
ACKNOWLEDGMENT
We would like to thank Dr. J.P. Gaudillère for helpful discussions.
Abbreviations:
- ADH
alcohol dehydrogenase
- ANP
anaerobic protein
- FK
fructokinase
- HK
hexokinase
- HPT
hypoxically pretreated or hypoxia pretreatment
- INV
invertase
- LDH
lactate dehydrogenase
- NBT
nitroblue tetrazolium
- NHPT
not hypoxically pretreated or no hypoxic pretreatment
- PMS
phenazine methosulfate
- SuSy
Suc synthase
LITERATURE CITED
- Bouny M, Saglio P. Glycolytic flux and hexokinase activities in anoxic maize root tips acclimated by hypoxic pretreatment. Plant Physiol. 1996;111:187–194. doi: 10.1104/pp.111.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bret-Harte MS, Silk WK. Non-vascular, symplasmic diffusion of sucrose cannot satisfy the carbon demands in the primary root tip of Zea mays. Plant Physiol. 1995;105:19–33. doi: 10.1104/pp.105.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourey PS. Genetic control of sucrose synthetase in maize endosperm. Mol Gen Genet. 1981;184:372–376. [Google Scholar]
- Chourey PS. Recombinants lacking in detectable levels of both sucrose synthases are functionally normal. Maize Genet Coop Newslett. 1988;62:62–63. [Google Scholar]
- Chourey PS, DeRobertis GA, Still PE. Altered tissue specificity of the revertant shrunken allele upon Dissociation (Ds) excision is associated with loss of expression and molecular rearrangement at the corresponding non-allelic isozyme locus in maize. Mol Gen Genet. 1988;214:300–306. [Google Scholar]
- Chourey PS, Latham MD, Still PE. Expression of two sucrose synthetase genes in endosperm and seedling cells of maize: evidence of tissue specific polymerization of protomers. Mol Gen Genet. 1986;203:251–255. [Google Scholar]
- Chourey PS, Taliercio EW. Epistatic interaction and functional compensation between the two tissue- and cell-specific SuSy genes in maize. Proc Natl Acad Sci USA. 1994;91:7917–7921. doi: 10.1073/pnas.91.17.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi MD, Zabaleta EJ, Pontis HG, Salerno GL. Sucrose synthase expression during cold acclimation in wheat. Plant Physiol. 1991;96:887–891. doi: 10.1104/pp.96.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta RT, Lin W, Sadler NL, Franceschi VR. Pathway of phloem unloading of sucrose in corn roots. Plant Physiol. 1983;72:362–367. doi: 10.1104/pp.72.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielminetti L, Alpi A, Perata P. Shrunken-1-encoded sucrose synthase is not required for the sucrose-ethanol transition in maize under anaerobic conditions. Plant Sci. 1996;119:1–10. [Google Scholar]
- Guglielminetti L, Perata P, Alpi A. Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Physiol. 1995;108:735–741. doi: 10.1104/pp.108.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hole DJ, Cobb BG, Hole PS, Drew MC. Enhancement of anaerobic respiration in root tips of Zea mays following low-oxygen (hypoxic) acclimation. Plant Physiol. 1992;99:213–218. doi: 10.1104/pp.99.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Cobb BG, Drew MC. Hypoxic induction of anoxia tolerance in root tips of Zea mays. Plant Physiol. 1989;91:837–841. doi: 10.1104/pp.91.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch EK, Nolte KD, Duke ER, McCarty DR, Avigne WT. Sugar levels modulate differential expression of maize sucrose synthase genes. Plant Cell. 1992;4:59–69. doi: 10.1105/tpc.4.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marana C, Garcia-Olmedo F, Carbonero P. Differential expression of two types of sucrose synthase-encoding genes in wheat in response to anaerobiosis, cold shock and light. Gene. 1990;88:167–172. doi: 10.1016/0378-1119(90)90028-p. [DOI] [PubMed] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelleschi S, Rocher JP, Prioul JL. Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Environ. 1997;20:493–503. [Google Scholar]
- Ricard B, Rivoal J, Spiteri A, Pradet A. Anaerobic stress induces the transcription and translation of sucrose synthase in rice. Plant Physiol. 1991;95:669–674. doi: 10.1104/pp.95.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Andrade FH, Anderson IC. Further evidence that cytoplasmic acidosis is a determinant of flooding intolerance in plants. Plant Physiol. 1985;77:492–494. doi: 10.1104/pp.77.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Callis J, Jardetsky O, Walbot V, Freeling M. Mechanism of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci USA. 1984;81:3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LJ, Chen Y-C, Chourey PS. Anaerobic treatment alters the cell specific expression of Adh-1, Sh, and Sus genes in roots of maize seedlings. Mol Gen Genet. 1989;218:33–40. [Google Scholar]
- Saglio PH, Drew MC, Pradet A. Metabolic acclimation to anoxia induced by low (2–4 kPa partial pressure) oxygen pretreatment (hypoxia) in root tips of Zea mays. Plant Physiol. 1988;86:61–66. doi: 10.1104/pp.86.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio PH, Pradet A. Soluble sugars, respiration and energy charge during aging of excised maize root tips. Plant Physiol. 1980;66:516–519. doi: 10.1104/pp.66.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio PH, Rancillac M, Bruzau F, Pradet A. Critical oxygen pressure for growth and respiration of excised and intact roots. Plant Physiol. 1984;76:151–154. doi: 10.1104/pp.76.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio PH, Raymond P, Pradet A. Metabolic activities and energy charge of excised maize root tips under anoxia. Plant Physiol. 1980;66:1053–1057. doi: 10.1104/pp.66.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B, Werr W, Starlinger P, Bennett DC, Zokolica M, Freeling M. The Shrunken gene on chromosome 9 of Zea mays L is expressed in various plant tissues and encodes an anaerobic protein. Mol Gen Genet. 1986;205:461–468. doi: 10.1007/BF00338083. [DOI] [PubMed] [Google Scholar]
- Stitt M. Plasmodesmata play an essential role in sucrose export from leaves: a step toward an integration of metabolic biochemistry and cell biology. Plant Cell. 1996;8:566–571. [Google Scholar]
- Taliercio EW, Chourey PS. Post-transcriptional control of sucrose synthase expression in anaerobic seedlings of maize. Plant Physiol. 1989;90:1359–1364. doi: 10.1104/pp.90.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanToai TT, Saglio PH, Ricard B, Pradet A. Developmental regulation of anoxic stress tolerance in maize. Plant Cell Environ. 1995;18:937–942. [Google Scholar]
- Warmbrodt RD. Studies of the root of Zea mays L—structure of the adventitious roots with respect to phloem unloading. Bot Gaz. 1985;146:169–180. [Google Scholar]
- Waters I, Morrell S, Greenway H, Colmer TD. Effects of anoxia on wheat seedlings. II. Influence of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation, and sugar levels. J Exp Bot. 1991;42:1437–1447. [Google Scholar]
- Xia JH, Saglio PH. Lactic acid efflux as a mechanism of hypoxic acclimation of maize root tips to anoxia. Plant Physiol. 1992;100:40–46. doi: 10.1104/pp.100.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JH, Saglio P, Roberts JKM. Nucleotide levels do not critically determine survival of maize root tips acclimated to a low-oxygen environment. Plant Physiol. 1995;108:589–595. doi: 10.1104/pp.108.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]