Abstract
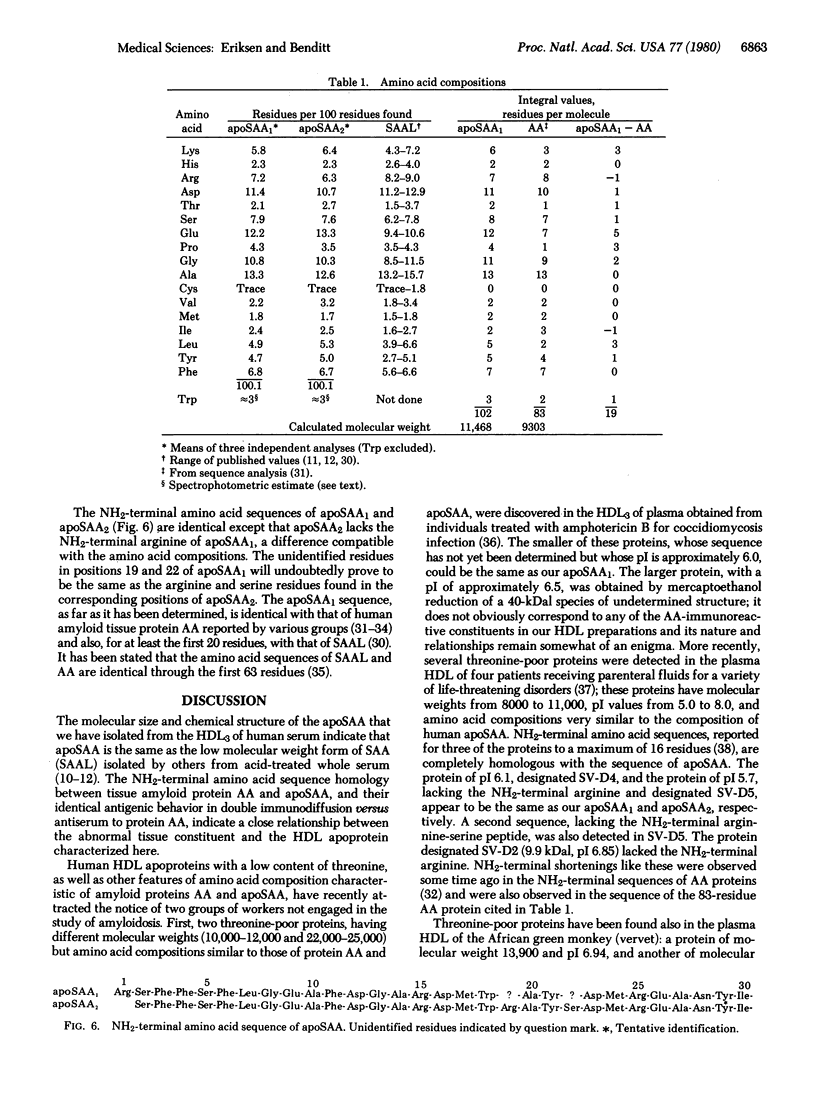
Two apoproteins immunologically related to the 9000-dalton abnormal tissue constituent known as amyloid protein AA were isolated from the lipoprotein density interval 1.125-1.21 g/cm3 (HDL3) of a pool of human serums by delipidation, gel filtration, and ion-exchange chromatography. Lesser amounts of the same apoproteins were isolated from the density interval 1.063-1.125 g/cm3 (HDL2). These apoproteins, designated apoSAA1 and apoSAA2, have molecular weights near 11,500, almost identical amino acid compositions, and slightly different isoelectric points. Their amino acid sequences are identical as far as determined (30 residues), except that apoSAA2 lacks the NH2-terminal arginine found in apoSAA1. The sequence is homologous with that of amyloid protein AA, which thus has residing in the plasma high density lipoproteins a potential precursor whose biological significance and function remain to be determined.
Full text
PDF
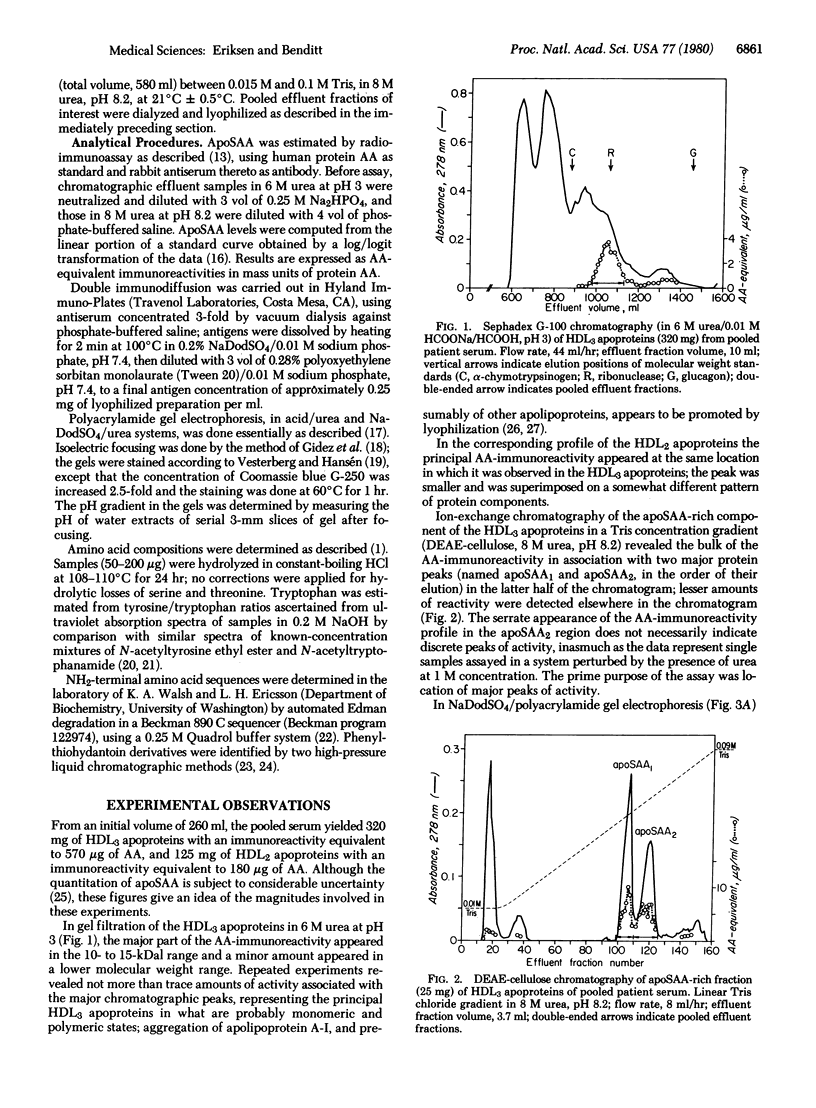
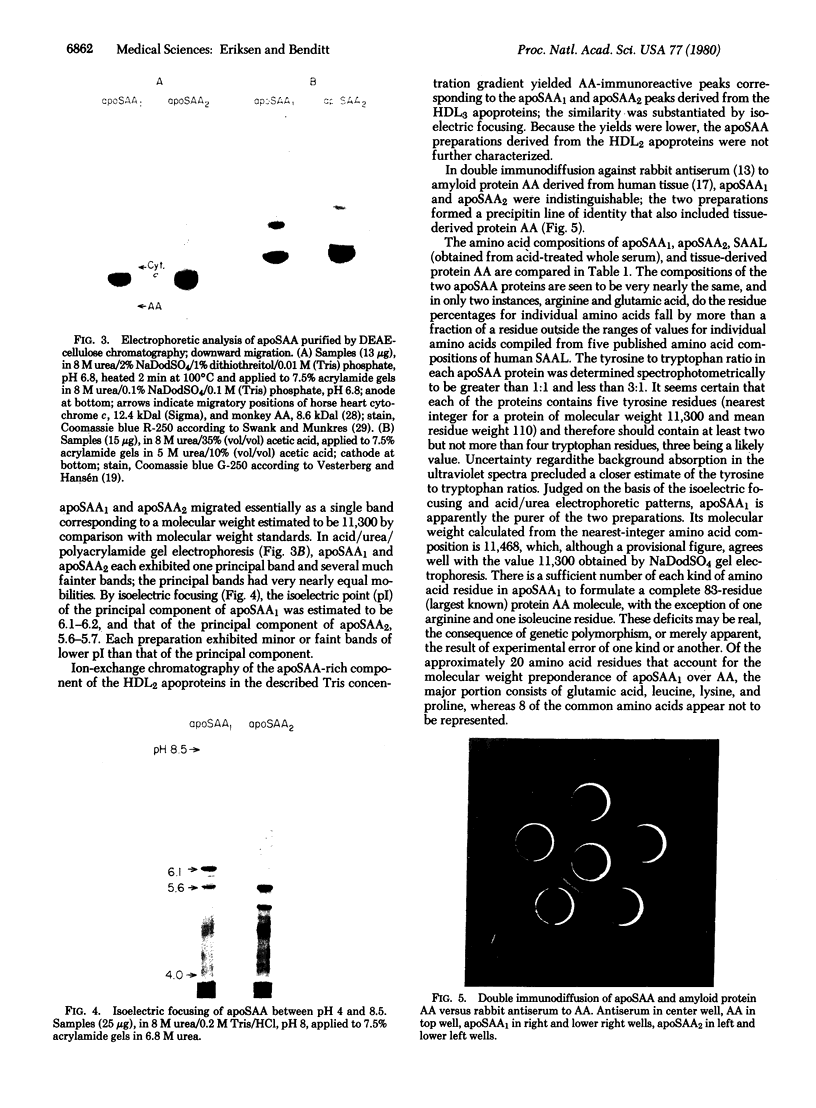

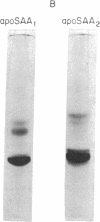
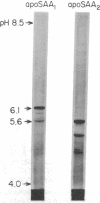
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Natvig J. B., Michaelsen T. E., Husby G. Isolation and characterization of amyloid-related serum protein SAA as a low molecular weight protein. Scand J Immunol. 1975;4(4):397–401. doi: 10.1111/j.1365-3083.1975.tb02642.x. [DOI] [PubMed] [Google Scholar]
- Anders R. F., Natvig J. B., Sletten K., Husby G., Nordstoga K. Amyloid-related serum protein SAA from three animal species: comparison with human SAA. J Immunol. 1977 Jan;118(1):229–234. [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Baumal R., Sklar S., Wilson B., Laskov R. Casein-induced murine amyloidosis: amyloidogenesis in vitro by monolayer spleen explants of casein-injected mice. Lab Invest. 1978 Dec;39(6):632–639. [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Chemical classes of amyloid substance. Am J Pathol. 1971 Oct;65(1):231–252. [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Chemical similarity among amyloid substances associated with long standing inflammation. Lab Invest. 1972 Jun;26(6):615–625. [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hanson R. H. Amyloid protein SAA is an apoprotein of mouse plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4092–4096. doi: 10.1073/pnas.76.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hermodson M. A., Ericsson L. H. The major proteins of human and monkey amyloid substance: Common properties including unusual N-terminal amino acid sequences. FEBS Lett. 1971 Dec 1;19(2):169–173. doi: 10.1016/0014-5793(71)80506-9. [DOI] [PubMed] [Google Scholar]
- Benson M. D., Cohen A. S. Serum amyloid A protein in amyloidosis, rheumatic, and enoplastic diseases. Arthritis Rheum. 1979 Jan;22(1):36–42. doi: 10.1002/art.1780220106. [DOI] [PubMed] [Google Scholar]
- Benson M. D., Kleiner E. Synthesis and secretion of serum amyloid protein A (SAA) by hepatocytes in mice treated with casein. J Immunol. 1980 Feb;124(2):495–499. [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Bridgen P. J., Cross G. A., Bridgen J. N-terminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature. 1976 Oct 14;263(5578):613–614. doi: 10.1038/263613a0. [DOI] [PubMed] [Google Scholar]
- Cohen A. S., Wegelius O. Classification of amyloid: 1979--1980. Arthritis Rheum. 1980 Jun;23(6):644–645. doi: 10.1002/art.1780230606. [DOI] [PubMed] [Google Scholar]
- Ein D., Kimura S., Terry W. D., Magnotta J., Glenner G. G. Amino acid sequence of an amyloid fibril protein of unknown origin. J Biol Chem. 1972 Sep 10;247(17):5653–5655. [PubMed] [Google Scholar]
- Gidez L. I., Swaney J. B., Murnane S. Analysis of rat serum apolipoproteins by isoelectric focusing. I. Studies on the middle molecular weight subunits. J Lipid Res. 1977 Jan;18(1):59–68. [PubMed] [Google Scholar]
- Gorevic P. D., Rosenthal C. J., Franklin E. C. Amyloid-related serum component (SAA)--studies in acute infections, medullary thyroid carcinoma, and postsurgery. Clin Immunol Immunopathol. 1976 Jul;6(1):83–93. doi: 10.1016/0090-1229(76)90063-5. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Kuhn R. W., Walsh K. A., Neurath H., Eriksen N., Benditt E. P. Amino acid sequence of monkey amyloid protein A. Biochemistry. 1972 Aug 1;11(16):2934–2938. doi: 10.1021/bi00766a002. [DOI] [PubMed] [Google Scholar]
- Hijmans W., Sipe J. D. Levels of the serum amyloid A protein (SAA) in normal persons of different age groups. Clin Exp Immunol. 1979 Jan;35(1):96–100. [PMC free article] [PubMed] [Google Scholar]
- Husby G., Natvig J. B. A serum component related to nonimmunoglobulin amyloid protein AS, a possible precursor of the fibrils. J Clin Invest. 1974 Apr;53(4):1054–1061. doi: 10.1172/JCI107642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby G., Natvig J. B., Michaelsen T. E., Sletten K., Höst H. Unique amyloid protein subunit common to different types of amyloid fibril. Nature. 1973 Aug 10;244(5415):362–364. doi: 10.1038/244362a0. [DOI] [PubMed] [Google Scholar]
- Johnson P. M., Husby G., Natvig J. B., Anders R. F., Linder E. Identification in human placentae of antigenic activity related to the amyloid serum protein SAA. Scand J Immunol. 1977;6(4):319–325. doi: 10.1111/j.1365-3083.1977.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Lavie G., Zucker-Franklin D., Franklin E. C. Elastase-type proteases on the surface of human blood monocytes: possible role in amyloid formation. J Immunol. 1980 Jul;125(1):175–180. [PubMed] [Google Scholar]
- Levin M., Franklin E. C., Frangione B., Pras M. The amino acid sequence of a major nonimmunoglobulin component of some amyloid fibrils. J Clin Invest. 1972 Oct;51(10):2773–2776. doi: 10.1172/JCI107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Pras M., Franklin E. C. Immunologic studies of the major nonimmunoglobulin protein of amyloid. I. Identification and partial characterization of a related serum component. J Exp Med. 1973 Aug 1;138(2):373–380. doi: 10.1084/jem.138.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder E., Lehto V. P., Virtanen I., Stenman S., Natvig J. B. Localization of amyloid-related serum protein SAA-like material to intermediate (10 nm) filaments of cultured human embryonal fibroblasts. J Exp Med. 1977 Oct 1;146(4):1158–1163. doi: 10.1084/jem.146.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke R. P., Sipe J. D., Pollock P. S., Ignaczak T. F., Glenner G. G. Isolation of a low-molecular-weight serum component antigenically related to an amyloid fibril protein of unknown origin. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1473–1476. doi: 10.1073/pnas.72.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmendier C. L., Christophe J., Ameryckx J. P. Separation and partial characterization of new apoproteins from human plasma high density lipoproteins. Clin Chim Acta. 1979 Dec 3;99(2):167–176. doi: 10.1016/0009-8981(79)90040-8. [DOI] [PubMed] [Google Scholar]
- Malmendier C. L., Paroutaud P., Ameryckx J. Partial amino acid sequence of three new apolipoproteins isolated from human high density lipoproteins. FEBS Lett. 1980 Jan 1;109(1):43–44. doi: 10.1016/0014-5793(80)81307-x. [DOI] [PubMed] [Google Scholar]
- Meretoja J., Natvig J. B., Husby G. Amyloid-related serum protein (SAA) in patients with inherited amyloidosis and certain viral conditions. Scand J Immunol. 1976;5(1-2):169–174. doi: 10.1111/j.1365-3083.1976.tb03005.x. [DOI] [PubMed] [Google Scholar]
- Møyner K., Sletten K., Husby G., Natvig J. B. An unusually large (83 amino acid residues) amyloid fibril protein AA from a patient with Waldenström's macroglobulinaemia and amyloidosis. Scand J Immunol. 1980;11(5):549–554. doi: 10.1111/j.1365-3083.1980.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Osborne J. C., Jr, Brewer H. B., Jr The plasma lipoproteins. Adv Protein Chem. 1977;31:253–337. doi: 10.1016/s0065-3233(08)60220-x. [DOI] [PubMed] [Google Scholar]
- Parks J. S., Rudel L. L. Isolation and characterization of high density lipoprotein apoproteins in the non-human primate (vervet). J Biol Chem. 1979 Jul 25;254(14):6716–6723. [PubMed] [Google Scholar]
- Rodbard D., Rayford P. L., Cooper J. A., Ross G. T. Statistical quality control of radioimmunoassays. J Clin Endocrinol Metab. 1968 Oct;28(10):1412–1418. doi: 10.1210/jcem-28-10-1412. [DOI] [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C., Frangione B., Greenspan J. Isolation and partial characterization of SAA-an amyloid-related protein from human serum. J Immunol. 1976 May;116(5):1415–1418. [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C. Variation with age and disease of an amyloid A protein-related serum component. J Clin Invest. 1975 Apr;55(4):746–753. doi: 10.1172/JCI107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal C. J., Sullivan L. Serum amyloid A: evidence for its origin in polymorphonuclear leukocytes. J Clin Invest. 1978 Dec;62(6):1181–1186. doi: 10.1172/JCI109237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer E. J., Eisenberg S., Levy R. I. Lipoprotein apoprotein metabolism. J Lipid Res. 1978 Aug;19(6):667–687. [PubMed] [Google Scholar]
- Selinger M. J., McAdam K. P., Kaplan M. M., Sipe J. D., Vogel S. N., Rosenstreich D. L. Monokine-induced synthesis of serum amyloid A protein by hepatocytes. Nature. 1980 Jun 12;285(5765):498–500. doi: 10.1038/285498a0. [DOI] [PubMed] [Google Scholar]
- Shore V. G., Shore B., Lewis S. B. Isolation and characterization of two threonine-poor apolipoproteins of human plasma high density lipoproteins. Biochemistry. 1978 May 30;17(11):2174–2179. doi: 10.1021/bi00604a023. [DOI] [PubMed] [Google Scholar]
- Skogen B., Børresen A. L., Natvig J. B., Berg K., Michaelsen T. E. High-density lipoprotein as carrier for amyloid-related protein SAA in rabbit serum. Scand J Immunol. 1979;10(1):39–45. doi: 10.1111/j.1365-3083.1979.tb01332.x. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Jaffe E., Pollock S., Sipe J., Glenner G. Amyloid AA protein. Cellular distribution and appearance. Am J Clin Pathol. 1977 Jun;67(6):540–544. doi: 10.1093/ajcp/67.6.540. [DOI] [PubMed] [Google Scholar]