Abstract
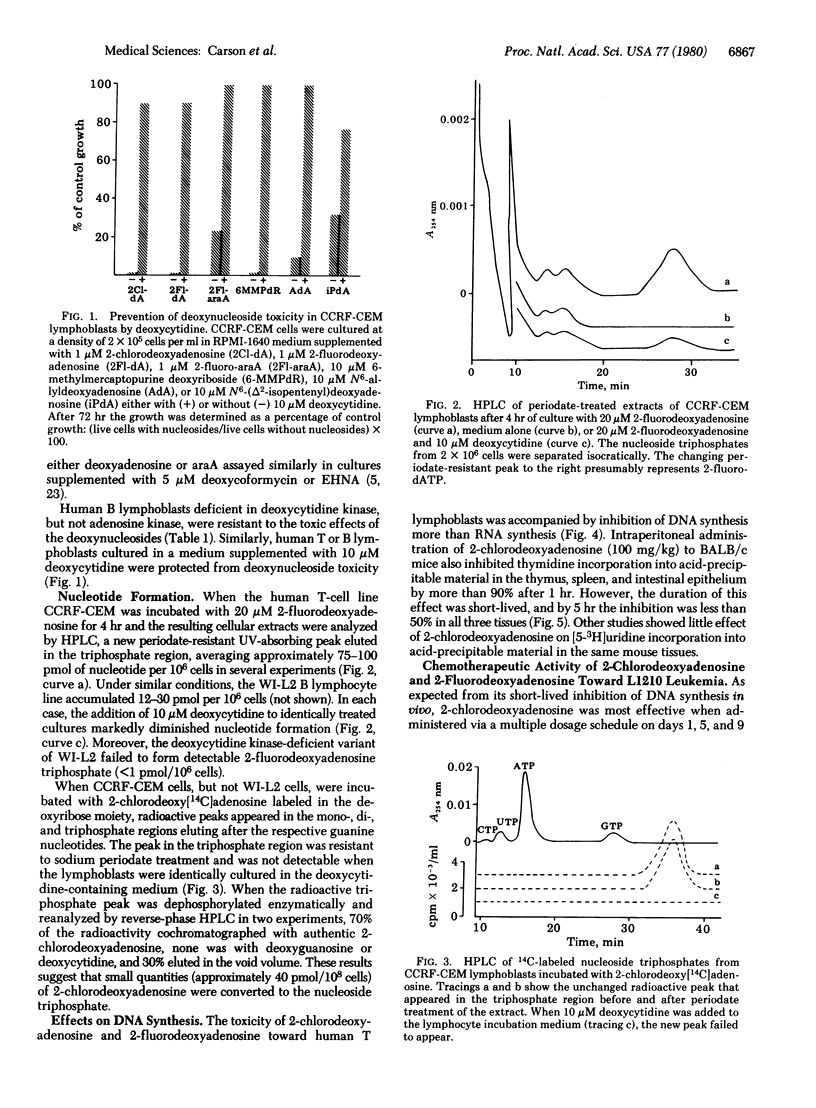
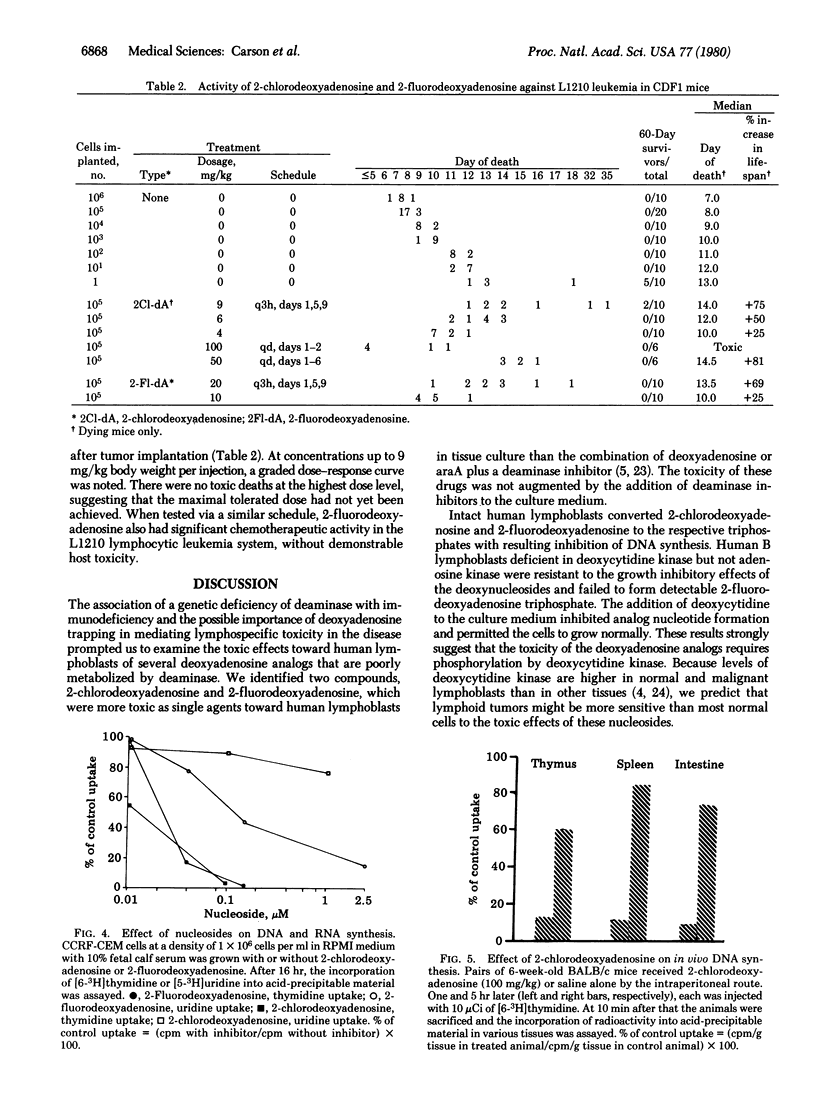
An inherited deficiency of adenosine deaminase (adenosine aminohydrolase, EC 3.5.4.4) produces selective lymphopenia and immunodeficiency disease in humans. Previous experiments have suggested that lymphospecific toxicity in this condition might result from the selective accumulation of toxic deoxyadenosine nucleotides by lymphocytes with high deoxycytidine kinase, levels and low deoxynucleotide dephosphorylating activity. The present experiments were designed to determine if deoxyadenosine analogs which are not substrates for adenosine deaminase might similarly be toxic toward lymphocytes and lymphoid tumors. Two such compounds, 2-chlorodeoxyadenosine and 2-fluorodeoxyadenosine, at concentrations of 3 nM and 0.15 microM, respectively, inhibited by 50% the growth of human CCRF-CEM malignant lymphoblasts in vitro. Each was phosphorylated in intact cells by deoxycytidine kinase accumulated as the nucleoside triphosphate, and inhibited DNA synthesis more than RNA synthesis. Both deoxynucleosides had significant chemotherapeutic activity against lymphoid leukemia L1210 in mice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cardinaud R. Nucleoside deoxyribosyltransferase from Lactobacillus helveticus. Methods Enzymol. 1978;51:446–455. doi: 10.1016/s0076-6879(78)51062-8. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Matsumoto S., Seegmiller J. E., Thompson L. Biochemical basis for the enhanced toxicity of deoxyribonucleosides toward malignant human T cell lines. Proc Natl Acad Sci U S A. 1979 May;76(5):2430–2433. doi: 10.1073/pnas.76.5.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Differential sensitivity of human leukemic T cell lines and B cell lines to growth inhibition by deoxyadenosine. J Immunol. 1978 Nov;121(5):1726–1731. [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Metabolism and toxicity of 9-beta-D-arabinofuranosyladenine in human malignant T cells and B cells in tissue culture. Adv Exp Med Biol. 1979;122B:299–307. doi: 10.1007/978-1-4684-8559-2_49. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Wasson D. B. Differences in deoxyadenosine metabolism in human and mouse lymphocytes. J Immunol. 1980 Jan;124(1):8–12. [PubMed] [Google Scholar]
- Christensen L. F., Broom A. D., Robins M. J., Bloch A. Synthesis and biological activity of selected 2,6-disubstituted-(2-deoxy- -and- -D-erythro-pentofuranosyl)purines. J Med Chem. 1972 Jul;15(7):735–739. doi: 10.1021/jm00277a010. [DOI] [PubMed] [Google Scholar]
- Cohen A., Hirschhorn R., Horowitz S. D., Rubinstein A., Polmar S. H., Hong R., Martin D. W., Jr Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- Dow L. W., Bell D. E., Poulakos L., Fridland A. Differences in metabolism and cytotoxicity between 9-beta-D arabinofuranosyladenine and 9-beta-D-arabinofuranosyl-2-fluoroadenine in human leukemic lymphoblasts. Cancer Res. 1980 May;40(5):1405–1410. [PubMed] [Google Scholar]
- Durham J. P., Ives D. H. Deoxycytidine kinase. I. Distribution in normal and neoplastic tissues and interrelationships of deoxycytidine and 1-beta-D-arabinofuranosylcytosine phosphorylation. Mol Pharmacol. 1969 Jul;5(4):358–375. [PubMed] [Google Scholar]
- Frederiksen S. Specificity of adenosine deaminase toward adenosine and 2'-deoxyadenosine analogues. Arch Biochem Biophys. 1966 Feb;113(2):383–388. doi: 10.1016/0003-9861(66)90202-5. [DOI] [PubMed] [Google Scholar]
- Garrett C., Santi D. V. A rapid and sensitive high pressure liquid chromatography assay for deoxyribonucleoside triphosphates in cell extracts. Anal Biochem. 1979 Nov 1;99(2):268–273. doi: 10.1016/s0003-2697(79)80005-6. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Hall R. H., Alam S. N., McLennan B. D. N6-(delta 2-isopentenyl)adenosine: its conversion to inosine, catalyzed by adenosine aminohydrolases from chicken bone marrow and calf intestinal mucosa. Can J Biochem. 1971 Jun;49(6):623–630. doi: 10.1139/o71-089. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Snyder F. F., Seegmiller J. E. Adenine and adenosine are toxic to human lymphoblast mutants defective in purine salvage enzymes. Science. 1977 Sep 23;197(4310):1284–1287. doi: 10.1126/science.197600. [DOI] [PubMed] [Google Scholar]
- Koller C. A., Mitchell B. S., Grever M. R., Mejias E., Malspeis L., Metz E. N. Treatment of acute lymphoblastic leukemia with 2'-deoxycoformycin: clinical and biochemical consequences of adenosine deaminase inhibition. Cancer Treat Rep. 1979 Nov-Dec;63(11-12):1949–1952. [PubMed] [Google Scholar]
- Mitchell B. S., Mejias E., Daddona P. E., Kelley W. N. Purinogenic immunodeficiency diseases: selective toxicity of deoxyribonucleosides for T cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5011–5014. doi: 10.1073/pnas.75.10.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyai K., Allen L. B., Huffman J. H., Sidwell R. W., Tolman R. L. Synthesis and anti-deoxyribonucleic acid virus activity of certain 9-beta-D-arabinofuranosyl-2-substituted adenine derivatives. J Med Chem. 1974 Feb;17(2):242–244. doi: 10.1021/jm00248a023. [DOI] [PubMed] [Google Scholar]
- Montgomery J. A., Hewson K. Nucleosides of 2-fluoroadenine. J Med Chem. 1969 May;12(3):498–504. doi: 10.1021/jm00303a605. [DOI] [PubMed] [Google Scholar]
- Moore E. C., Hurlbert R. B. Regulation of mammalian deoxyribonucleotide biosynthesis by nucleotides as activators and inhibitors. J Biol Chem. 1966 Oct 25;241(20):4802–4809. [PubMed] [Google Scholar]
- Skipper H. E., Schabel F. M., Jr, Wilcox W. S. Experimental evaluation of potential anticancer agents. XXI. Scheduling of arabinosylcytosine to take advantage of its S-phase specificity against leukemia cells. Cancer Chemother Rep. 1967 Jun;51(3):125–165. [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Cohen A., Martin D. W., Jr Deoxyadenosine metabolism and cytotoxicity in cultured mouse T lymphoma cells: a model for immunodeficiency disease. Cell. 1978 Jun;14(2):365–375. doi: 10.1016/0092-8674(78)90122-8. [DOI] [PubMed] [Google Scholar]
- Wortmann R. L., Mitchell B. S., Edwards N. L., Fox I. H. Biochemical basis for differential deoxyadenosine toxicity to T and B lymphoblasts: role for 5'-nucleotidase. Proc Natl Acad Sci U S A. 1979 May;76(5):2434–2437. doi: 10.1073/pnas.76.5.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]


