Abstract
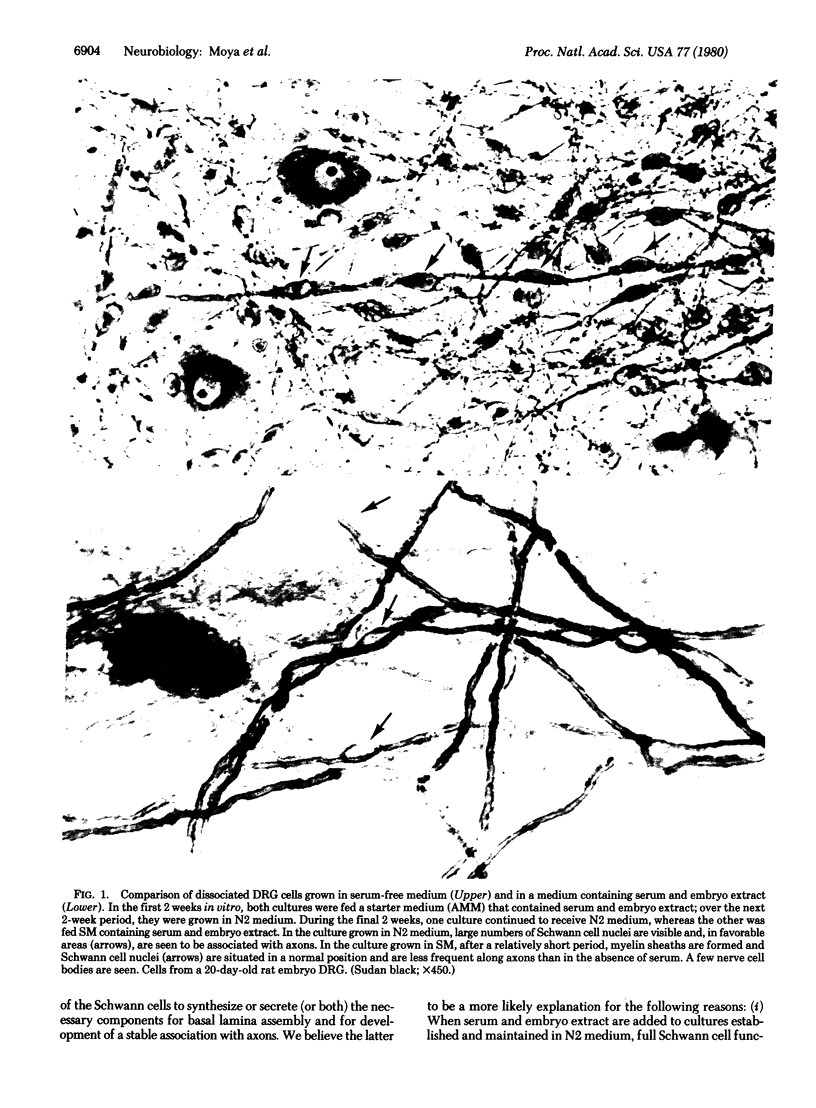
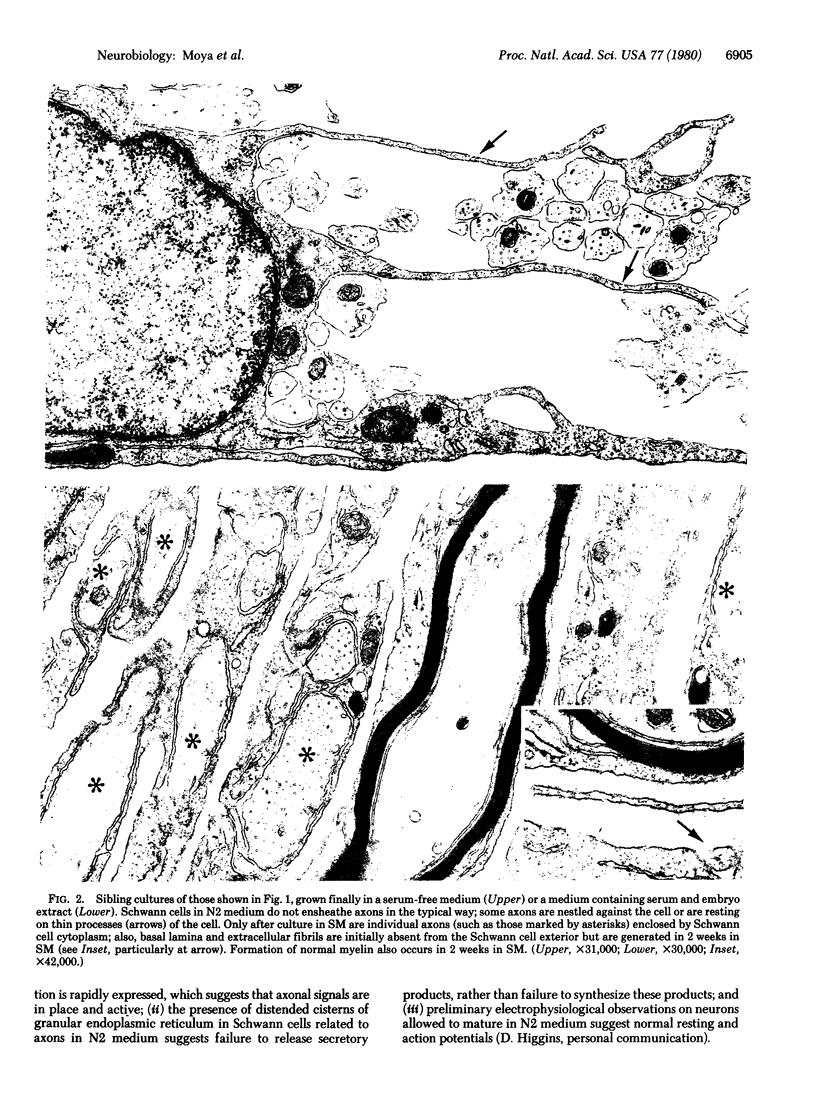
Primary cultures of dorsal root ganglia cells from 18- to 21-day rodent embryos were studied for their ability to express Schwann cell function in a defined medium lacking serum and embryo extract. It was confirmed that Schwann cells, but not fibroblasts, are able to proliferate in response to contact with axons when cultured in this defined medium. We here report that in this medium, however, differentiation of Schwann cells was arrested before completion of ensheathment and before initiation of myelin formation. Electron microscopic analysis confirmed this ensheathment failure and showed that the extracellular matrix components (basal lamina and thin collagenous fibrils) normally produced by axon-related Schwann cells had not been formed. This absence of extracellular matrix, as well as the presence in the Schwann cell of an increased cytoplasmic granularity (observed in the light microscope) and numerous distended cisterns of rough endoplasmic reticulum, suggest a failure in Schwann cell secretion. However, within one week after addition of serum and embryo extract to the culture medium, the ensheathment failure was corrected and myelination occurred; electron microscopic observations showed the presence of basal lamina and collagen fibrils in association with Schwann cells. These results suggest the presence in serum or embryo extract (or both) of factors necessary for the full expression of Schwann cell function (although a similar requirement is not present for the expression of oligodendrocyte function in culture). We propose that these observations indicate a linkage between Schwann cell secretion and axonal ensheathment, including myelin formation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billings-Gagliardi S., Webster H. F., O'Connell M. F. In vivo and electron microscopic observations on Schwann cells in developing tadpole nerve fibers. Am J Anat. 1974 Nov;141(3):375–391. doi: 10.1002/aja.1001410308. [DOI] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge M. B., Bunge R. P., Peterson E. R., Murray M. R. A light and electron microscope study of long-term organized cultures of rat dorsal root ganglia. J Cell Biol. 1967 Feb;32(2):439–466. doi: 10.1083/jcb.32.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge M. B. Initial endocytosis of perioxidase or ferritin by growth cones of cultured nerve cells. J Neurocytol. 1977 Aug;6(4):407–439. doi: 10.1007/BF01178226. [DOI] [PubMed] [Google Scholar]
- Bunge M. B., Williams A. K., Wood P. M., Uitto J., Jeffrey J. J. Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J Cell Biol. 1980 Jan;84(1):184–202. doi: 10.1083/jcb.84.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge R. P., Wood P. Studies on the transplantation of spinal cord tissue in the rat. I. The development of a culture system for hemisections of embryonic spinal cord. Brain Res. 1973 Jul 27;57(2):261–276. doi: 10.1016/0006-8993(73)90135-2. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Honegger P., Lenoir D., Favrod P. Growth and differentiation of aggregating fetal brain cells in a serum-free defined medium. Nature. 1979 Nov 15;282(5736):305–308. doi: 10.1038/282305a0. [DOI] [PubMed] [Google Scholar]
- Jaros E., Bradley W. G. Atypical axon-Schwann cell relationships in the common peroneal nerve of the dystrophic mouse: an ultrastructural study. Neuropathol Appl Neurobiol. 1979 Mar-Apr;5(2):133–147. doi: 10.1111/j.1365-2990.1979.tb00666.x. [DOI] [PubMed] [Google Scholar]
- Manthorpe M., Skaper S., Varon S. Purification of mouse Schwann cells using neurite-induced proliferation in serum-free monolayer culture. Brain Res. 1980 Sep 8;196(2):467–482. doi: 10.1016/0006-8993(80)90410-2. [DOI] [PubMed] [Google Scholar]
- PETERSON E. R., MURRAY M. R. Modification of development in isolated dorsal root ganglia by nutritional and physical factors. Dev Biol. 1960 Oct;2:461–476. doi: 10.1016/0012-1606(60)90028-2. [DOI] [PubMed] [Google Scholar]
- PETERSON E. R., MURRAY M. R. Myelin sheath formation in cultures of avian spinal ganglia. Am J Anat. 1955 May;96(3):319–355. doi: 10.1002/aja.1000960302. [DOI] [PubMed] [Google Scholar]
- Salzer J. L., Bunge R. P., Glaser L. Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J Cell Biol. 1980 Mar;84(3):767–778. doi: 10.1083/jcb.84.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J. L., Bunge R. P. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol. 1980 Mar;84(3):739–752. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J. L., Williams A. K., Glaser L., Bunge R. P. Studies of Schwann cell proliferation. II. Characterization of the stimulation and specificity of the response to a neurite membrane fraction. J Cell Biol. 1980 Mar;84(3):753–766. doi: 10.1083/jcb.84.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto V. J., Uitto J., Kao W. W., Prockop D. J. Procollagen polypeptides containing cis-4-hydroxy-L-proline are overglycosylated and secreted as nonhelical pro-gamma-chains. Arch Biochem Biophys. 1978 Jan 15;185(1):214–221. doi: 10.1016/0003-9861(78)90161-3. [DOI] [PubMed] [Google Scholar]
- Webster H. D., Martin R., O'Connell M. F. The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study. Dev Biol. 1973 Jun;32(2):401–416. doi: 10.1016/0012-1606(73)90250-9. [DOI] [PubMed] [Google Scholar]
- Wood P. M., Bunge R. P. Evidence that sensory axons are mitogenic for Schwann cells. Nature. 1975 Aug 21;256(5519):662–664. doi: 10.1038/256662a0. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Separation of functional Schwann cells and neurons from normal peripheral nerve tissue. Brain Res. 1976 Oct 22;115(3):361–375. doi: 10.1016/0006-8993(76)90355-3. [DOI] [PubMed] [Google Scholar]
- Yu R. C., Bunge R. P. Damage and repair of the peripheral myelin sheath and node of Ranvier after treatment with trypsin. J Cell Biol. 1975 Jan;64(1):1–14. doi: 10.1083/jcb.64.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]