Abstract
In this study the interplay of mitochondria and peroxisomes in photorespiration was simulated in a reconstituted system of isolated mitochondria and peroxisomes from spinach (Spinacia oleracea L.) leaves. The mitochondria oxidizing glycine produced serine, which was reduced in the peroxisomes to glycerate. The required reducing equivalents were provided by the mitochondria via the malate-oxaloacetate (OAA) shuttle, in which OAA was reduced in the mitochondrial matrix by NADH generated during glycine oxidation. The rate of peroxisomal glycerate formation, as compared with peroxisomal protein, resembled the corresponding rate required during leaf photosynthesis under ambient conditions. When the reconstituted system produced glycerate at this rate, the malate-to-OAA ratio was in equilibrium with a ratio of NADH/NAD of 8.8 × 10−3. This low ratio is in the same range as the ratio of NADH/NAD in the cytosol of mesophyll cells of intact illuminated spinach leaves, as we had estimated earlier. This result demonstrates that in the photorespiratory cycle a transfer of redox equivalents from the mitochondria to peroxisomes, as postulated from separate experiments with isolated mitochondria and peroxisomes, can indeed operate under conditions of the very low reductive state of the NADH/NAD system prevailing in the cytosol of mesophyll cells in a leaf during photosynthesis.
Leaf peroxisomes, a specialized form of cell organelles, play a vital role in photosynthesis and photorespiration (Tolbert, 1980). They are involved in the formation of glycerate from the glycolate derived from the oxygenase activity of Rubisco. Since the ratio of oxygenation to carboxylation during photosynthesis in a leaf will be 0.2 to 0.5 (Sharkey, 1988), the metabolic flux of glycolate through the leaf peroxisomes is very high (Heupel et al., 1991; Reumann et al., 1994). In mitochondria and chloroplasts the compartmentation of metabolism is due to the function of boundary membranes in which the inner membrane contains specific translocators for a controlled passage of metabolites. This is different in leaf peroxisomes, where the latency of enzymes and the compartmentation of the conversion of glycolate to Gly and of Ser to glycerate were found to be unaffected by osmotic shock. In peroxisomes the compartmentation of metabolites is apparently not due to the boundary function of the peroxisomal membrane but is the result of the arrangement of the peroxisomal matrix proteins as multienzyme complexes, allowing metabolite channeling (Heupel and Heldt, 1994). Instead of specific translocators, the leaf peroxisomal membrane contains a porin, allowing the passage of a large variety of negatively charged intermediates of photorespiratory metabolism. This differs from the porins of the outer membranes of leaf mitochondria and chloroplasts (Flügge and Benz, 1984; Schmid et al., 1992) in containing a binding site with a high affinity to dicarboxylic acids (Reumann et al., 1995, 1996, 1998) and thus resembles some prokaryote-specific porins.
The conversion of Ser to glycerate in peroxisomes requires reducing equivalents in the form of NADH for the reduction of hydroxypyruvate. Studies of isolated spinach (Spinacia oleracea L.) peroxisomes indicated that the import of reduced equivalents occurred exclusively through the malate-OAA shuttle from the cytosol but not directly by NADH (Reumann et al., 1994). This can be explained in terms of a diffusion resistance of the highly aggregated matrix proteins. The diffusion flux of a molecule is proportional to its concentration difference and inversely proportional to the square of the Mr. Because the concentration of malate in the cytosol of the mesophyll cells in intact leaves is more than 1000 times higher than that of NADH (1 mm malate versus 0.7 μm NADH, Heineke et al., 1991), and the square of the Mr of malate is 25 times lower than that of NADH, the diffusion of malate across the peroxisomal matrix is expected to occur about 30,000 times faster than the diffusion of NADH. Moreover, the permeability properties of the leaf peroxisomal porin will favor the passage of malate and OAA over NADH and NAD (Reumann et al., 1996).
Leaf mitochondria are able to export reducing equivalents via the malate-OAA shuttle at high rates (Douce and Bonner, 1972; Day and Wiskich, 1984; Ebbighausen et al., 1985). Alternatively, chloroplasts are able to export reducing equivalents generated by photosynthesis by the malate-OAA shuttle (Hatch et al., 1984). Therefore, both mitochondria and chloroplasts are able to meet the requirement of NADH for hydroxypyruvate reduction in peroxisomes. It has been estimated from experiments with isolated spinach leaf mitochondria that 25 to 50% of the NADH needed for hydroxypyruvate reduction in peroxisomes might be provided by mitochondria (Krömer and Heldt, 1991; Hanning and Heldt, 1993) and the rest by the chloroplasts. In mesophyll cells of intact, illuminated leaves, the reductive state of NADH/NAD has been found to be on the order of 10−3 (Heineke et al., 1991). The question remained whether a redox transfer from the mitochondria to the peroxisomes, as postulated from experiments performed with isolated mitochondria or peroxisomes, can operate at physiological rates under such conditions. To answer this question we used a reconstituted system of mitochondria and peroxisomes, in which the mitochondria oxidized Gly to provide the peroxisomes with Ser and reducing equivalents via the malate-OAA shuttle. Our results demonstrate that mitochondria are capable of supplying malate for sustaining high rates of peroxisomal glycerate production. The assay of metabolite levels in the steady state revealed that the redox supply from the mitochondria to the peroxisomes via the malate-OAA shuttle can occur at a very low redox state known to occur in the cytosol of mesophyll cells in intact illuminated leaves performing photosynthesis under ambient conditions.
MATERIALS AND METHODS
Spinach (Spinacia oleracea, U.S. hybrid 424, Ferry-Morse Seed Company, Mountain View, CA) was grown in growth chambers at 19°C for 9 h in light in hydroponic culture. The illumination was about 350 μmol quanta m−2 s−1 with tungsten and mercury lamps (Heupel et al., 1991). Deribbed mature leaves of 2-month-old plants were used for isolating peroxisomes or mitochondria. For partial purification of glycerate kinase according to the method of Schmitt and Edwards (1983), 12- to 16-d-old-plants of pea (Pisum sativum, var Kleine Rheinländerin) grown with supplementary tungsten lighting were used.
Isolation of Peroxisomes and Mitochondria
Peroxisomes were isolated as described previously using a discontinuous Percoll gradient (Reumann et al., 1995). The intactness of peroxisomes, as judged by the latency of HPR (Heupel et al., 1991), ranged from 87 to 95%. Mitochondria were isolated and purified using a medium containing Percoll, as described earlier (Krömer and Heldt, 1991). The intactness of mitochondria, as judged by the latency of Cyt c oxidase (Neuberger et al., 1982; Krömer and Heldt, 1991), ranged from 95 to 98%. The average specific activities of these preparations were 15 U of HPR mg−1 peroxisomal protein and 600 nanoatom [O] mg−1 mitochondrial protein min−1 (Gly oxidation).
Glycerate Formation
Peroxisomes (and/or mitochondria) were incubated at 20°C in a reaction medium containing 50 mm KH2PO4 (pH 7.5), 0.25 m mannitol, 2 mm glycolate, 1 mm malate, 1 mm NAD, 0.5 mm OAA, 10 mm Gly, 0.05 mm CoA, 0.1 mm thiamine PPi, and 1 mm ADP. The following compounds were included, when mentioned: 15 mm Ser, 0.25 mm Glu, and 10 U mL−1 GOT. The reaction was initiated by peroxisomes equivalent to 10 to 12 μg of peroxisomal protein mL−1 and mitochondria equivalent to 125 to 150 μg of mitochondrial protein mL−1, when present. At appropriate intervals, the reaction was terminated by the transfer of a 500-μL aliquot to a tube containing 100 μL of 10% (v/v) perchloric acid and 50 mm EDTA. Further details of neutralization of the acidified reaction mixture were as described earlier (Heupel et al., 1991).
Reconstitution of Peroxisomes and Mitochondria
The specific activity of each organelle preparation, simultaneously made from the same set of spinach plants, was determined using a typical marker: HPR for peroxisomes (Heupel et al., 1991) and Gly-dependent respiratory O2 uptake for mitochondria (Hanning and Heldt, 1993). In the reconstituted system mitochondria catalyzing 0.5 μmol Gly oxidation (equivalent to 0.5 μatom [O] consumed min−1 mL−1) were added for each unit of peroxisomes catalyzing the reduction of 1 μmol hydroxypyruvate min−1 mL−1.
The peroxisomal preparations were of high purity, and the mitochondrial contamination was negligible (<1%; Reumann et al., 1995). However, the mitochondrial preparation was slightly contaminated with peroxisomes. Therefore, the experiments were also run with the sets of mitochondria alone. The rates of glycerate formation by the mixture of peroxisomes plus mitochondria were corrected by subtracting the background rates of glycerate production by the mitochondrial preparation.
Determination of Glycerate and Other Metabolites
Glycerate was assayed by an enzymatic coupled assay using partially purified glycerate kinase from osmotically shocked pea chloroplasts (Heupel et al., 1991). The levels of other metabolites (oxoglutarate, Glu, Asp, and malate) were determined by standard procedures (Bergmeyer et al., 1983; Heineke et al., 1991). All of the enzymatic analyses of metabolites were done using a dual-wavelength spectrophotometer (model ZFF-22, Sigma). Protein was estimated by the method of Lowry et al. (1951).
All of the experiments were run at least three times on different days. The data of a typical experiments are represented in each case.
RESULTS
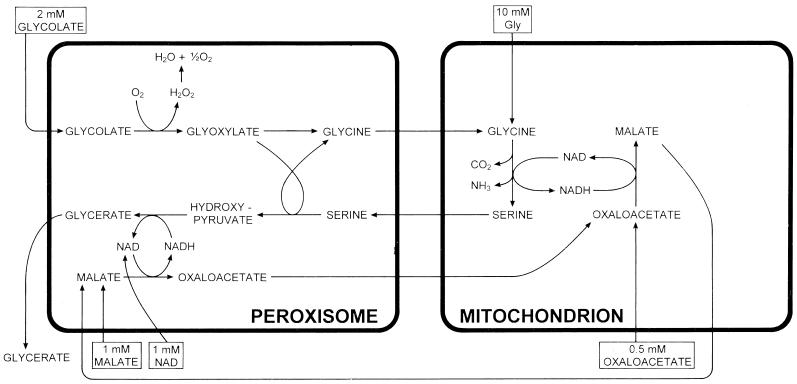
Isolated, intact leaf peroxisomes are able to produce glycerate when glycolate, Ser, malate, and NAD are provided externally (Heupel et al., 1991; Reumann et al., 1994). To study the interplay of mitochondria and peroxisomes in photorespiration, we combined isolated mitochondria and peroxisomes from spinach leaves to a reconstituted system that is able to form glycerate from glycolate (Fig. 1). The optimal ratio of peroxisomes to mitochondria, as indicated in “Materials and Methods,” was determined from the stimulatory effect of mitochondria on peroxisomal glycerate synthesis (data not shown).
Figure 1.
A flow chart of possible movement of metabolites between peroxisomes and mitochondria in the reconstituted system during glycerate formation. The patterns of glycerate formation and metabolite levels are illustrated in Table I.
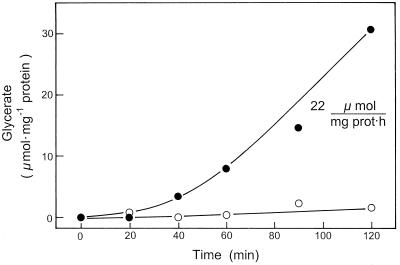
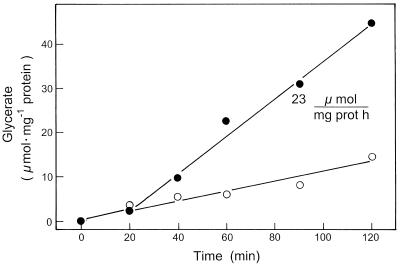
When peroxisomes were incubated with glycolate, NAD, a low concentration of malate (1 mm), and OAA (0.5 mm), but no Ser added, the rate of their glycerate formation was very low. But in the presence of mitochondria, glycerate formation increased, reaching high linear rates after an initial lag period of about 40 min (Fig. 2). At the times indicated, the concentrations of malate, aspartate, 2-oxoglutarate, and glutamate were assayed in deproteinized samples of the reconstituted system. The assay of metabolite concentrations in the reaction mixture showed that Ser levels increased from low levels (< 0.1 mm) at the start of incubation to 0.2 mm and 0.8 mm at 20 and 90 min of incubation in the medium containing the mixture of peroxisomes and mitochondria (data not shown). However, peroxisomes alone formed no Ser (data not shown). The initial rate of glycerate formation was slightly increased when 15 mm Ser was present during incubation (Fig. 3). The system did not require glutamate. However, the rates of glycerate production were low when only peroxisomes were present and were increased almost 4-fold in the presence of mitochondria. In this system the stimulating function of mitochondria on peroxisomal glycerate formation was due to the reduction of OAA enabling the delivery of reducing equivalents via the malate-OAA shuttle.
Figure 2.
Time course of glycerate formation by isolated peroxisomes alone (○) or by reconstituted system of peroxisomes plus mitochondria (•) in the absence of externally added Ser. The rates of glycerate formation by the latter system (peroxisomes plus mitochondria) were corrected for the background rates by mitochondria alone, as described in detail in the text. The medium contained 2 mm glycolate, 1 mm malate, 1 mm NAD, 0.5 mm OAA, 10 mm Gly, and 1 mm ADP. prot, Protein.
Figure 3.
Time course of glycerate formation in the presence of 15 mm Ser, by peroxisomes alone (○), or by a reconstituted system of peroxisomes plus mitochondria (•). The rates of glycerate formation by the latter system (peroxisomes plus mitochondria) were corrected for the background rates by mitochondria alone, as described in detail in the text. The medium contained 15 mm Ser in addition to the components described in the legend of Figure 2. prot, Protein.
Our main objective was to determine the levels of OAA and malate in the reconstituted system in a steady state, as indicated by the linear rates of glycerate production. This would reveal the redox state of the medium, reflecting that of the cytosol, when glycerate formation is optimal. It is very difficult to make accurate estimations of OAA because it is a very unstable substance. As expected, our attempts to determine directly OAA in the medium always yielded underestimates. We have therefore included 0.25 mm glutamate (along with 10 U mL−1 GOT) so as to maintain an equilibrium of OAA with glutamate, aspartate, and 2-oxoglutarate. The concentrations of OAA were calculated from the GOT equilibrium, (oxoglutarate × Asp)/(Glu × 6.61), as carried out by Heineke et al. (1991), based on the equilibrium constant of K = 6.61 (Veech et al., 1969). Under these conditions, we obtained with peroxisomes alone, and peroxisomes plus mitochondria, essentially the same time course of glycerate synthesis as shown in Figure 3 (data not shown). The rate of glycerate formation by peroxisomes alone was less than one-sixth of that when mitochondria were also present.
In the system containing peroxisomes alone, the malate content did not change much from the original level of 1 mm, and the ratio of malate to OAA remained as low as 5 (Table I). However, in the reconstituted system of mitochondria and peroxisomes, the level of malate increased from the initial 1 mm (at the beginning of incubation) to almost 1.4 mm by 20 min and then decreased slightly. At the same time, the level of OAA decreased to 13 μm by 40 min and stayed low. At 40 and 90 min, when the rate of glycerate formation was maximal, the ratio of malate to OAA was 101 and 110, respectively.
Table I.
Correlation between the rate of glycerate formation by the reconstituted system of peroxisomes and mitochondria and the concentration ratio of malate to OAA
| Metabolite | Peroxisomes
Alone
|
Peroxisomes + Mitochondria
|
||
|---|---|---|---|---|
| 40 mina | 20 min | 40 min | 90 min | |
| mm | ||||
| Malate | 1.08 | 1.37 | 1.31 | 1.21 |
| Aspartate | 0.236 | 0.115 | 0.109 | 0.088 |
| Glutamate | 0.048 | 0.082 | 0.147 | 0.220 |
| 2-Oxoglutarate | 0.273 | 0.187 | 0.113 | 0.180 |
| OAA (calculated) | 0.20 | 0.040 | 0.013 | 0.011 |
| Malate/OAA | 5.4 | 34 | 101 | 110 |
| μmol mg−1 peroxisomal protein | ||||
| Rate of glycerate formation | 2.6 | 5.7 | 25 | 25 |
The experimental conditions were the same as in Figure 2, the only difference being that the medium also contained 0.25 mm glutamate and 10 U/mL GOT. The concentrations of aspartate, glutamate, 2-oxoglutarate, and malate were determined enzymatically. The concentration of OAA was evaluated from the concentrations of aspartate, 2-oxoglutarate, and glutamate according to the GOT equilibrium (see Methods).
Time after start of reaction.
Intactness of organelles was essential for the beneficial interaction of peroxisomes and mitochondria during glycerate formation. Therefore, control experiments were performed in the presence of 0.1% (v/v) Triton X-100. In the presence of detergent, the rate of glycerate formation by the reconstituted system of peroxisomes plus mitochondria decreased by 95% (data not shown), demonstrating that high rates of glycerate formation were dependent on the intactness of the peroxisomes and mitochondria.
DISCUSSION
In the reconstituted system of isolated peroxisomes and mitochondria, the rate of glycerate formation was consistently high and sustainable, even when Ser was not added (Fig. 2). The addition of Ser only slightly enhanced glycerate formation (Fig. 3). The rate of glycerate formation with peroxisomes alone was quite low (Figs. 2 and 3; Table I), indicating that mitochondrial metabolism was required to achieve high rates of glycerate production. The data in Table I demonstrate the dynamic status of metabolites and the effective movement between the medium and these two organelles. A model of the flow of metabolites between the peroxisomes and mitochondria in our experimental system is shown in Figure 1.
A comparison of the malate-to-OAA ratio with the rate of glycerate formation in the same system (Table I) suggests that high rates of glycerate production were achieved as the ratio of malate to OAA increased. The ratio of malate to OAA was about 100 when the system was in equilibrium (producing glycerate at linear rates) during the 40 to 90 min of incubation (Table I). When malate dehydrogenase is at equilibrium, according to the following equation,
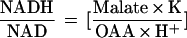 |
where K = 2.78 × 10−12 (Veech et al., 1969) and H+ = 10−7.5, a malate-to-OAA ratio of 100 corresponds to an NADH:NAD ratio of 8.8 × 10−3. In previous studies in which nonaqueous subcellular fractionation was used, the NADH-to-NAD ratio in the cytosol of illuminated spinach leaves was estimated to be on the order of 10−3 (Heineke et al., 1991). Taking into account that some error is involved in these estimations of redox potentials, the present results indicate that the reduction of hydroxypyruvate in the peroxisomes can proceed at the very low NADH-to-NAD ratio prevailing in the cytosol of mesophyll cells of illuminated, intact leaves.
After the initial lag phase, the average rate of glycerate formation by the reconstituted system of peroxisomes and mitochondria reached 25 μmol mg−1 peroxisomal protein h−1 (Table I). Assuming a ratio of 1.04 mg peroxisomal protein mg−1 chlorophyll in spinach leaf (Heupel et al., 1991), the rate of glycerate formation in our system can be calculated to be 26 μmol mg−1 chlorophyll h−1. The rate of glycerate formation in a photosynthesizing leaf at ambient CO2 is expected to be about 20 μmol mg−1 chlorophyll h−1 (Heupel et al., 1991; Reumann et al., 1994). Thus, the determined rate of glycerate formation is close to the physiological demand.
ACKNOWLEDGMENTS
We wish to acknowledge the kind help of Dr. Dieter Heineke, Dr. Ralf Heupel, Dr. Iris Hanning, Ms. Helma Lindemann (for help with preparation of peroxisomes/mitochondria, amino acid analyses, and for stimulating discussions), and Ms. Monica Raabe (for excellent technical assistance).
Abbreviations:
- GOT
glutamate oxaloacetate transaminase
- HPR
hydroxypyruvate reductase
- OAA
oxaloacetate
- U
μmol min−1
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (H.W.H.) and a fellowship from the Alexander von Humboldt-Stiftung to A.S.R.
LITERATURE CITED
- Bergmeyer HU (1983) Methods of Enzymatic Analysis, Ed 3. Verlag Chemie, Weinheim, Germany
- Day DA, Wiskich JT. Transport processes in isolated plant mitochondria. Physiol Veg. 1984;22:241–261. [Google Scholar]
- Douce R, Bonner WD. Oxaloacetate control of Krebs cycle oxidation in purified plant mitochondria. Biochem Biophys Res Commun. 1972;47:619–624. doi: 10.1016/0006-291x(72)90923-0. [DOI] [PubMed] [Google Scholar]
- Ebbighausen H, Chen J, Heldt HW. Oxaloacetate translocator in plant mitochondria. Biochim Biophys Acta. 1985;810:184–199. [Google Scholar]
- Flügge U-I, Benz R. Pore forming activity in the outer membrane of the chloroplast envelope. FEBS Lett. 1984;169:85–89. [Google Scholar]
- Hanning I, Heldt HW. On the function of mitochondrial metabolism during photosynthesis in spinach leaves (Spinacia oleracea L.). Partitioning between respiration and export of redox equivalents and precursors for nitrate assimilation products. Plant Physiol. 1993;103:1147–1154. doi: 10.1104/pp.103.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD, Dröscher L, Flügge UI, Heldt HW. A specific translocator for oxaloacetate transport in chloroplasts. FEBS Lett. 1984;178:15–19. [Google Scholar]
- Heineke D, Riens B, Grosse H, Hoferichter P, Peter U, Flügge UI, Heldt HW. Redox transfer across the inner chloroplast envelope membrane. Plant Physiol. 1991;95:1131–1137. doi: 10.1104/pp.95.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heupel R, Heldt HW. Protein organization in the matrix of leaf peroxisomes. A multienzyme complex involved in photorespiratory metabolism. Eur J Biochem. 1994;220:165–172. doi: 10.1111/j.1432-1033.1994.tb18611.x. [DOI] [PubMed] [Google Scholar]
- Heupel R, Markgraf T, Robinson DG, Heldt HW. Compartmentation studies on spinach leaf peroxisomes. Evidence for channeling of photorespiratory metabolites in peroxisomes devoid of intact boundary membrane. Plant Physiol. 1991;96:971–979. doi: 10.1104/pp.96.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krömer S, Heldt HW. Respiration of pea leaf mitochondria and redox transfer between the mitochondrial and extramitochondrial compartment. Biochim Biophys Acta. 1991;1057:42–50. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Neuberger M, Journet E-P, Bligny R, Carde JP, Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982;217:312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Reumann S, Heupel R, Heldt HW. Compartmentation studies on spinach leaf peroxisomes. II. Evidence for the transfer of reductant from the cytosol to peroxisomal compartment via malate-oxaloacetate shuttle. Planta. 1994;193:167–173. [Google Scholar]
- Reumann S, Maier E, Benz R, Heldt HW. The membrane of leaf peroxisomes contains a porin-like channel. J Biol Chem. 1995;270:17559–17565. doi: 10.1074/jbc.270.29.17559. [DOI] [PubMed] [Google Scholar]
- Reumann S, Maier E, Benz R, Heldt HW. A specific porin is involved in the malate shuttle of leaf peroxisomes. Biochem Soc Trans. 1996;24:754–757. doi: 10.1042/bst0240754. [DOI] [PubMed] [Google Scholar]
- Reumann S, Maier E, Benz R, Heldt HW (1998) Permeability properties of the porin of spinach leaf peroxisomes. Eur J Biochem (in press) [DOI] [PubMed]
- Schmid A, Krömer S, Heldt HW, Benz R. Identification of two general diffusion channels in the outer membrane of pea mitochondria. Biochem Biophys Acta. 1992;1112:174–180. doi: 10.1016/0005-2736(92)90389-4. [DOI] [PubMed] [Google Scholar]
- Schmitt MR, Edwards GE. Glycerate kinase from leaves of C3 plants. Arch Biochem Biophys. 1983;224:332–341. doi: 10.1016/0003-9861(83)90217-5. [DOI] [PubMed] [Google Scholar]
- Sharkey DT. Estimating the rate of photorespiration in leaves. Physiol Plant. 1988;73:147–152. [Google Scholar]
- Tolbert NE (1980) Microbodies—peroxisomes and glyoxysomes. In NE Tolbert, ed, The Biochemistry of Plants, Vol 1. Academic Press, New York, pp 359–388
- Veech RL, Eggleston LV, Krebs HA. The redox state of the nicotinamide-adenine-dinucleotide phosphate in the cytoplasm of rat liver. Biochem J. 1969;115:609–619. doi: 10.1042/bj1150609a. [DOI] [PMC free article] [PubMed] [Google Scholar]