Abstract
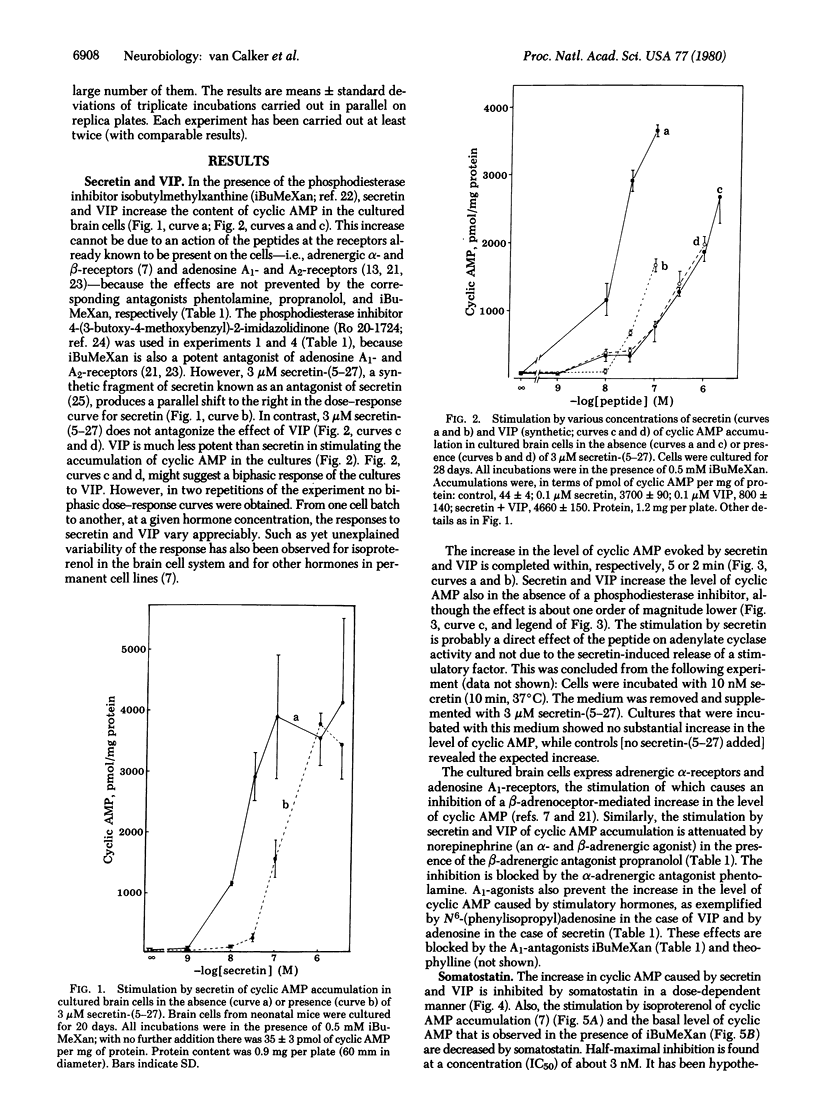
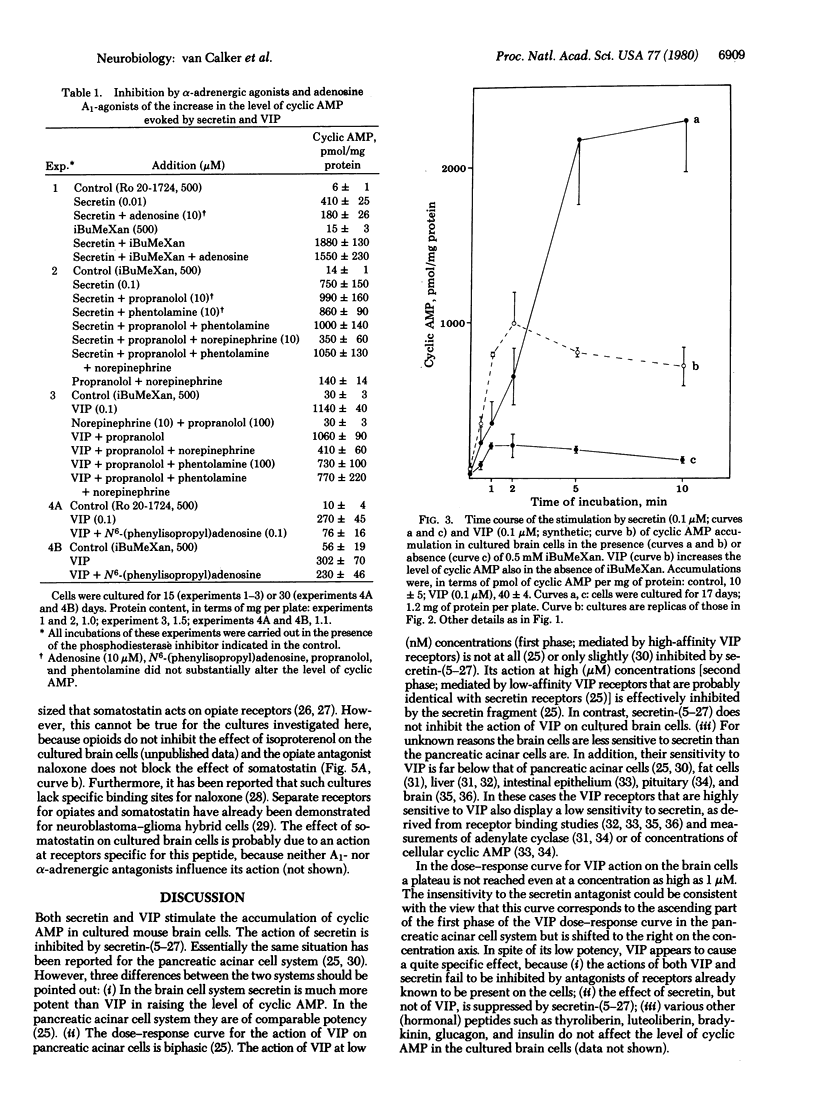
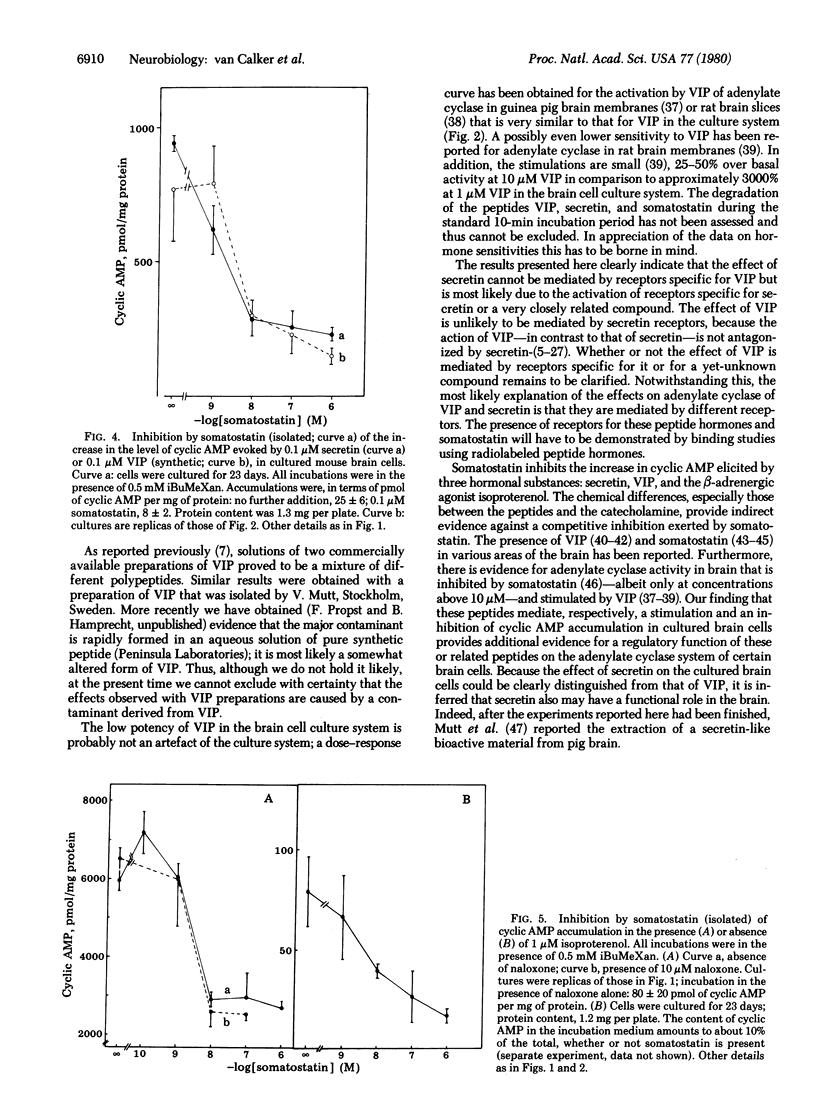
Secretin stimulates the accumulation of cyclic AMP (half maximally stimulating concentration: 10-20 nM) in cultured mouse brain cells mainly consisting of glioblasts. Vasoactive intestinal peptide (VIP) is much less potent in raising the level of cyclic AMP in these cultures. The effect of secretin but not that of VIP is inhibited by secretin-(5-27), a synthetic antagonist of secretin. Stimulation of the adrenergic alpha-receptors and the adenosine A1-receptors present on the cells attenuates the increase in cyclic AMP evoked by secretin and VIP. Somatostatin at low concentrations inhibits the accumulation of cyclic AMP (half-maximally inhibitory concentration: 3 nM), in the absence or presence of secretin, VIP, or isoproterenol. The results suggest that secretin might regulate the concentration of cyclic AMP in brain and provoke the question of a possible involvement of glial cells in the action of peptide hormones in the brain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bock E., Jorgensen O. S., Dittmann L., Eng L. F. Determination of brain-specific antigens in short term cultivated rat astroglial cells and in rat synaptosomes. J Neurochem. 1975 Dec;25(6):867–870. doi: 10.1111/j.1471-4159.1975.tb04419.x. [DOI] [PubMed] [Google Scholar]
- Bock E., Moller M., Nissen C., Sensenbrenner M. Glial fibrillary acidic protein in primary astroglial cell cultures derived from newborn rat brain. FEBS Lett. 1977 Nov 15;83(2):207–211. doi: 10.1016/0014-5793(77)81006-5. [DOI] [PubMed] [Google Scholar]
- Booher J., Sensenbrenner M. Growth and cultivation of dissociated neurons and glial cells from embryonic chick, rat and human brain in flask cultures. Neurobiology. 1972;2(3):97–105. [PubMed] [Google Scholar]
- Borghi C., Nicosia S., Giachetti A., Said S. I. Vasoactive intestinal polypeptide (VIP) stimulates adenylate cyclase in selected areas of rat brain. Life Sci. 1979 Jan 1;24(1):65–70. doi: 10.1016/0024-3205(79)90281-9. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Breen G. A., de Vellis J. Regulation of glycerol phosphate dehydrogenase by hydrocortisone in dissociated rat cerebral cell cultures. Dev Biol. 1974 Dec;41(2):255–266. doi: 10.1016/0012-1606(74)90304-2. [DOI] [PubMed] [Google Scholar]
- Brownstein M., Arimura A., Sato H., Schally A. V., Kizer J. S. The regional distribution of somatostatin in the rat brain. Endocrinology. 1975 Jun;96(6):1456–1461. doi: 10.1210/endo-96-6-1456. [DOI] [PubMed] [Google Scholar]
- Bryant M. G., Polak M. M., Modlin I., Bloom S. R., Albuquerque R. H., Pearse A. G. Possible dual role for vasoactive intestinal peptide as gastrointestinal hormone and neurotransmitter substance. Lancet. 1976 May 8;1(7967):991–993. doi: 10.1016/s0140-6736(76)91863-8. [DOI] [PubMed] [Google Scholar]
- Desbuguois B., Laudat M. H., Laudat P. Vasoactive intestinal polypeptide and glucagon: stimulation of adenylate cyclase activity via distinct receptors in liver and fat cell membranes. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1187–1194. doi: 10.1016/0006-291x(73)90590-1. [DOI] [PubMed] [Google Scholar]
- Desbuquois B. The interaction of vasoactive intestinal polypeptide and secretin with liver-cell membranes. Eur J Biochem. 1974 Aug 1;46(3):439–450. doi: 10.1111/j.1432-1033.1974.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., Christophe J. Characterization of VIP-sensitive adenylate cyclase in guinea pig brain. FEBS Lett. 1977 Nov 1;83(1):76–80. doi: 10.1016/0014-5793(77)80645-5. [DOI] [PubMed] [Google Scholar]
- Gainer H. Peptides and neuronal function. Adv Biochem Psychopharmacol. 1976;15:193–210. [PubMed] [Google Scholar]
- Gardner J. D., Rottman A. J., Natarajan S., Bodanszky M. Interaction of secretin5-27 and its analogues with hormone receptors on pancreatic acini. Biochim Biophys Acta. 1979 Apr 3;583(4):491–503. doi: 10.1016/0304-4165(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Giachetti A., Said S. I., Reynolds R. C., Koniges F. C. Vasoactive intestinal polypeptide in brain: localization in and release from isolated nerve terminals. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3424–3428. doi: 10.1073/pnas.74.8.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamprecht B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Int Rev Cytol. 1977;49:99–170. doi: 10.1016/s0074-7696(08)61948-8. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Efendic S., Johansson O., Luft R., Arimura A. Immunohistochemical localization of somatostatin (growth hormone release-inhibiting factor) in the guinea pig brain. Brain Res. 1974 Nov 8;80(1):165–169. doi: 10.1016/0006-8993(74)90737-9. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Nicoll R. A., Vale W. W. Neurobiology of peptides. Neurosci Res Program Bull. 1978 Jun;16(2):209–370. [PubMed] [Google Scholar]
- Laburthe M., Prieto J. C., Amiranoff B., Dupont C., Hui Bon Hoa D., Rosselin G. Interaction of vasoactive intestinal peptide with isolated intestinal epithelial cells from rat. 2. Characterization and structural requirements of the stimulatory effect of vasoactive intestinal peptide on production of adenosine 3':5'-monophosphate. Eur J Biochem. 1979 May 15;96(2):239–248. doi: 10.1111/j.1432-1033.1979.tb13034.x. [DOI] [PubMed] [Google Scholar]
- Lim R., Mitsunobu K., Li W. K. Maturation-stimulation effect of brain extract and dibutyryl cyclic AMP on dissociated embryonic brain cells in culture. Exp Cell Res. 1973 Apr;79(1):243–246. [PubMed] [Google Scholar]
- Lim R., Turriff D. E., Troy S. S., Moore B. W., Eng L. F. Glia maturation factor: effect on chemical differentiation of glioblasts in culture. Science. 1977 Jan 14;195(4274):195–196. doi: 10.1126/science.188136. [DOI] [PubMed] [Google Scholar]
- Martin J. B., Audet J., Saunders A. Effects of somatostatin and hypothalamic ventromedial lesions on GH release induced by morphine. Endocrinology. 1975 Apr;96(4):839–847. doi: 10.1210/endo-96-4-839. [DOI] [PubMed] [Google Scholar]
- Mutt V., Carlquist M., Tatemoto K. Secretin-like bioactivity in extracts of porcine brain. Life Sci. 1979 Nov 12;25(20):1703–1707. doi: 10.1016/0024-3205(79)90472-7. [DOI] [PubMed] [Google Scholar]
- Propst F., Moroder L., Wünsch E., Hamprecht B. The influence of secretin, glucagon and other peptides, of amino acids, prostaglandin endoperoxide analogues and diazepam on the level of adenosine 3',5'-cyclic monophosphate in neuroblastoma x glioma hybrid cells. J Neurochem. 1979 May;32(5):1495–1500. doi: 10.1111/j.1471-4159.1979.tb11090.x. [DOI] [PubMed] [Google Scholar]
- Quik M., Iversen L. L., Bloom S. R. Effect of vasoactive intestinal peptide (VIP) and other peptides on cAMP accumulation in rat brain. Biochem Pharmacol. 1978;27(18):2209–2213. doi: 10.1016/0006-2952(78)90079-5. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Conlon T. P., Gardner J. D. Interaction of porcine vasoactive intestinal peptide with dispersed pancreatic acinar cells from the guinea pig. Structural requirements for effects of vasoactive intestinal peptide and secretin on cellular adenosine 3':5'-monophosphate. J Biol Chem. 1976 Aug 10;251(15):4635–4639. [PubMed] [Google Scholar]
- Robberecht P., De Neef P., Lammens M., Deschodt-Lanckman M., Christophe J. P. Specific binding of vasoactive intestinal peptide to brain membranes from the guinea pig. Eur J Biochem. 1978 Sep 15;90(1):147–154. doi: 10.1111/j.1432-1033.1978.tb12585.x. [DOI] [PubMed] [Google Scholar]
- Said S. I., Rosenberg R. N. Vasoactive intestinal polypeptide: abundant immunoreactivity in neural cell lines and normal nervous tissue. Science. 1976 May 28;192(4242):907–908. doi: 10.1126/science.1273576. [DOI] [PubMed] [Google Scholar]
- Schousboe A., Fosmark H., Svenneby G. Taurine uptake in astrocytes cultured from dissociated mouse brain hemispheres. Brain Res. 1976 Oct 29;116(1):158–164. doi: 10.1016/0006-8993(76)90258-4. [DOI] [PubMed] [Google Scholar]
- Schousboe A., Svenneby G., Hertz L. Uptake and metabolism of glutamate in astrocytes cultured from dissociated mouse brain hemispheres. J Neurochem. 1977 Dec;29(6):999–1005. doi: 10.1111/j.1471-4159.1977.tb06503.x. [DOI] [PubMed] [Google Scholar]
- Shapiro D. L. Morphological and biochemical alterations in foetal rat brain cells cultured in the presence of monobutyryl cyclic AMP. Nature. 1973 Jan 19;241(5386):203–204. doi: 10.1038/241203a0. [DOI] [PubMed] [Google Scholar]
- Sheppard H., Wiggan G. Analogues of 4-(3,4-dimethoxybenzyl)-2-imidazolidinone as potent inhibitors of rat erythrocyte adenosine cyclic 3',5'-phosphate phosphodiesterase. Mol Pharmacol. 1971 Jan;7(1):111–115. [PubMed] [Google Scholar]
- Taylor D. P., Pert C. B. Vasoactive intestinal polypeptide: specific binding to rat brain membranes. Proc Natl Acad Sci U S A. 1979 Feb;76(2):660–664. doi: 10.1073/pnas.76.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius L. Somatostatin and ACTH are peptides with partial antagonist-like selectivity for opiate receptors. Eur J Pharmacol. 1976 Jul;38(1):211–213. doi: 10.1016/0014-2999(76)90221-1. [DOI] [PubMed] [Google Scholar]
- Traber J., Glaser T., Brandt M., Klebensberger W., Hamprecht B. Different receptors for somatostatin and opioids in neuroblastoma X glioma hybrid cells. FEBS Lett. 1977 Sep 15;81(2):351–354. doi: 10.1016/0014-5793(77)80552-8. [DOI] [PubMed] [Google Scholar]
- Van Calker D., Müller M., Hamprecht B. Adrenergic alpha- and beta-receptors expressed by the same cell type in primary culture of perinatal mouse brain. J Neurochem. 1978 Apr;30(4):713–718. doi: 10.1111/j.1471-4159.1978.tb10776.x. [DOI] [PubMed] [Google Scholar]
- Wu P. H., Durden D. A., Hertz L. Net production of gamma-aminobutyric acid in astrocytes in primary cultures determined by a sensitive mass spectrometric method. J Neurochem. 1979 Feb;32(2):379–390. doi: 10.1111/j.1471-4159.1979.tb00361.x. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine inhibits the accumulation of cyclic AMP in cultured brain cells. Nature. 1978 Dec 21;276(5690):839–841. doi: 10.1038/276839a0. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


