Abstract
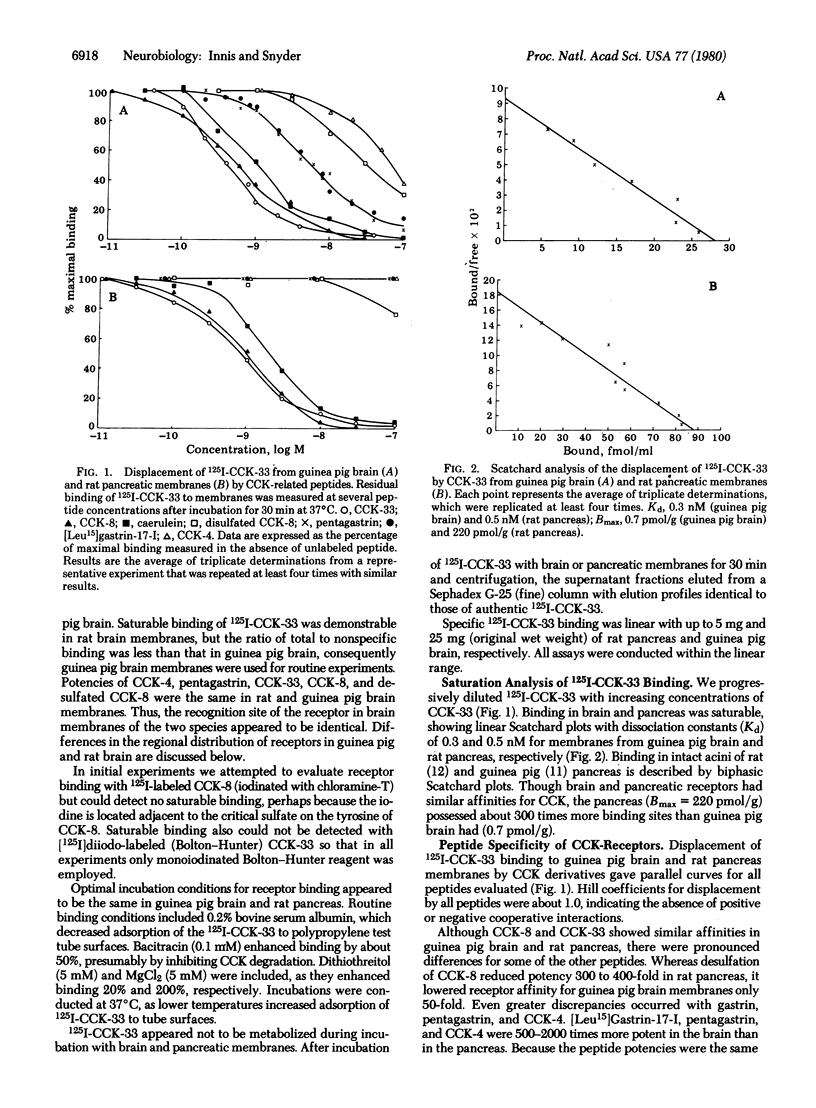
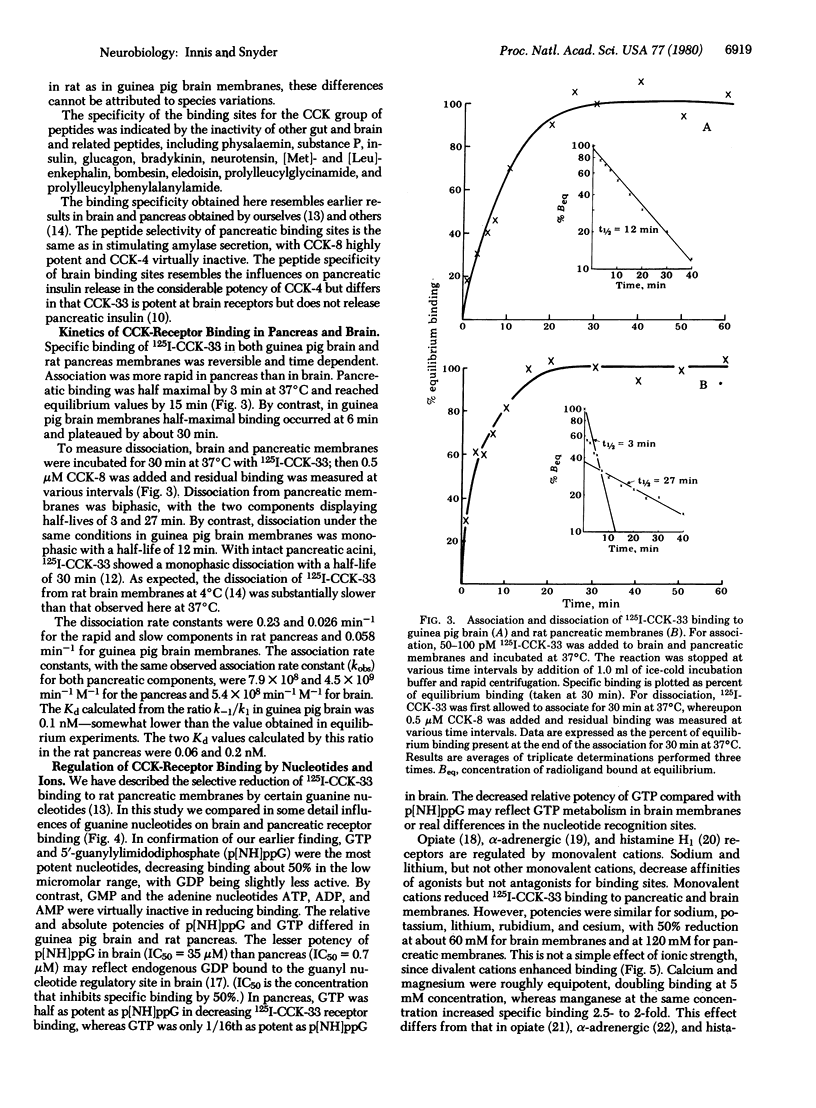
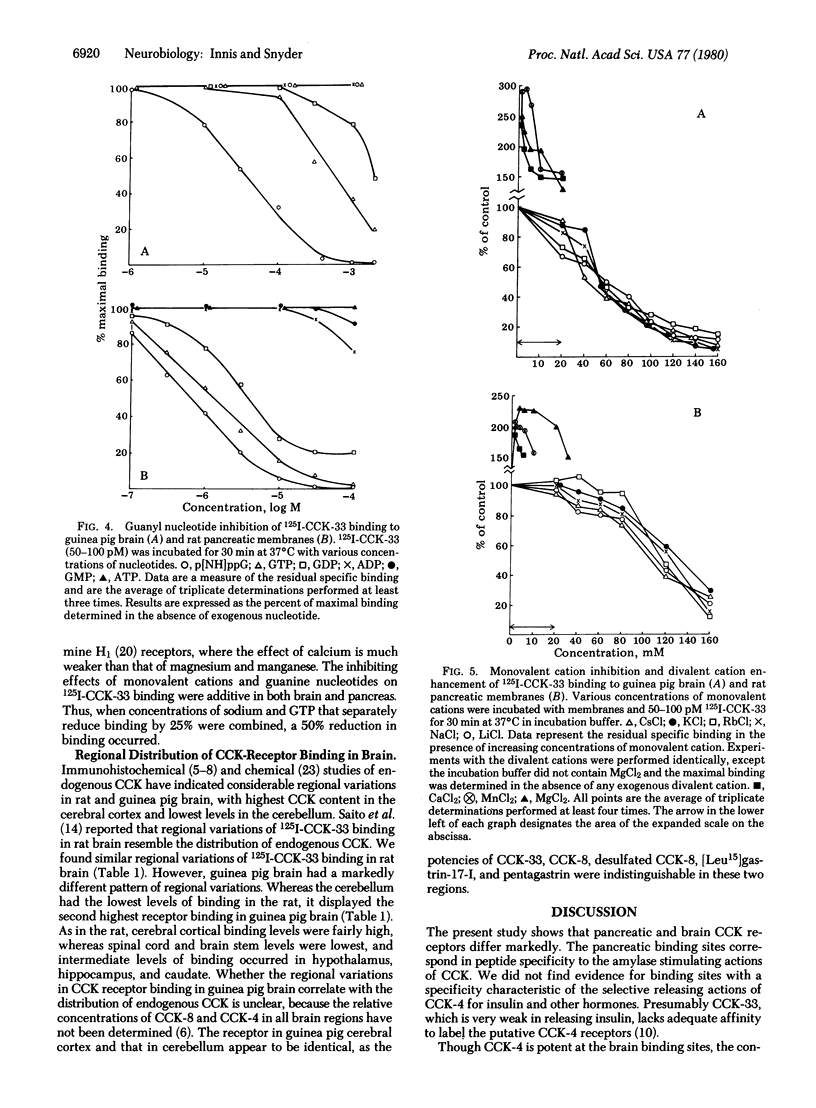
125I-Labeled (Bolton-Hunter) cholecystokinin triacontatriapeptide (CCK-33) binds saturably and reversibly to distinct receptors in brain and pancreatic membranes. The peptide specificity of pancreatic CCK binding is the same as that for pancreatic amylase release. In brain, gastrin and pentagastrin display nanomolar affinity for binding sites, whereas in pancreas these two peptides are virtually inactive. Though these differences indicate that brain and pancreas possess distinct CCK receptors, the two tissues show some similarities. Both pancreas and brain receptors show greater sensitivity to sulfated than to desulfated COOH-terminal octapeptide of CCK and display dissociation constants of 0.3-9.5 nM. The pancreas possesses about 300 times more binding sites than does brain. CCK binding in both brain and pancreas is enhanced by divalent cations and reduced by monovalent cations. Receptor binding in both tissues is regulated in a selective fashion by guanyl nucleotides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang K. J., Cooper B. R., Hazum E., Cuatrecasas P. Multiple opiate receptors: different regional distribution in the brain and differential binding of opiates and opioid peptides. Mol Pharmacol. 1979 Jul;16(1):91–104. [PubMed] [Google Scholar]
- Chang R. S., Synder S. H. Histamine H1-receptor binding sites in guinea pig brain membranes: regulation of agonist interactions by guanine nucleotides and cations. J Neurochem. 1980 Apr;34(4):916–922. doi: 10.1111/j.1471-4159.1980.tb09666.x. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Hahne W. F. Calcium transport in dispersed acinar cells from rat pancreas. Biochim Biophys Acta. 1977 Dec 15;471(3):466–476. doi: 10.1016/0005-2736(77)90050-5. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Baukal A., Catt K. J. Angiotensin II receptors in bovine adrenal cortex. Modification of angiotensin II binding by guanyl nucleotides. J Biol Chem. 1974 Jan 25;249(2):664–666. [PubMed] [Google Scholar]
- Greenberg D. A., U'Prichard D. C., Sheehan P., Snyder S. H. alpha-Noradrenergic receptors in the brain: differential effects of sodium on binding of [3H]agonists and [3H]antagonists. Brain Res. 1978 Jan 27;140(2):378–384. doi: 10.1016/0006-8993(78)90472-9. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Corrêa F. M., Uhl G. R., Schneider B., Snyder S. H. Cholecystokinin octapeptide-like immunoreactivity: histochemical localization in rat brain. Proc Natl Acad Sci U S A. 1979 Jan;76(1):521–525. doi: 10.1073/pnas.76.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R. B., Synder S. H. Cholecystokinin receptor binding in brain and pancreas: regulation of pancreatic binding by cyclic and acyclic guanine nucleotides. Eur J Pharmacol. 1980 Jul 11;65(1):123–124. doi: 10.1016/0014-2999(80)90221-6. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2079–2083. doi: 10.1073/pnas.77.4.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. M., Nielsen T. B., Preston M. S., Rodbell M. The role of the guanine nucleotide exchange reaction in the regulation of the beta-adrenergic receptor and in the actions of catecholamines and cholera toxin on adenylate cyclase in turkey erythrocyte membranes. J Biol Chem. 1980 Feb 10;255(3):988–995. [PubMed] [Google Scholar]
- Larsson L. I., Childers S., Snyder S. H. Met- and Leu-enkephalin immunoreactivity in separate neurones. Nature. 1979 Nov 22;282(5737):407–410. doi: 10.1038/282407a0. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F. Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res. 1979 Apr 13;165(2):201–218. doi: 10.1016/0006-8993(79)90554-7. [DOI] [PubMed] [Google Scholar]
- Lopatin R. N., Gardner J. D. Effects of calcium and chelating agents on the ability of various agonists to increase cyclic GMP in pancreatic acinar cells. Biochim Biophys Acta. 1978 Nov 1;543(4):465–475. doi: 10.1016/0304-4165(78)90301-x. [DOI] [PubMed] [Google Scholar]
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Lorén I., Alumets J., Håkanson R., Sundler F. Distribution of gastrin and CCK-like peptides in rat brain. An immunocytochemical study. Histochemistry. 1979 Feb 21;59(4):249–257. doi: 10.1007/BF00689607. [DOI] [PubMed] [Google Scholar]
- Muller J. E., Straus E., Yalow R. S. Cholecystokinin and its COOH-terminal octapeptide in the pig brain. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3035–3037. doi: 10.1073/pnas.74.7.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak G. W., Snowman A. M., Snyder S. H. Selective enhancement of [3H]opiate agonist binding by divalent cations. Mol Pharmacol. 1975 Nov;11(6):735–744. [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978 Jun 10;253(11):4022–4030. [PubMed] [Google Scholar]
- Rehfeld J. F., Larsson L. I., Goltermann N. R., Schwartz T. W., Holst J. J., Jensen S. L., Morley J. S. Neural regulation of pancreatic hormone secretion by the C-terminal tetrapeptide of CCK. Nature. 1980 Mar 6;284(5751):33–38. doi: 10.1038/284033a0. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Deschodt-Lanckman M., Woussen-Colle M. C., De Neef P., Camus J. C., Christophe J. Butyryl derivatives of cyclic GMP interfere with the biological and the immunological properties of the pancreozymin-gastrin family of peptides. Mol Pharmacol. 1980 Mar;17(2):268–274. [PubMed] [Google Scholar]
- Saito A., Sankaran H., Goldfine I. D., Williams J. A. Cholecystokinin receptors in the brain: characterization and distribution. Science. 1980 Jun 6;208(4448):1155–1156. doi: 10.1126/science.6246582. [DOI] [PubMed] [Google Scholar]
- Sankaran H., Deveney C. W., Goldfine I. D., Williams J. A. Preparation of biologically active radioiodinated cholecystokinin for radioreceptor assay and radioimmunoassay. J Biol Chem. 1979 Oct 10;254(19):9349–9351. [PubMed] [Google Scholar]
- Sankaran H., Goldfine I. D., Deveney C. W., Wong K. Y., Williams J. A. Binding of cholecystokinin to high affinity receptors on isolated rat pancreatic acini. J Biol Chem. 1980 Mar 10;255(5):1849–1853. [PubMed] [Google Scholar]
- Schneider B. S., Monahan J. W., Hirsch J. Brain cholecystokinin and nutritional status in rats and mice. J Clin Invest. 1979 Nov;64(5):1348–1356. doi: 10.1172/JCI109591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz I., Stolze H. H. The exocrine pancreas: the role of secretagogues, cyclic nucleotides, and calcium in enzyme secretion. Annu Rev Physiol. 1980;42:127–156. doi: 10.1146/annurev.ph.42.030180.001015. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Goodman R. R. Multiple neurotransmitter receptors. J Neurochem. 1980 Jul;35(1):5–15. doi: 10.1111/j.1471-4159.1980.tb12483.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Speir G. R., Johnson L. R. Mucosal gastrin receptor. I. Assay standardization and fulfillment of receptor criteria. Am J Physiol. 1979 Sep;237(3):E284–E294. doi: 10.1152/ajpendo.1979.237.3.E284. [DOI] [PubMed] [Google Scholar]
- U'Prichard D. C., Snyder S. H. Interactions of divalent cations and guanine nucleotides at alpha 2-noradrenergic receptor binding sites in bovine brain mechanisms. J Neurochem. 1980 Feb;34(2):385–394. doi: 10.1111/j.1471-4159.1980.tb06608.x. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Lotstra F., De Mey J., Gilles C. Immunohistochemical localization of cholecystokinin- and gastrin-like peptides in the brain and hypophysis of the rat. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1190–1194. doi: 10.1073/pnas.77.2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Signeau J. C., Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975 Oct 16;257(5527):604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]



