Abstract
Previous studies have demonstrated that certain flavonoids can have an inhibitory effect on angiotensin-converting enzyme (ACE) activity, which plays a key role in the regulation of arterial blood pressure. In the present study, 17 flavonoids belonging to five structural subtypes were evaluated in vitro for their ability to inhibit ACE in order to establish the structural basis of their bioactivity. The ACE inhibitory (ACEI) activity of these 17 flavonoids was determined by fluorimetric method at two concentrations (500 µM and 100 µM). Their inhibitory potencies ranged from 17 to 95% at 500 µM and from 0 to 57% at 100 µM. In both cases, the highest ACEI activity was obtained for luteolin. Following the determination of ACEI activity, the flavonoids with higher ACEI activity (i.e., ACEI >60% at 500 µM) were selected for further IC50 determination. The IC50 values for luteolin, quercetin, rutin, kaempferol, rhoifolin and apigenin K were 23, 43, 64, 178, 183 and 196 µM, respectively. Our results suggest that flavonoids are an excellent source of functional antihypertensive products. Furthermore, our structure-activity relationship studies show that the combination of sub-structures on the flavonoid skeleton that increase ACEI activity is made up of the following elements: (a) the catechol group in the B-ring, (b) the double bond between C2 and C3 at the C-ring, and (c) the cetone group in C4 at the C-ring. Protein-ligand docking studies are used to understand the molecular basis for these results.
Introduction
Cardiovascular disease (CVD) is the most important cause of death among the industrialized societies [1]. Hypertension, which is estimated to affect one-third of the Western population, is one of the major risk factors for CVD [2]. In spite of its significance, hypertension remains poorly controlled [3], and approximately two-thirds of hypertension is undetected or inadequately treated [4]. Lifestyle modifications, including changes in dietary habits, have substantial effects on risk factors for CVD, such as hypertension [5].
The renin–angiotensin–aldosterone system is a key factor in the maintenance of arterial blood pressure. One of its main components is the angiotensin-converting enzyme (ACE) [EC 3.4.15.1] [6], which is a glycosylated zinc dipeptidyl-carboxypeptidase whose main function is to regulate arterial blood pressure and electrolyte balance through the renin–angiotensin–aldosterone system [7]. There are two isoforms of ACE that are transcribed from the same gene in a tissue-specific manner. In somatic tissues, ACE exists as a glycoprotein composed of a single large polypeptide chain of 1,277 amino acids, whereas in sperm cells, it occurs as a lower-molecular-mass glycoform of 701 amino acids. The somatic form consists of two homologous domains (the N and C domains), each of which contains an active site with a conserved HEXXH zinc-binding motif [8], where the Zn2+ is bound to the two motif histidines as well as to a glutamate 24 residue downstream the last motif histidine [9]. The testis ACE (tACE) is identical to the C-terminal half of somatic ACE, with the exception of a unique 36-residue sequence that constitutes its amino terminus [10]. The two domains differ in their substrate specificities, inhibitor and chloride activation profiles, and physiological functions [11]. Thus, mice expressing only the N domain of ACE show a low blood pressure phenotype that is very similar to ACE knockout mice [12], and ACE inhibition with an N-domain-specific inhibitor (i.e., RXP407) has no effect on blood pressure regulation [13]. On the other hand, mice that are homozygous for a mutation that inactivates the somatic ACE N domain, but not the C domain, retained a phenotype that was indistinguishable from that of wild-type mice with regards to blood pressure and renal function [14]. Therefore, the inhibition of the C domain appears to be necessary and sufficient for the control of blood pressure and cardiovascular function, which suggests that the C domain is the dominant angiotensin-converting site. As an exopeptidase, ACE catalyzes the conversion of angiotensin I into the potent vasoconstrictor angiotensin II [15]. In addition, ACE catalyzes the inactivation of the vasodilator bradykinin [16]. Therefore, the inhibition of this enzyme can generate an antihypertensive effect. In fact, synthetic ACE inhibitors, such as captopril and enalapril, are widely used for the treatment of cardiovascular and renal disease, for the secondary prevention of coronary artery disease, and for the treatment of heart failure [17]. However, side effects such as cough, angioneurotic edema and deleterious effects in pregnancy have been associated with the clinical use of ACE inhibitors [18], [19]. Therefore, the investigation of new, natural product-based ACE inhibitors could greatly benefit hypertensive patients.
A number of extracts and compounds obtained from plants have been identified as in vitro ACE inhibitors [20], [21]. These beneficial effects have largely been ascribed to the presence of flavonoid molecules, which generation of chelate complexes within the active center of ACE [22]. Flavonoids are polyphenol molecules of low molecular weight; the basic structure is a 2-phenyl benzopyrone in which the three-carbon bridge between the phenyl groups is usually cyclized oxygen [23], [24]. Flavonoids can be differentiated into several subfamilies according to their degree of unsaturation and the degree of oxidation of the oxygenated heterocycle and can be characterized as flavanones, flavones, flavonols, isoflavones, flavanols (essentially, flavan-3-ols) and anthocyanidins, all of which are the most relevant for the human diet [23], [25]. Different studies have revealed the important role that flavonoid structure plays in its biological function; the position and number of substituents in the flavonoid basic structure significantly affects the antiproliferative, cytotoxic, antioxidant, and anti-enzymatic activities of such molecules [26]–[28].
Previous studies have shown that certain flavonoids exhibit a capacity to inhibit different zinc metalloproteinases [29], [30], including ACE. In fact, micromolar concentrations of different flavonoids, such as anthocyanins [31], [32], flavones [33], flavonols [33]–[35], and flavanols [36], have been shown to inhibit 50% of ACE activity. Furthermore, the ACE-inhibitory (ACEI) activity of different foods and plant extracts rich in flavonoids has also been demonstrated by in vitro [37], [38], studies and by in vivo studies in hypertensive rats [39], [40] and humans [41]. The preliminary structure-activity relationships (SAR) studied in some flavonoids (flavanols and flavonols) generally attribute the observed effect either to the distribution of free hydroxyl groups [33], [35], [42] or in the number of monomers units forming the corresponding procyanidins [36]. However, the key molecular flavonoid sub-structures that dictate effective ACE inhibition activity have not yet been characterized.
The objective of this work was to define the key flavonoid structural elements that are required for ACE inhibition activity through the determination of the ability of 17 flavonoids belonging to five structural subtypes (i.e., 5 flavanones, 2 flavan-3-ols, 1 isoflavone, 6 flavones and 3 flavonols; including potassium salts for 1 flavanone and for 1 flavone) to inhibit ACE. To achieve this goal, the in vitro ACE inhibition activity of these 17 flavonoids was measured, and the corresponding results were used to establish SAR for these molecules. Afterwards, protein-ligand docking studies were used to describe the molecular basis for most significant SAR results.
Materials and Methods
Chemicals
o-aminobenzoylglycyl-p-nitro-phenylalanylproline (o-ABz-Gly-Phe(NO2)-Pro) was purchased from Bachem Feinchemikalien (Bubendorf, Switzerland). Five units of Angiotensin-I Converting Enzyme from rabbit lung and ZnCl2 were obtained from Sigma (Barcelona, Spain). All flavonoids (assay >90% purity) used in this study were kindly provided by Nutrafur S.A. (Murcia, Spain), except for the catechin, luteolin and genistein, which came from Sigma-Aldrich Química (Barcelona, Spain). Flavonoids were solubilized in dimethyl sulfoxide (DMSO) and prepared daily. In all experiments, the final concentration of DMSO was 0.4%. Distilled water was obtained from a Millipore Milli-Q® system.
Preparation of Solutions
The 0.45 mM buffered substrate solution (o-Abz-Gly-p-Phe(NO2)-Pro) and 150 mM Tris-acid buffer solution containing 1.125 M NaCl (pH 8.3) were prepared daily. Flavonoid solutions (100 and 500 µM) were prepared in DMSO (0.4%) daily. The 0.1 U/mL ACE solution stock was prepared in glycerol: water (1∶1), aliquoted and stored at −20°C. The 0.1 µM ZnCl2 stock solution was prepared and stored at 4°C. The ACE working solution was prepared daily by diluting it in 150 mM Tris buffer (pH 8.3) containing 0.1 µM ZnCl2.
Chromatographic Analysis and Quantification of Flavonoid Compounds
For the elucidation and quantification of the main flavonoids present in each sample, we modified a previously published method [27]. All the samples were dissolved in DMSO in the ratio of 5 mg/mL, and the resulting solutions were filtered through a 0.45 µm nylon membrane. The HPLC equipment was a Hewlett-Packard Series HP 1100 equipped with a diode array detector. The stationary phase was a C18 LiChrospher 100 analytical column (250 × 4 mm i.d.) with a particle size of 5 µm (Merck, Darmstadt, Germany) thermostated at 30°C. The flow rate was 1 mL/min and the absorbance changes were simultaneously monitored at 280 and 340 nm. The mobile phases for chromatographic analysis were: (A) acetic acid: water (2.5∶97.5) and (B) acetonitrile. A linear gradient was run from 95% (A) and 5% (B) to 75% (A) and 25% (B) for 20 min; changed to 50% (A) and (B) for 20 min (40 min, total time); changed to 20% (A) and 80% (B) for 10 min (50 min, total time), and finally re-equilibrated for 10 min (60 min, total time) to the initial composition. Table 1 resumes the global HPLC profile of the different samples used in this study.
Table 1. HPLC analysis of the flavonoid samples used in the current study.
| Main content of the minor flavonoids | |||
| Compound | content (%)1 | (%)2 | Other minor flavonoids |
| Naringenin | 94.7 | Naringin (0.6) | Naringenin-7-glucoside |
| Naringenin K | 93.8 | Naringin (0.9) | Naringenin-7-glucoside |
| Naringin | 95.2 | Narirutin (1.1) | Poncirin, Naringenin |
| Apigenin | 97.2 | Rhoifolin (0.7) | Apigenin-7-glucoside |
| Apigenin K | 96.5 | Rhoifolin (0.8) | Apigenin-7-glucoside |
| Rhoifolin | 96.1 | Naringin (0.4) | Apigenin |
| Genistein | 97.2 | Daidzein (1.1) | Genistin |
| Luteolin | 95.9 | Eriodictyol (0.8) | Luteolin-7-glucoside |
| Hesperetin | 94.7 | Hesperidin (1.2) | Hesperetin-7-glucoside |
| Diosmetin | 93.8 | Diosmin (2) | Hesperidin |
| Diosmin | 95.2 | Hesperidin (1.8) | Hesperetin |
| Catechin | 98.1 | Other catechins | |
| Epicatechin | 98.5 | Other catechins | |
| Quercetin | 95.7 | Rutin (1.5) | Isoquercitrin |
| Rutin | 97.1 | Isoquercitrin (1.1) | Quercetin |
| Kaempferol | 96.8 | Quercetin (0.8) | Kaempferol-3-glucoside |
Absolute value as is.
The reference % assay is referred to the absolute content as is of the main flavonoid.
Measurement of ACE-inhibitory Activity
ACEI activity was measured by a fluorimetric assay following the method of Sentandreu and Toldrá [43] with some modifications [44]. A volume of 160 µL of 0.45 mM buffered substrate solution in 150 mM Tris-acid buffer containing 1.125 M NaCl, (pH 8.3) was mixed with 40 µL of the flavonoid solution (with 0.4% DMSO for the blank samples) and 40 µL of ACE solution (0.1 U/mL), and the mixture was incubated at 37°C. Fluorescence was measured after 30 min in 96-well microplates (Thermo Scientific, Rochester, NY) using a multiscan microplate fluorimeter (Biotek. FL×800). Microplates (Thermo Scientific, Rochester, NY) were used in this assay. The excitation and emission wavelengths were 360 and 430 nm, respectively. The activity of each sample was tested in technical and biological triplicate.
The ACEI activity was calculated using the following formula:
where A is the fluorescence without the flavonoid solution, B is the fluorescence without ACE and C is the fluorescence in the presence of both ACE and the flavonoid solution. A flavonoid solution of 500 µM was selected on the basis of previous studies [33], [35], and the concentration of 100 µM was chosen because it is within the physiological concentration range. An ACEI activity higher than 60% at 500 µM concentration was used as a selection criterion for the IC50 (the flavonoid concentration required to inhibit the original ACE activity by 50%). The IC50 of each selected flavonoid was tested in technical and biological triplicate. The results from three experiments are expressed as the mean ± SD and were performed in different platelet samples.
Molecular Modeling Studies
Flavonoid structures were either obtained from ChemSpider (http://www.chemspider.com/) or drawn with Marvin Sketch v5.9.0 (ChemAxon Kft., Budapest, Hungary; http://www.chemaxon.com/). All flavonoid structures were further set up with LigPrep v2.5 (Schrödinger LLC, Portland, USA; http://www.schrodinger.com) following three steps: (1) using Epik software [45] to generate all possible protonation states within a pH range of 4.0±7.0 and selecting the “add metal binding states” option to generate possible ligand-metal binding states among metalloproteins; (2) generating tautomers at the previously given pH range; and (3) determining chiralities from the ligand’s 3D structure.
All the protein-ligand docking studies performed in this work were performed with Glide v5.7 (Schrödinger LLC., Portland, USA; http://www.schrodinger.com) [46], [47] with extra precision (i.e., with GlideXP; [48]). Before docking the flavonoids, the shape and properties of the ACE binding site were represented by several different sets of fields on a grid. This grid was made of a box that has default dimensions around the location of the experimental pose of the inhibitor (i.e., RXPA380), and the inhibitor forms a complex with tACE at PDB file 2OC2 [49]. No constraints were set while building the grid. Default settings for the rest of the grid set-up options were used. During the protein-ligand docking, a maximum number of 5 poses per ligand were obtained. Then, the most reliable pose was selected (irrespective of its glide score) by taking advantage of the information provided by (1) the other experimental complexes between ACE and ACE inhibitors available in the PDB (Protein Cate Base) (http://www.pdb.org) and (2) the SAR results obtained in the current study for flavonoids. No constraints were imposed during the docking except for those flavonoids in which all docked poses were far from the area predicted for either luteolin or quercetin. In those cases, the AC ring location of their selected poses was used to restrict their docking (i.e., luteolin was used during apigenin and diosmetin docking, whereas quercetin was used to restrict the docking of rutin and kaempferol). Restricted docking with rhoifolin failed as a consequence of the steric hindrance between the ACE binding site and the 7-O-glycoside substituent; therefore, no docking results are reported for rhoifolin. The results for predicted poses were compared with known experimental poses for ACE inhibitors by comparing their intermolecular interactions with the ACE binding site. With that aim, LigPlot diagrams for the experimental ACE-inhibitor complexes were obtained from the PDBsum website (http://www.ebi.ac.uk/pdbsum/) and compared with equivalent diagrams derived from LigPlot+ [50] for predicted ACE-flavonoid complexes.
Results and Discussion
Seventeen flavonoids were evaluated for their ACEI activity. The structures of all compounds studied are represented in Figure 1. All the flavonoids were studied at concentrations of 100 and 500 µM (see Figures 2A and 2B, respectively). The maximum inhibitory potencies were 57% at 100 µM and 95% at 500 µM. At both concentrations, the highest ACEI activity was exhibited by luteolin. The relative inhibitory potencies for the most active flavonoids (i.e., ACEI higher than 30%) were luteolin>apigenin K>rutin>rhoifolin>quercetin>kaempferol>apigenin>diosmetin>narigenin K>epicatechin>genistein>hesperetin and diosmin for 500 µM; and luteolin>kaempferol>rutin>rhoifolin>quercetin for 100 µM. The rest of the flavonoids exhibited ACEI activities lower than 30%. The IC50 value was obtained for each flavonoid that exhibited an ACEI activity higher than 60% at 500 µM (i.e., luteolin, apigenin K, rutin, rhoifolin, quercetin and kaempferol; see Table 2). These IC50 values were found to be in the 23 to 196 µM range (with luteolin being the flavonoid with the highest ability to inhibit ACE activity).
Figure 1. Structures of the different flavonoids used in this study.
Figure 2. (A) Effect of different flavonoids on Angiotensin Converting Enzyme (ACE) activity.
Purified lung ACE was preincubated at 37°C for 30 min in the presence of 100 µM of flavonoids or DMSO as a control. The results are expressed as the percentage of ACE inhibition. The plot represents the mean result ± SD from three experiments. (B) Effect of different flavonoids on Angiotensin Converting Enzyme (ACE) activity. Purified lung ACE was preincubated at 37°C for 30 min in the presence of 500 µM of flavonoids or DMSO as control. The results are expressed as the percentage of ACE inhibition. The plot represents the mean ± SD from three experiments.
Table 2. IC50 values obtained for the selected flavonoids.
| IC50 value1 (µM) | |
| Apigenin K | 196 |
| Rhoifolin (apigenin 7-O-glycoside) | 183 |
| Kaempferol | 178 |
| Rutin (quercetin 3-O-glycoside) | 64 |
| Quercetin | 43 |
| Luteolin | 23 |
The IC50 value represents the concentration of each compound that inhibits ACE activity by 50%.
In recent years, flavonoids have gained a great amount of interest with regards to their potential for cardiovascular protection. In fact, many epidemiological studies associate an increased consumption of foods and beverages rich in flavonoids with a reduced risk of CVD death [51]–[53]. Additionally, several of these flavonoids or their derivatives (i.e., diosmin, rutin and quercetin) are widely used as pharmaceutical agents for their vasoprotective properties (i.e., Daflon 500 and Venorutom) [54].
Flavonoids are based on the structure of phenyl-benzopyrone and differ from one another in terms of hydroxyl, methoxyl or glycosylated substituents, the position of the benzenoide (B-ring) substituent relative to the C-ring, the degree of unsaturation and the types of sugars that are attached [55]. We evaluated the inhibitory effects on the ACE activity of a group of flavonoids from five different structural types (see Figure 1). The inhibitory effects of certain flavonoids on ACE activity that have been reported in other studies were confirmed [37]. Many of the flavonoids that were tested could inhibit ACE in the micromolar range [22], [56]. However, as was expected, significant differences were observed in the ACEI activity depending on the flavonoid structure [25], [36]. Although the ACEI activity of these flavonoids does not reach the potency of drugs commonly used in the treatment of hypertension, food products with moderate ACEI activity (i.e., an ACEI index higher than 70%) may be considered as naturally functional foods [57] if it is also taken into account that the regular dietary intake of polyphenols could be as high as 1 g/day [58], [59]. Moreover, functional foods containing these natural compounds would not be expected to have the side effects associated with synthetic drugs used in hypertension control [60].
Our evaluation of the abilities of different flavonoids to inhibit the activity of ACE confirmed that the principal structural features for their inhibitory activity are as follows: (a) the double bond between C2 and C3 at the C-ring; (b) the catechol group in the B-ring (3′,4′-dihydroxy) [61]; and (c) the cetone group at the C4 carbon on the C-ring [which is a functional group that has been observed to be essential for inhibiting ACE [62]. According to these general considerations, we analyzed and evaluated the SAR derived from our results. Our data confirm that a distinguishing feature for ACE inhibition by flavonoids is the presence of an unsaturated 2–3 bond conjugated with a 4-oxo- function, aside from the 3′,4′-catechol B-ring pattern, as occurs mainly in luteolin, quercetin and rutin. However, it is important to analyze the specific, qualitative and quantitative influence of each one of these three sub-structures in the SAR results.
The Significance of the C2 = C3 Double Bond in the C-ring: Flavone vs. Flavanone
As previously mentioned, the presence of a C2–C3 double bond seems to be essential for the molecule to exert a significant ACEI activity. Two main factors would explain this fact. First, the molecular electronic distribution would allow the maintenance of a definitive structural conjugation, from the B-ring to the A and C rings, in contrast to the flavanone structure, with which this definitive structural conjugation is not possible. Second, the spatial, or the maintenance of a nearly planar structure, would disappear if this bond was saturated, producing a flavonoid skeleton with an obtuse angle, which would be variable depending on the rest of the constituents of the molecule. Our data confirm previous findings that suggest that a nearly planar flavonoid structure is an important factor in the inhibition of ACE [55]. In fact, all flavanones included in this study, both aglycones (naringenin and hesperetin) and glycosides (naringin), are not as effective as flavones on ACEI activity. This difference can be observed, more specifically, by comparing the results between the flavone apigenin and its corresponding structurally similar flavanone, naringenin (and, although the difference is on another scale, between diosmetin and hesperetin) where the absence of the C2 = C3 double bond in naringenin involves a 91% reduction of ACEI activity at 100 µM vs. apigenin (see Figures 2A and 3).
Figure 3. Structural diagram which quantitatively assesses the effect of the addition or elimination of different structural elements from the flavonoid core on the ACEI activity of luteolin at 100 µM.
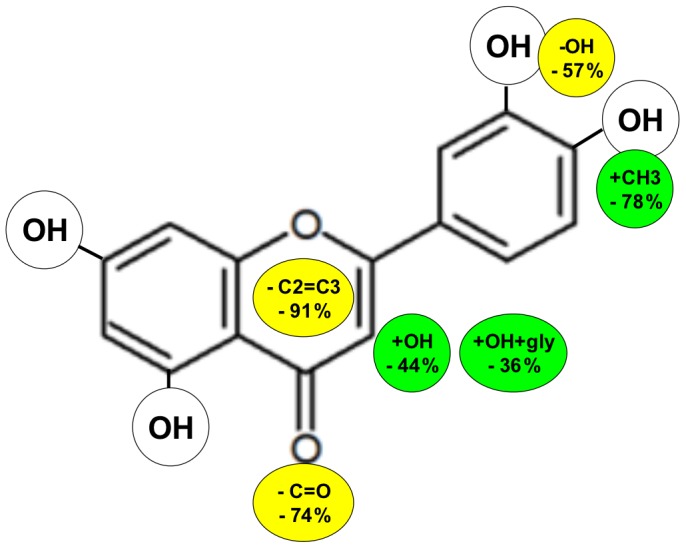
According to these data, the significance order was: double bond C2 = C3 (absence: −91% activity by comparing naringenin vs. apigenin) >4′-O-methoxylation (presence: −78% by comparing diosmetin vs. luteolin) ≈ 4-carbonyl group (absence: −74% by comparing epicatechin vs. luteolin) >3′-hydroxylation (absence: −57% by comparing apigenin vs. luteolin) >3-hydroxylation (presence: −44% by comparing quercetin vs. luteolin) >3-O-glycosylation (presence: -36% by comparing rutin vs. luteolin).
B-ring Pattern: Catechol Group vs. Monohydroxy Group and O-methylation
The presence of several hydroxyl groups in the flavonoids seems be important for the extent of inhibition of the zinc metalloproteinases [29]. Additionally, the exact position of this group has been revealed to be very important for ACE inhibition. Hydroxylation at the 4′-position of the B ring seems to be of particular relevance, and in addition, the presence of a catechol group in the B ring (3′,4′-dihydroxy) appears to be fundamental to achieving an increased ACE inhibitory activity, as occurred in luteolin (as well as quercetin and rutin), which presented the highest ACEI efficiency (see Figure 2 and Table 2). Luteolin has also been described as the most effective flavonoid for inhibiting other metalloproteinases (aminopeptidases), such as MMP-1 and MMP-2 [30]. Consequently, the presence of a catechol at the B-ring should be considered to be very important; indeed, the absence of the 3′ hydroxyl group in apigenin causes a 57% reduction of ACEI activity at 100 µM relative to the luteolin (see Figures 2A and 3). A similar reduction of activity occurs with the flavonols quercetin and kaempferol, where the absence of catechol in the kaempferol resulted in a 4-fold increase in the IC50 relative to quercetin (see Table 2).
Additionally, the characteristic esterification of flavonoids in the 4′ position significantly reduces ACEI activity, as occurs when the 4′-hydroxyl group of luteolin is methylated to generate diosmetin (an esterification that causes a 78% reduction of ACEI activity at 100 µM; see Figures 2A and 3).
The Absence of a Carbonyl Group in C4 (C-ring)
The absence of this functional group represents an important reduction in the capacity to inhibit ACE activity. This fact was evident in the case of flavan-3-ols, catechin and epicatechin in comparing their ACEI activities with the inhibition exerted by the luteolin, although all of them have a catechol structure in their B ring. However, it is important to keep in mind that the disappearance of the carbonyl group is simultaneously accompanied by the disappearance of the double bond C2 = C3, the structural significance of which has been previously mentioned. This effect is perhaps related to the loss of planar spatial structure [26], [62].
The Significance of 3-hydroxylation of the C-ring: Flavone vs. Flavonol
According to all the structural aspects mentioned above, the ACEI activity of flavonoids are related to a specific electronic distribution, the minimal alteration of this electronic distribution on the flavonoid skeleton, such as the transformation of flavone into flavonol by the addition of a hydroxyl group in the C-3 position of the C ring, thus, luteolin to quercetin, produced a 44% decrease in the ACEI activity at 100 µM (see Figures 2A and 3). However, a similar reduction in ACE activity does not occur when comparing the activity of apigenin and kaempferol because in this case, the flavonol exerts an increase in the ACEI activity with respect to the flavone at both the tested concentrations. This suggests that the absence of a catechol group in the B ring of apigenin and kaempferol also modifies the global electronic distribution, altering the significance of the hydroxylation at C-3.
The Significance of Glycosylation at 7-O (A-ring) and 3-O (C-ring) Positions
These two positions are the most typical locations for glycosyl radicals in the flavonoid skeleton (generally, rhamnose-glucose-aglycon). Table 2 shows that the flavonoids rhoifolin (7-O-gly) and rutin (3-O-gly) were almost as effective as their corresponding aglycons (i.e., apigenin and quercetin, respectively) indicating that glycosylations at positions 7 or 3 do not produce steric hindrances that hamper them from binding to ACE in a way that is similar to the actions of their own aglycons.
These data show similarities in activity levels for the naringin-naringenin tests but demonstrate the opposite effects for diosmin-diosmetin (see Figure 2). This behavioral difference could be related to differences in the glycosylation pattern: neohesperidosyl (rha-1-2-glu) for rhoifolin and naringin, and rutinosyl (rha-1–6-glu) for diosmin. The neohesperidosyl structure has greater electronic interaction with the flavonoid skeleton (including intramolecular hydrogen bonds), with consequent influence on the overall electronic distribution. The fact that the 7-O-glucoside flavonoids (glucose-aglycon) were equal or even stronger inhibitors than the aglycons has already been reported with relation to the inhibition of the zinc metalloproteinases MMP-2 and MMP-9 [30].
The Significance of the Position of B-ring in C-ring: Flavone (2-B-ring) vs. Isoflavone (3-B-ring)
The data from this study do not allow a definite assessment of ACEI activity in the tested compounds. Although the values obtained for the isoflavone genistein and the flavone apigenin show differences at 500 µM (40% vs. 58%, respectively), the responses were similar at the 100 µM concentration.
The Possible Significance of Potassium Salts
More research is needed to evaluate the use of flavonoids in which the hydroxyls in positions 7 and 4′ are ionized (R-O-K). At 100 µM, apigenin and naringenin showed similar effects to those of their potassium salts, while at 500 µM, their potassium salts showed a significantly higher ACEI activity.
IC50 Values for Selected Compounds
Independent of the comparative analysis of the results obtained at 100 and 500 µM, the determination of the IC50 value for six selected compounds allows us to confirm the importance of the sub-structural elements of the SAR (see Table 2). Thus, the relative IC50 ratios of these compounds ([i.e., luteolin (1)>quercetin (1.87)>rutin (2.78)>kaempferol (7.74)>rhoifolin (7.96)>apigenin K (8.52)] confirm the importance of the presence of the B-ring catechol group combined with the double bond and the carbonyl group of the C ring. In fact, the absence of the above-mentioned catechol group for kaempferol, rhoifolin and apigenin supposes an increase of the IC50 up to values that are most likely within a range of physiological concentrations. Therefore, to reach the concentration of flavonoids necessary to inhibit the ACE, it would be necessary to apply them as pharmaceuticals agents or as dietary supplements which contain a sufficient flavonoid concentration to obtain in vivo efficacy. The apparent influence of 3-O-glycosylation (rutin vs. quercetin) would seem to be more logical, in that the existence of a certain steric impediment inhibits the interaction of the flavonoid with the active center of the enzyme, although the values for both flavonols are reasonably similar.
Prediction of Complexes between tACE and the Flavonoids with the Highest ACEI Activity
The intermolecular interactions between ACE’s binding site residues and the predicted poses for the flavonoids with the highest ACEI activity are shown in Table 3 (together with results for experimental complexes at PDB between tACE and ACE inhibitors). The results in that table show how these flavonoids form intermolecular interactions with some of the ACE’s binding site regions (i.e., S2′, S2′/S1′ and S1) that are also involved in the binding of known ACE inhibitors. At this point, it is remarkable how these flavonoids share most of the intermolecular interactions at the ACE’s S1 site that have been found for synthetic ACE inhibitors (e.g., lisinopril, enalaprilat, captopril; see Table 3) and, therefore, suggest that binding to the ACE’s S1 subsite is essential for ACEI activity.
Table 3. Intermolecular interactions between ACE inhibitors and the tACE binding site.
| LISINOPRIL | ENALAPRILAT | CAPTOPRIL | RXPA380 | SELENOCAPTOPRIL | KAF | KAW | lisW-S | FII-A | LUTEOLIN | QUERCETIN | RUTIN | KAEMPFEROL | ||
| S2′ | Gln281 | ✓ | ✓ | NE2 | NE2 | NE2 | NE2 | ✓ | H | H | ||||
| Thr282 | ✓ | ✓ | ✓ | ✓ | ||||||||||
| His353 | NE2 | NE2 | NE2 | NE2 | NE2 | NE2 | NE2 | NE2 | NE2 | ✓ | ✓ | ✓ | ✓ | |
| Glu376 | ✓ | ✓ | ||||||||||||
| Asp453 | ✓ | |||||||||||||
| Lys511 | NZ | NZ | NZ | NZ | NZ | NZ | NZ | NZ | NZ | |||||
| His513 | NE2 | NE2 | NE2 | NE2 | NE2 | NE2 | NE2 | NE2 | NE2 | |||||
| Tyr520 | OH | OH | OH | OH | OH | OH | OH | OH | OH | |||||
| S2′/S1′ | Ser284 | |||||||||||||
| Val379 | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Val380 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| S1′ | Glu162 | ✓ | OE2 | |||||||||||
| Asn277 | ✓ | |||||||||||||
| Asn374 | ||||||||||||||
| Asp377 | OD1 | |||||||||||||
| S1 | Glu143 | |||||||||||||
| Val351 | ||||||||||||||
| Ala354 | O | O | ✓ | ✓ | ✓ | ✓ | ✓ | O | ✓ | ✓ | ✓ | O | O | |
| Ser355 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Trp357 | ||||||||||||||
| Lys368 | ||||||||||||||
| Glu384 | OE2 | OE2 | OE2 | OE2 | ✓ | OE2 | OE2 | OE2 | OE2 | ✓ | ✓ | ✓ | ||
| Phe512 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Ser516 | ||||||||||||||
| Tyr523 | OH | OH | ✓ | OH | ✓ | OH | OH | OH | OH | OH | OH | OH | ✓ | |
| S1/S2 | Val518 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| S2 | Phe391 | ✓ | ✓ | ✓ | ✓ | |||||||||
| Glu403 | ||||||||||||||
| Arg522 | ||||||||||||||
| Other | Thr166 | ✓ | ||||||||||||
| Trp279 | ✓ | |||||||||||||
| Ala356 | N | N | N | N | ||||||||||
| His383 | ✓ | ✓ | ✓ | NE2 | ✓ | NE2 | NE2 | NE2 | NE2 | ✓ | ||||
| His387 | NE2 | ✓ | ✓ | NE2 | ✓ | ✓ | NE2 | NE2 | ||||||
| His410 | ✓ | ✓ | ✓ | |||||||||||
| Glu411 | ✓ | ✓ | ✓ | OE1 | ✓ | ✓ | OE1 | |||||||
| Asp415 | ✓ | ✓ | OD2 | ✓ | OD1 | ✓ | ✓ | |||||||
| Lys454 | NZ | NZ | ||||||||||||
| Phe457 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Phe527 | ✓ | ✓ | ✓ |
Data used for lisinopril, enalaprilat, captopril, RXPA380, selenocaptopril, KAF, KAW, lisW-S and FII-A was obtained from the LigPlot+ diagrams that are available at the PDBsum resource (http://www.ebi.ac.uk/pdbsum/) for PDB files 1O86, 1UZE, 1UZF, 2OC2, 2YDM, 3BKK, 3BKL, 3L3N and 2XY9, respectively. Data for luteolin, quercetin, rutin and kaempferol was obtained by applying LigPlot+ to the structure of their predicted complexes with tACE. Hydrophobic contacts are indicated by a check mark whereas hydrogen bonds are indicated with the label of the protein atom that is involved.
Figure 4 shows the predicted pose for luteolin at the tACE binding site and its location relative to the experimental poses for the ACE inhibitors lisinopril, captopril and enalaprilat. In that pose, the two B-ring hydroxyls of luteolin are able to make charge-charge interactions with the active site Zn2+ (in a way that is similar to the interactions made by the carboxylic acid from lisinopril and enalaprilat; see Figure 4). Interestingly, the charge-charge interaction with the 3′ hydroxyl is stronger than that of with the 4′ interaction (i.e., the distance between the Zn2+ ion and the hydroxyl oxygen is 2.1 and 4.3 Å for 3′ and 4′, respectively) which could explain why apigenin (see Figure 1) shows a decrease of approximately 50% of ACE inhibition relative to luteolin (see Figure 2).
Figure 4. Best predicted pose for luteolin (panel A) at the tACE binding site and the relative location to experimental poses for the ACE inhibitors lisinopril (panel B), enalaprilat (panel C), and captopril (panel D).
All of the panels in this figure are in the same relative orientation to allow for easier comparisons between the poses. Residues at the ACE binding site are colored according to the subsite where they belong (i.e., residues from the S2′, S2′/S1′, S1′, S1 and S1/S2 subsites are colored in red, cyan, magenta, green, brown, white and yellow, respectively). Other important residues that have not been classified in any pocket are colored in white. Carbon atoms for the ligands are shown in yellow to make them more easily distinguishable from the binding site residues. Dashed lines are used to show intermolecular hydrogen bonds (in red) or charge-charge interactions (in blue).
Conclusions
In this study, we have demonstrated that changes in the flavonoid active core affect its capacity to inhibit the ACE, in a way that is similar to what has been described for other zinc metalloproteinases [29], [30]. We provide additional examples of flavonoid structure–activity relationships and establish the structural features needed for the ACEI activity of flavonoids. We show that at the physiological flavonoid concentration (i.e., 100 µM), the relative effect of the different substructures on ACEI activity is as follows: double bond C2 = C3>4′-O-methoxylation ≈ 4-carbonyl group>3′-hydroxylation >3-hydroxylation >3-O-glycosylation. Through this study we can assess the influence of different structural groups, at the steric level, on inductive-mesomeric effects and the flavonoid molecular skeleton. Finally, we would like to remark that it is clear that the application of these flavonoids as inhibitors of ACE in vivo may be useful as nutritional supplements or in pharmaceutical formulations to obtain a sufficient dose/response efficacy.
Acknowledgments
We thank Zara Pons and María Margalef for their technical assistance.
Funding Statement
This study was supported by grants from the Ministerio de Educación y Ciencia of the Spanish Government(AGL 2008-00387/ALI) and from the Universiti Rovira i Virgili - Banco Santander (2011 LINE-12). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Heart Association (2004) Heart Disease and Stroke Statistics: 2004 Update. Dallas, TX, American Heart Assosiation.
- 2. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, et al. (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223. [DOI] [PubMed] [Google Scholar]
- 3. Mancia G, Bombelli M, Lanzarotti A, Grassi G, Cesana G, et al. (2002) Systolic vs. diastolic blood pressure control in the hypertensive patients of the PAMELA population. Arch Intern Med 162: 582–586. [DOI] [PubMed] [Google Scholar]
- 4. Romero JR (2007) Prevention of ischemic stroke: overview of traditional risk factors. Curr Drug Targets 8: 794–801. [DOI] [PubMed] [Google Scholar]
- 5. Maruthur NM, Wang NY, Appel LJ (2009) Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation 119: 2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ondetti MA, Rubin B, Cushman DW (1977) Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science 196: 441–444. [DOI] [PubMed] [Google Scholar]
- 7. Coates D (2003) The angiotensin converting enzyme (ACE). Int J Biochem Cell Biol 35: 769–773. [DOI] [PubMed] [Google Scholar]
- 8. Soubrier F, Alhen-Gelas F, Huber J, Allegrini J, John M, et al. (1988) Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci 85: 9386–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams TA, Corvol P, Soubrier F (1994) Identification of two active site residues in human angiotensin I-converting enzyme. J Biol Chem 47: 29430–29434. [PubMed] [Google Scholar]
- 10. Ehlers MR, Fox EA, Strydom DJ, Riordan JF (1989) Molecular cloning of human testicular angiotensin-converting enzyme: the testis isozyme is identical to the C-terminal half of endothelial angiotensin-converting enzyme. Proc Natl Acad Sci 86: 7741–7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei L, Clauser E, Alhenc-Gelas F, Corvol P (1992) The two homologous domains of human angiotensin I-converting enzyme interact differently with competitive inhibitors. J Biol Chem 19: 13398–13405. [PubMed] [Google Scholar]
- 12. Esther CR, Marino EM, Howard TE, Machaud A, Corvol P, et al. (1997) The critical role of tissue angiotensin-converting enzyme as revealed by gene targeting in mice. J Clin Invest 99: 2375–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Junot C, Gonzales MF, Ezan E, Cotton J, Vazeux G, et al. (2001) RXP 407, a selective inhibitor of the N-domain of angiotensin I-converting enzyme, blocks in vivo the degradation of hemoregulatory peptide acetyl-Ser-Asp-Lys-Pro with no effect on angiotensin I hydrolysis. J Pharmacol Exp Ther 297: 606–611. [PubMed] [Google Scholar]
- 14. Fuchs S, Xiao HD, Cole JM, Adams JW, Frenzel K, et al. (2004) Role of the N-terminal catalytic domain of angiotensin-converting enzyme investigated by targeted inactivation in mice. J Biol Chem 279: 15946–15953. [DOI] [PubMed] [Google Scholar]
- 15. Skeggs L, Kahn J, Shumway N (1956) The preparation and function of the hypertension-converting enzyme. J Exp Med 103: 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dorer FE, Kahn JR, Lentz KE, Levine M, Skeggs LT (1974) Hydrolysis of bradykinin by angiotensin-converting enzyme. Circ Res 34: 824–827. [DOI] [PubMed] [Google Scholar]
- 17. Pfeffer MA, Frohlich ED (2006) Improvements in clinical outcomes with the use of angiotensin-converting enzyme inhibitors: cross-fertilization between clinical and basic investigation. Am J Physiol Heart Circ Physiol 291: H2021–H2025. [DOI] [PubMed] [Google Scholar]
- 18. Israili ZH, Hall WD (1992) Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 117: 234–242. [DOI] [PubMed] [Google Scholar]
- 19. Opie LH (1996) ACE inhibitors in pregnancy–how to avoid the sting in the tail. S Afr Med J 86: 326–327. [PubMed] [Google Scholar]
- 20. Nyman U, Joshi P, Madsen LB, Pedersen TB, Pinstrup M, et al. (1998) Ethnomedical information and in vitro screening for angiotensin-converting enzyme inhibition of plants utilized as traditional medicines in Gujarat, Rajasthan and Kerala (India). J Ethnopharmacol 60: 247–263. [DOI] [PubMed] [Google Scholar]
- 21. Park PJ, Je JY, Kim SK (2003) Angiotensin I converting enzyme (ACE) inhibitory activity of hetero-chitooligosaccharides prepared from partially different deacetylated chitosans. J Agric Food Chem 51: 4930–4934. [DOI] [PubMed] [Google Scholar]
- 22. Loizzo MR, Said A, Tundis R, Rashed K, Statti GA, et al. (2007) Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excels (Roxb) (Simaroubaceae). Phytother Res 21: 32–36. [DOI] [PubMed] [Google Scholar]
- 23. Corradini E, Foglia P, Giansanti P, Gubbiotti R, Samperi R, et al. (2011) Flavonoids: chemicalproperties and analytical methodologies of identification and quantitation in foods and plants. Nat Prod Res 5: 469–495. [DOI] [PubMed] [Google Scholar]
- 24. Hughes R, Croley T, Metcalfe C, March R (2001) A tandem mass spectrometric study of selected characteristic flavonoids. International Journal of Mass Spectrometry 210/211: 371–385. [Google Scholar]
- 25. Fraga CG, Galleano M, Verstraeten SV, Oteiza PI (2010) Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Aspects Med 31: 435–445. [DOI] [PubMed] [Google Scholar]
- 28. Amic D, Davidovic-Amic D, Beslo D, Rastija V, Lucia B, et al. (2007) SAR and QSAR of the antioxidant activity of flavonoids. Curr Med Chem 14: 827–45. [DOI] [PubMed] [Google Scholar]
- 29. Parellada J, Suárez G, Guinea M (1998) Inhibition of zinc metallopeptidases by flavonoids and related phenolic compounds: structure-activity relationships. J Enzyme Inhib 13: 347–359. [DOI] [PubMed] [Google Scholar]
- 30. Ende C, Gebhardt R (2004) Inhibition of matrix metalloproteinase-2 and -9 activities by selected flavonoids. Planta Med 70: 1006–1008. [DOI] [PubMed] [Google Scholar]
- 31. Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, et al. (2010) vInhibition of angiotensin converting enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J Ethnopharmacol 127: 7–10. [DOI] [PubMed] [Google Scholar]
- 32. Kwon EK, Lee DY, Lee H, Kim DO, Baek NI, et al. (2010) Flavonoids from the buds of Rosa damascena inhibit the activity of 3-hydroxy-3-methylglutaryl-coenzyme a reductase and angiotensin I-converting enzyme. J Agric Food Chem 58: 882–866. [DOI] [PubMed] [Google Scholar]
- 33. Loizzo MR, Said A, Tundis R, Rashed K, Statti GA, et al. (2007) Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excels (Roxb) (Simaroubaceae). Phytother Res 21: 32–36. [DOI] [PubMed] [Google Scholar]
- 34. Kiss A, Kowalski J, Melzig MF (2004) Compounds from Epilobium angustifolium inhibit the specific metallopeptidases ACE, NEP, and APN. Planta Med 70: 919–923. [DOI] [PubMed] [Google Scholar]
- 35. Oh H, Kang DG, Kwon JW, Kwon TO, Lee SY, et al. (2004) Isolation of angiotensin converting enzyme (ACE) inhibitory flavonoids from Sedum sarmentosum. Biol Pharm Bull 27: 2035–2037. [DOI] [PubMed] [Google Scholar]
- 36. Ottaviani JI, Actis-Goretta L, Villordo JJ, Fraga CG (2006) Procyanidin structure defines the extent and specificity of angiotensin I converting enzyme inhibition. Biochimie 88: 359–365. [DOI] [PubMed] [Google Scholar]
- 37. Actis-Goretta L, Ottaviani JI, Fraga CG (2006) Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agric Food Chem 54: 229–234. [DOI] [PubMed] [Google Scholar]
- 38. Balasuriya BWN, Rupasinghe HPV (2011) Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Functional Foods in Health and Disease 5: 172–188. [Google Scholar]
- 39. Quiñones M, Sanchez D, Muguerza B, Miguel M, Aleixandre A (2011) Mechanisms for antihypertensive effect of CocoanOX, a polyphenol-rich cocoa powder, in spontaneously hypertensive rats. Food Res Int 44: 1203–1208. [Google Scholar]
- 40. Gasparotto J, Junior A, Gasparotto FM, Lourenço EL, Crestani S, et al. (2011) Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: evidence for the inhibition of angiotensin converting enzyme. J Ethnopharmacol 134: 363–372. [DOI] [PubMed] [Google Scholar]
- 41. Aviram M, Dornfeld L (2001) Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 158: 195–198. [DOI] [PubMed] [Google Scholar]
- 42. Bormann H, Melzig MF (2000) Inhibition of metallopeptidases by flavonoids and related compounds. Pharmazie 55: 129–132. [PubMed] [Google Scholar]
- 43. Sentandreu MA, Toldrá F (2006) A fluorescence-based protocol for quantifying angiotensin-converting enzyme activity. Nat Protoc 1: 2423–2437. [DOI] [PubMed] [Google Scholar]
- 44. Quirós A, del Mar Contreras M, Ramos M, Amigo L, Recio I (2009) Stability to gastrointestinal enzymes and structure-activity relationship of beta-casein-peptides with antihypertensive properties. Peptides 30: 1848–1853. [DOI] [PubMed] [Google Scholar]
- 45. Shelley JC, Cholleti A, Frye LL, Greenwood JR, Timlin MR, et al. (2007) Epik: a software program for pK(a ) prediction and protonation state generation for drug-like molecules. J Comput Aided Mol Des 12: 681–691. [DOI] [PubMed] [Google Scholar]
- 46. Friesner RA, Banks JL, Murphy RB, Beard HS, Frye LL, et al. (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 7: 1739–49. [DOI] [PubMed] [Google Scholar]
- 47. Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, et al. (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 7: 1750–1759. [DOI] [PubMed] [Google Scholar]
- 48. Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, et al. (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 21: 6177–6196. [DOI] [PubMed] [Google Scholar]
- 49. Anthony CS, Corradi HR, Schwager SL, Redelinghuys P, Goergiadis D, et al. (2010) The N domain of human angiotensin-I-converting enzyme: the role of N-glycosylation and the crystal structure in complex with an N domain-specific phosphinic inhibitor, RXP407. J Biol Chem 285: 35685–35693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 10: 2778–2786. [DOI] [PubMed] [Google Scholar]
- 51. Liu Z, Ma LP, Zhou B, Yang L, Liu ZL (2000) Antioxidative effects of green tea polyphenols on free radical initiated and photosensitized peroxidation of human low density lipoprotein. Chem Phys Lipids 106: 53–63. [DOI] [PubMed] [Google Scholar]
- 52. Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm E, et al. (2001) The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med 134: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 53. Kris-Etherton P, Keen C (2002) Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr Opin Lipidol 13: 41–49. [DOI] [PubMed] [Google Scholar]
- 54. Gohel MS, Davies AH (2009) Pharmacological agents in the treatment of venous disease: an update of the available evidence. Curr Vasc Pharmacol 3 303–308. [DOI] [PubMed] [Google Scholar]
- 55. Xu YC, Leung SW, Yeung DK, Hu LH, Chen GH, et al. (2007) Structure-activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry 68: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 56. Olszanecki R, Bujak-Gizycka B, Madej J, Suski M, Wołkow PP, et al. (2008) Kaempferol, but not resveratrol inhibits angiotensin converting enzyme. J Physiol Pharmacol 59: 387–392. [PubMed] [Google Scholar]
- 57. Meisel H, Goepfert A, Günther S (1997) ACE-inhibitory activities in milk products. Milchwissenschaft 52: 307–311. [Google Scholar]
- 58. Kuhnau J (1976) The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet 24: 117–191. [PubMed] [Google Scholar]
- 59. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79: 727–747. [DOI] [PubMed] [Google Scholar]
- 60. FitzGerald RJ, Meisel H (2000) Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. Br J Nutr 84: 33S–37S. [DOI] [PubMed] [Google Scholar]
- 61. Saragusti AC, Ortega MG, Cabrera JL, Estrin DA, Marti MA, et al. (2010) Inhibitory effect of quercetin on matrix metalloproteinase 9 activity molecular mechanism and structure-activity relationship of the flavonoid-enzyme interaction. Eur J Pharmacol 644: 138–145. [DOI] [PubMed] [Google Scholar]
- 62. Sartor L, Pezzato E, Dell’Aica I, Caniato R, Biggin S, et al. (2002) Inhibition of matrix-proteases by polyphenols: chemical insights for anti-inflammatory and anti-invasion drug design. Biochem Pharmacol 64: 229–237. [DOI] [PubMed] [Google Scholar]





