Abstract
Objectives
Both ketamine and ethanol are N-methyl-D-aspartate (NMDA) receptor antagonists. Ketamine has rapid antidepressant properties in major depressive disorder (MDD) as well as bipolar depression. In individuals with MDD, a positive family history of alcohol dependence (FHP) was associated with greater improvement in depressive symptoms after ketamine administration compared to individuals whose family history of alcohol dependence was negative (FHN). This study investigated whether FHP influences ketamine’s antidepressant and perceptual effects in individuals with bipolar depression.
Methods
A post-hoc analysis was conducted on 33 subjects with DSM-IV bipolar disorder (BD) type I or II depression pooled from two previously published studies. All subjects had undergone a double-blind, randomized, crossover trial of a single intravenous infusion of ketamine (0.5 mg/kg) combined with lithium or valproate therapy. Subjects were rated at baseline; at 40, 80, 120, and 230 min; and at Days 1, 2, 3, 7, 10, and 14 post-infusion. The primary outcome measure was Montgomery-Åsberg Depression Rating Scale (MADRS) scores. Patients were categorized as FHP when they reported at least one first-degree relative with alcohol dependence. Measures of psychosis, dissociation, and dysphoria were also collected.
Results
After ketamine infusion, subjects with FHP showed significantly greater improvement on MADRS scores than FHN subjects. In addition, patients with FHP had attenuated psychotomimetic and dissociative scores compared to FHN patients.
Conclusions
FHP appears to predict a more sustained antidepressant response to ketamine in individuals with BD. Family history of alcoholism may be an important consideration in the development of glutamatergic-based therapies for depression.
Keywords: alcohol, bipolar disorder, depression, family history, ketamine, N-methyl-D-aspartate, predictors of response
Substance use disorders, particularly alcohol dependence, are highly prevalent in individuals diagnosed with bipolar disorder (BD) (1). In addition, the presence of substance abuse or alcoholism significantly affects the course and prognosis of BD (2–6). Recent evidence from diverse studies suggests that glutamatergic dysfunction may be involved in the pathophysiology of both BD (7, 8) and alcoholism (9–11), as well as both disorders concomitantly (12). Furthermore, the glutamatergic system, particularly the N-methyl-D-aspartate (NMDA) receptor complex, has been investigated as a putative target for the development of novel treatments for both disorders (7, 13–18).
For mood disorders, much of the recent work in this area has centered on ketamine, an NMDA receptor antagonist with rapid (within hours) antidepressant effects in both major depressive disorder (MDD) and bipolar depression. Notably, both ketamine and ethanol are NMDA receptor antagonists and show dose-related similarities with regard to their subjective effects in healthy subjects—an effect that appears to be mediated by positive family history of alcohol dependence (FHP) (19, 20). In healthy subjects undergoing ketamine infusion, individuals with FHP showed an attenuated response in terms of perceptual alterations and dysphoric mood compared to those with a negative family history of alcohol dependence (FHN) (20). Alcohol-dependent patients also appear to have a blunted behavioral affect in response to ketamine, suggesting cross-tolerance between alcohol and ketamine (19). Relative to healthy subjects, ketamine administration in recovering ethanol-dependent patients was associated with reduced psychotomimetic symptoms and dysphoric mood (11).
A previous study from our laboratory examined whether FHP would mediate the subjective and behavioral effects of ketamine in individuals with MDD. Indeed, consistent with the extant literature, we found that treatment-resistant MDD patients with FHP had fewer dysphoric and psychotomimetic effects in response to ketamine administration than MDD patients with FHN. In addition, we found that FHP patients had a significantly better initial antidepressant response to ketamine (21). Thus, in patients with MDD, FHP predicted a differential response to the perceptual disturbances, dysphoric effects, and antidepressant effects associated with ketamine.
To date, no studies have investigated the link between FHP and ketamine’s antidepressant properties in patients with bipolar depression. Nevertheless, identifying predictors of robust treatment response could improve research efforts to develop glutamatergic modulators for the treatment of mood disorders. Thus, this study sought to determine whether the dysphoric, psychotomimetic, and antidepressant effects associated with ketamine are altered in FHP individuals with bipolar depression; the study combines samples from two recently published controlled studies investigating the antidepressant properties of ketamine in individuals with bipolar depression (7, 8). The relationship between personal history of alcohol dependence and ketamine’s effects was also examined.
Materials and methods
This study was a post-hoc analysis that combined data from two identical but independent controlled, crossover studies investigating the use of ketamine in bipolar I or II depression without psychotic features (7, 8); pooled data were available for 33 subjects (18–65 years old).
Subject population, study design, and main outcome measures
Details regarding the subjects in each study have been previously published (7, 8). Briefly, at the time of the initial studies, all subjects were inpatients at the National Institute of Mental Health (NIMH) Mood Disorders Research Unit (Bethesda, MD, USA). Subjects were required to have a score of 20 or higher on the Montgomery-Åsberg Depression Rating Scale (MADRS) at screening and at the start of each ketamine or placebo infusion. Subjects had no diagnosis of substance abuse or dependence for at least three months, as assessed by the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID), Patient Version (22); individuals with a personal history of alcohol dependence before this period of time were not excluded.
Diagnostic information about participants' relatives was ascertained by a clinician-administered interview to assess family history of alcohol dependence and mood disorders. This interview was augmented in most cases by the subject’s medical records and by interviews with individuals, including family members, who knew the subject well. FHP was defined as the presence of at least one affected first-degree relative with a history of alcoholism. Individual history of alcohol abuse or dependence was determined by SCID.
Both studies were identically-designed, double-blind, randomized, crossover, placebo-controlled studies conducted to assess the efficacy and safety of a single intravenous infusion of the NMDA antagonist ketamine combined with lithium or valproate monotherapy in the treatment of bipolar I or II depression. During the entirety of both studies, patients were required to take either lithium or valproate within a specified therapeutic range and were not allowed to receive any other psychotropic medications (including benzodiazepines) or structured psychotherapy. Eligible patients were either continued on their mood stabilizer (lithium or divalproex sodium), started on, or switched to one of these for the purpose of the study. If a patient was taking both lithium and divalproex sodium or had never been treated with a mood stabilizer at the time of the screening, preference was giving to using lithium during the study. Following non-response to open treatment with lithium or valproate for a minimum of four weeks at therapeutic levels and a two-week drug-free period (except for treatment with lithium or valproate), subjects received intravenous infusions of saline solution and 0.5 mg/kg ketamine hydrochloride two weeks apart using a randomized, double-blind, crossover design.
Ratings were obtained 60 min prior to the infusion and at 40, 80, 110, and 230 min post-infusion. Subjects were also rated at Days 1, 2, 3, 7, 10, and 14 post-infusion. The MADRS was the primary outcome measure; secondary outcome measures included the 17-item Hamilton Rating Scale for Depression (HDRS) (23), the Brief Psychiatric Rating Scale (BPRS) (24), and the Clinician Administered Dissociative States Scale (CADSS) (25), which was used to measure the dissociative symptoms that may occur with ketamine infusions.
Statistical analysis
A linear mixed model with restricted maximum likelihood estimation and a first order autoregressive covariance structure was used to examine changes in depression over time in patients receiving ketamine. All main effects and interactions were included with time, drug, and family history of drug dependence factors, but the baseline outcome was included as a covariate as a main effect only. The fixed intercept was included, but no random effects were included. Bonferroni-adjusted simple effects tests were used to examine significant omnibus main effects and interactions. Significance was evaluated at p < 0.05, two-tailed.
Results
Ketamine and family history of alcohol dependence
Twelve (36%) of the 33 patients had FHP. FHP and FHN groups did not differ on most demographic and course of illness characteristics (Table 1); however the FHP group had a longer duration of illness, greater history of lifetime substance abuse or dependence, and more patients taking valproate than the FHN group. No group differences were noted in plasma ketamine and norketamine levels at 40 min post-infusion. A similar proportion of patients in the FHP (75%) and FHN (81%) groups completed the studies (χ2 = 0.16, p = 0.69).
Table 1.
Demographic and clinical characteristics
| Positive family history (n = 12) |
No family history (n = 21) |
p-value | |
|---|---|---|---|
| Age, years, mean (SD) | 50.7 (6.6) | 44.7 (12.7) | 0.14 |
| Length of illness, years, mean (SD) | 34.6 (8.7) | 25.9 (10.1) | 0.02 |
| Length of current depressive episode, months, mean (SD) | 12.2 (9.7) | 20.8 (24.7) | 0.26 |
| Clinical ratings at baseline, mean (SD) | |||
| MADRS | 33.7 (4.3) | 33.6 (5.7) | 0.98 |
| HDRS | 21.6 (2.9) | 22.3 (4.6) | 0.64 |
| CADSS | 2.6 (1.5) | 4.9 (7.6) | 0.32 |
| BPRS | |||
| Total | 36.4 (5.4) | 36.7 (5.1) | 0.90 |
| Dysphoric | 20.5 (2.8) | 20.9 (3.5) | 0.74 |
| Negative | 6.5 (3.3) | 6.4 (2.8) | 0.95 |
| Positive | 9.9 (1.6) | 9.6 (1.9) | 0.60 |
| Bipolar I disorder, n (%) | 7 (58) | 13 (62) | 0.84 |
| Education (college graduate), n (%) | 5 (42) | 10 (48) | 0.74 |
| Sex, female, n (%) | 8 (67) | 12 (57) | 0.59 |
| Lifetime diagnosis, n (%) | |||
| Anxiety disorder | 5 (42) | 11 (52) | 0.55 |
| Alcohol dependence | 6 (50) | 5 (24) | 0.13 |
| Substance abuse or dependence | 10 (83) | 5 (24) | < 0.001 |
| Family history of mood disorder, n (%) | 10 (83) | 19 (91) | 0.55 |
| Lifetime history, n (%) | |||
| Suicide attempt | 7 (58) | 9 (43) | 0.39 |
| Abuse | |||
| Emotional | 3 (25) | 6 (29) | 0.83 |
| Physical | 2 (17) | 4 (19) | 0.87 |
| Sexual | 4 (33) | 7 (33) | 1.00 |
| Mood stabilizer, n (%) | |||
| Lithium | 5 (42) | 16 (76) | 0.05 |
| Valproate | 7 (58) | 5 (24) |
SD = standard deviation; MADRS = Montgomery-Åsberg Depression Rating Scale; HDRS = Hamilton Rating Scale for Depression; CADSS = Clinician Administered Dissociative States Scale; BPRS = Brief Psychiatric Rating Scale.
For the MADRS, the linear mixed model showed a significant interaction between drug and FHP [F(1,112) = 4.96, p = 0.03]; the three-way interaction with time was not significant. While MADRS scores were significantly improved in both groups in response to ketamine (p’s < 0.001), post-hoc tests indicated that the FHP group had significantly lower scores than the FHN group [p = 0.03; d = 0.19, 95% confidence interval (CI): 0.02–0.37] (Fig. 1). Overall, 74% of patients had at least a 50% decrease from baseline MADRS scores, and 55% had a MADRS score below 10 at some point post-infusion.
Fig. 1.
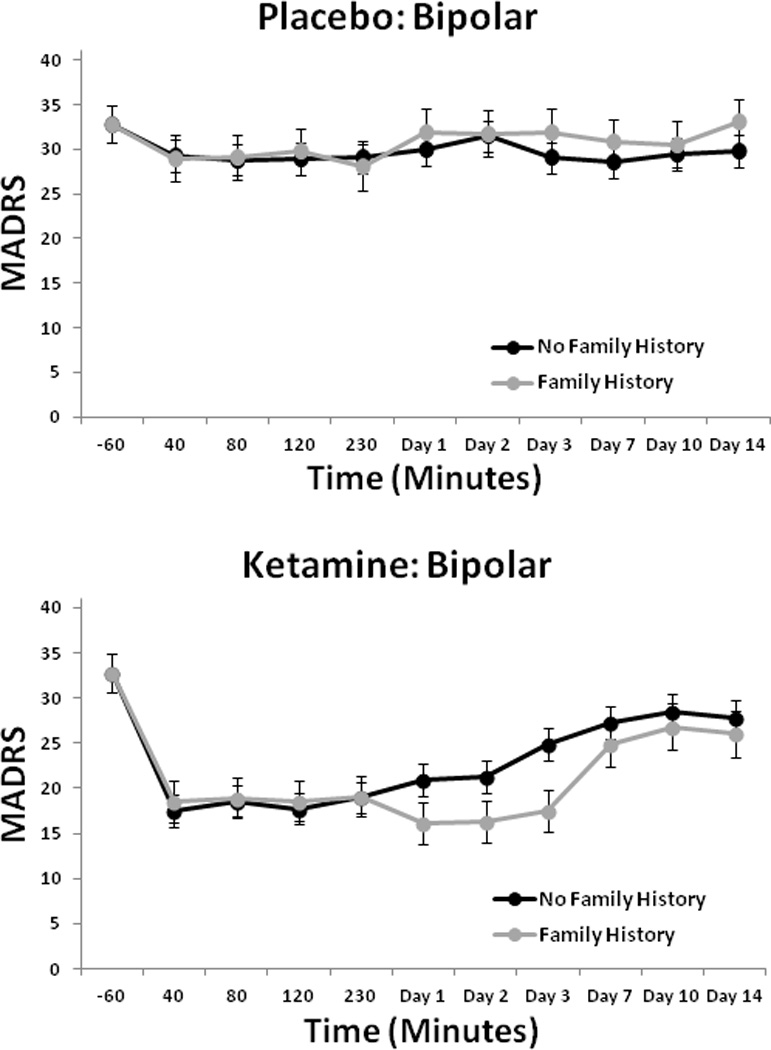
Montgomery-Åsberg Depression Rating Scale (MADRS) scores over two weeks in patients with bipolar depression with or without family history of alcohol dependence who received placebo or ketamine (n = 33).
The linear mixed model for the HDRS showed a significant interaction between drug and FHP [F(1,163) = 5.17, p = 0.02], but the three-way interaction with time was not significant. Post-hoc tests indicated that the FHP group had significantly lower scores than the FHN group (p = 0.01; d = 0.25, 95% CI: 0.05–0.45) (Fig. 2). Analysis of BPRS scores showed a significant interaction between drug and FHP for Total BPRS score [F(1,186) = 5.25, p = 0.02] and Positive Symptom subscale score [F(1,223) = 8.04, p = 0.005] (Fig. 3), but not for the Dysphoria or the Negative Symptom subscale scores. The FHP group had significantly lower total scores in response to ketamine than the FHN group (Total: p = 0.02). For the Positive Symptom subscale score, the FHP group had significantly lower scores on ketamine than placebo (p < 0.001), but the FHN group did not. The CADSS analysis found a significant three-way interaction between drug, FHP, and time [F(9,366) = 2.33, p = 0.01]. Post-hoc tests showed that the FHP group had significantly lower scores in response to ketamine than the FHN group at 40 min only (p < 0.001) (Fig. 4); comparisons at all other time points were nonsignificant.
Fig. 2.
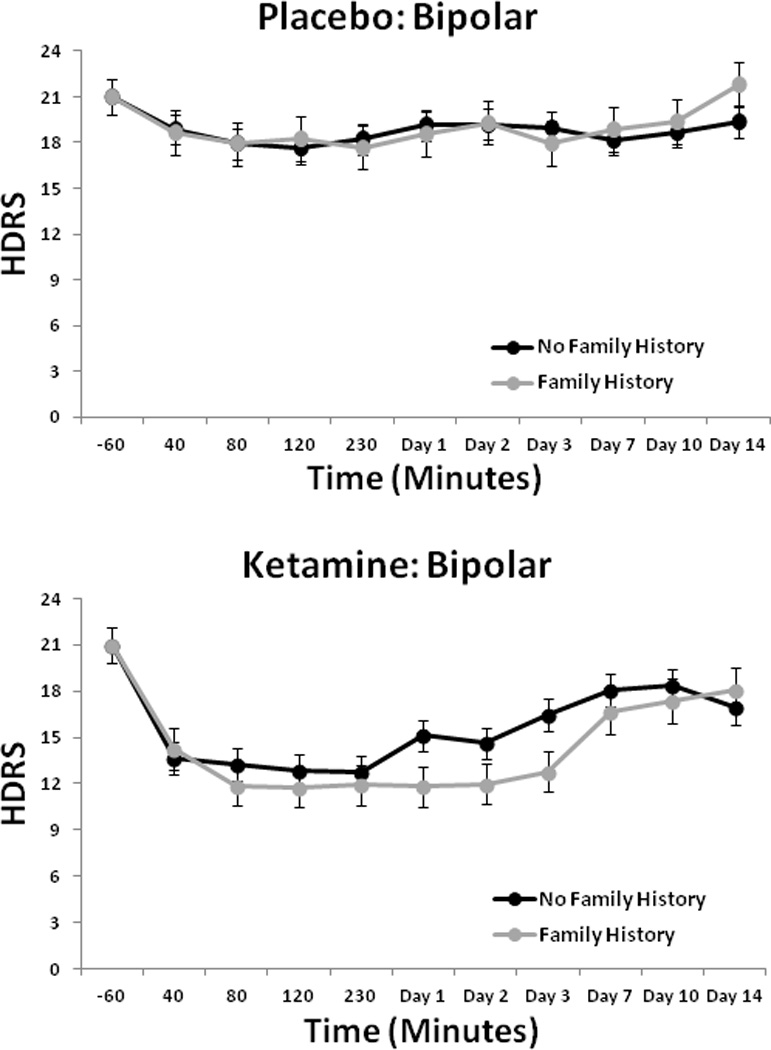
Hamilton Depression Rating Scale (HDRS) scores over two weeks in patients with bipolar depression with or without family history of alcohol dependence who received placebo or ketamine (n = 33).
Fig. 3.
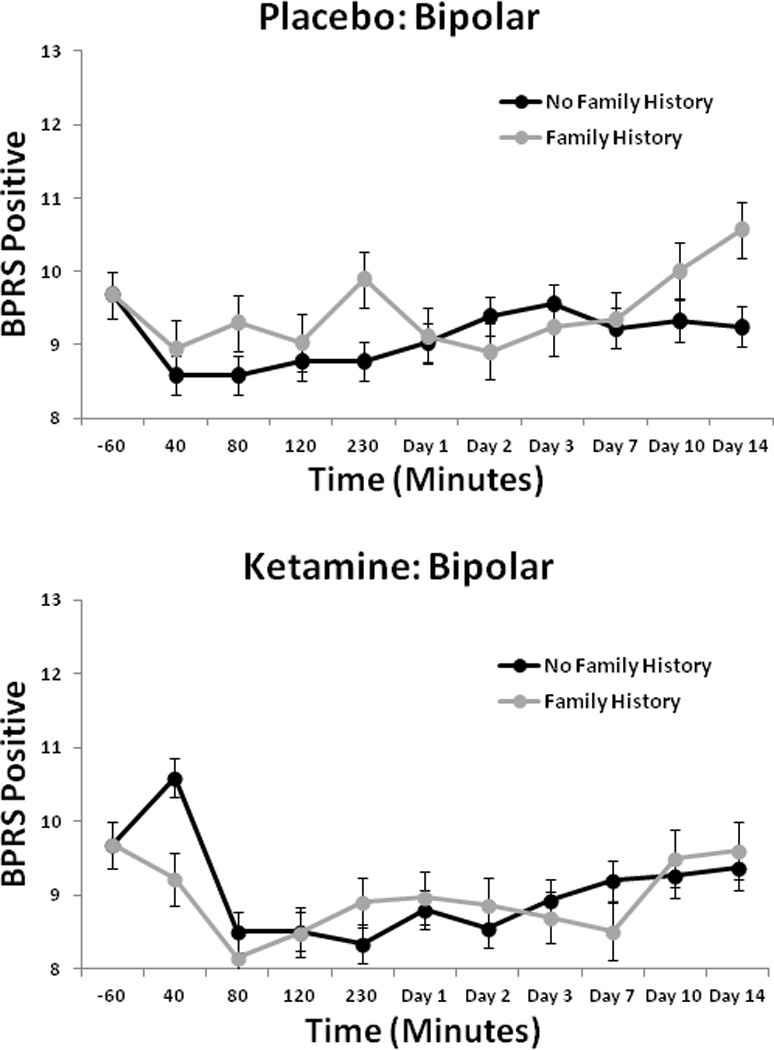
Brief Psychiatric Rating Scale (BPRS) positive scores over two weeks in patients with bipolar depression with or without family history of alcohol dependence who received placebo or ketamine (n = 33).
Fig. 4.
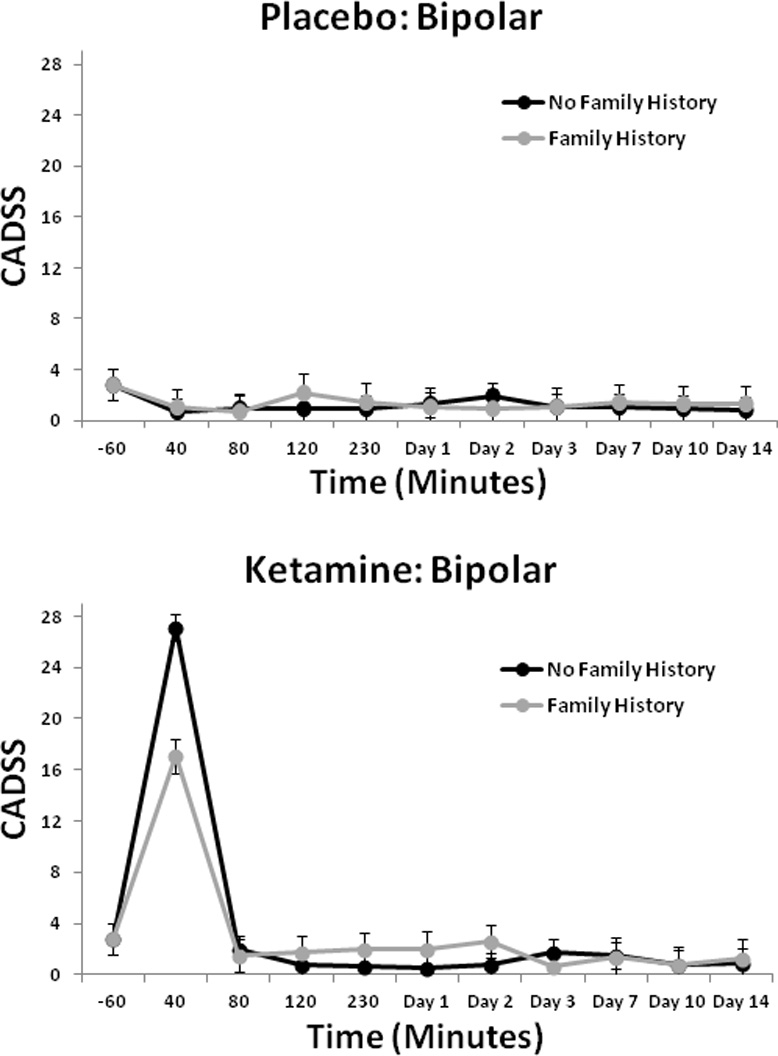
Clinician Administered Dissociative States Scale (CADSS) positive scores over two weeks in patients with bipolar depression with or without family history of alcohol dependence who received placebo or ketamine (n = 33). ***p < 0.001.
After Bonferroni corrections were applied for all six of the secondary analyses (HDRS, BPRS Total, BPRS Positive, BPRS Dysphoria, BPRS Negative, and CADSS), only the results for the BPRS Positive Symptom subscale remained significant.
Ketamine and personal history of alcohol dependence
When personal history of alcohol dependence was substituted for family history with MADRS as the outcome measure, a significant drug-by-history interaction was observed [F(1,88) = 4.40, p = 0.04]; no three-way interaction was noted. Those with a personal history of alcohol dependence had significantly lower scores in response to ketamine than those with no personal history of alcohol dependence (p = 0.04).
With regard to the secondary analyses, a significant interaction was noted on the BPRS between personal history of alcohol dependence and drug [F(1,209) = 16.08, p < 0.001]; no three-way interaction was observed. Those with a positive personal history of alcohol dependence had a greater post-infusion decrease in BPRS Total score than those without a personal history (p = 0.001). Results were similar for the BPRS Positive Symptom [history × drug: F(1,209) = 5.04, p = 0.03], Dysphoria [history × drug: F(1,155) = 9.17, p = 0.003], and Negative Symptom subscales [history × drug: F(1,269) = 27.26, p < 0.001]. Patients with a personal history of alcohol dependence had lower CADSS scores in general; a main effect for personal history of alcohol dependence was observed [F(1,177) = 7.14, p = 0.008], but the two- and three-way interactions were not significant. No significant results were found with the HDRS.
After Bonferroni corrections were applied for all six of the secondary analyses (HDRS, BPRS Total, BPRS Positive, BPRS Dysphoria, BPRS Negative, and CADSS), results for the CADSS as well as BPRS Total, BPRS Dysphoria, and BPRS Negative Symptom subscales remained significant.
Discussion
The principal finding of this post-hoc analysis was that individuals with treatment-resistant bipolar depression and FHP showed significantly greater and more durable improvements in depressive symptoms in response to a single intravenous infusion of an NMDA antagonist than BD subjects with FHN. These results support our previous open-label study (21), which found that treatment-resistant patients with MDD and FHP had a better and more rapid antidepressant response to ketamine than FHN patients. That study only examined antidepressant effects for 230 min post-infusion; however, the present study observed that improvement occurred through Day 3 in individuals with bipolar depression. In addition, personal history of alcohol dependence in these BD patients was associated with greater improvement in MADRS scores than no personal history of alcohol dependence.
With regard to perceptual disturbances and dysphoric effects, we found a blunting of perceptual responses to ketamine in those with FHP, as indicated by lower scores on the CADSS and Positive Symptom subscale of the BPRS. Individuals with a personal history of alcohol dependence also had lower scores on the CADSS as well as on BPRS Positive Symptom and Dysphoria subscales than individuals with no personal history of alcohol dependence. In contrast, our previous study of MDD patients found no decrease in BPRS Positive Symptom subscale scores in those with FHP (21); CADSS data were not collected. Compared to the studies by Phelps and colleagues (21) and Petrakis and colleagues (20), the present study did not find lower dysphoria in FHP individuals. This disparity could be due to differences in the population studied (BD vs. MDD), the controlled nature of the present study, or the concomitant use of mood stabilizer, which may have protected against dysphoria. Another possibility for this disparity is the more stringent FHP requirements of the present study, which only classified an individual as having FHP if he or she had a first-degree relative with alcohol dependence; in contrast, our previous study of MDD patients categorized FHP individuals as those with either a first- or second-degree relative with alcohol dependence.
Previous studies have shown that glutamatergic system alterations may be involved in the pathophysiology of alcohol dependence (26), as well as BD concomitant with alcoholism (12). For example, one magnetic resonance spectroscopy (MRS) study found that BD patients in long-term remission from alcoholism showed reduced dorsolateral prefrontal cortex glutamate levels, as well as glutamate plus glutamine levels, compared to BD adults who never developed alcoholism. The authors suggested that these results were consistent with a defect in the glutamatergic system that might function as a risk factor for the development of alcoholism in individuals with BD (12). Schumann and colleagues (26) investigated the link between alcohol dependence and 21 single nucleotide polymorphisms (SNPs) from 10 genes involved in glutamatergic pathways and found a significant association between risky drinking behavior in adolescents and the genes encoding mGluR5 and the NR2A subunit of the NMDA receptor. The authors suggested that a genetic variation in the NR2A gene that expresses the NMDA receptor could be involved in susceptibility to alcohol dependence. Because ketamine is a partial NR2A antagonist, it is possible that this genetic variation may render FHP patients more responsive to the antidepressant effects of ketamine (26, 27). This suggests that alterations in NMDA receptors in patients with a genetic heritability to alcoholism could be a distinct neurobiological subtype that leads to altered response to ketamine, and thus by extension may affect response to other NMDA antagonists being studied in mood disorders. Future studies will need to explore genetic influences on the co-occurrence of alcoholism and BD, as well as treatment response to ketamine.
The pooled patient group used in this study had several strengths. First, subjects were hospitalized for an average of seven weeks prior to the first ketamine infusion, permitting sufficient time to characterize them, establish family history of alcohol dependence, ensure that no alcohol consumption took place, and document the stability of depressive symptoms during their current episode. Second, both studies were randomized and controlled. Third, all subjects had been completely free of psychotropic medications (except for lithium or valproate) for at least two weeks prior to infusion. However, these findings should also be interpreted in the context of the following limitations. First, although we did our best to ensure the validity of family history reports, this method is not as accurate as interviewing each relative; however, self-report of mood and alcohol use disorders is a well-established method in family history studies. Second, it is possible that the improved response to ketamine in FHP patients resulted from the use of lithium or valproate rather than ketamine. This seems unlikely, however, given that subjects had not responded to prospective trials of these medications lasting on average six weeks. Third, the differences observed here may have been due to the demographic differences between the FHP and FHN groups, though it should be noted that controlling for these factors did not affect study results. Finally, this study was post-hoc and as such requires confirmation in a prospective controlled study.
Taken together, these findings support the hypothesis that response to the NMDA antagonist ketamine in patients with bipolar depression is mediated by family history of alcoholism. This finding warrants the search for SNPs within the glutamatergic system that might affect response to NMDA antagonists. As an example, such strategies have led to the identification of successful predictors of response to naltrexone in the treatment of alcohol dependence (28).
Acknowledgements
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH) and by the Brain and Behavior Research Foundation. CAZ had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Ioline Henter provided outstanding editorial assistance.
Footnotes
Disclosures
A patent application for the use of ketamine in depression has been submitted listing CAZ among the inventors; he has assigned his rights on the patent to the U.S. Government but will share a percentage of any royalties that may be received by the government. DAL, LI, NB, JF-C, DM, CAM, and CC have no conflicts of interest to disclose, financial or otherwise.
References
- 1.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salloum IM, Thase ME. Impact of substance abuse on the course and treatment of bipolar disorder. Bipolar Disord. 2000;2:269–280. doi: 10.1034/j.1399-5618.2000.20308.x. [DOI] [PubMed] [Google Scholar]
- 3.Ostacher MJ, Perlis RH, Nierenberg AA, et al. Impact of substance use disorders on recovery from episodes of depression in bipolar disorder patients: prospective data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2010;167:289–297. doi: 10.1176/appi.ajp.2009.09020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black DW, Winokur G, Nasrallah A. Mortality in patients with primary unipolar depression, secondary unipolar depression, and bipolar affective disorder: a comparison with general population mortality. Int J Psychiatry Med. 1987;17:351–360. doi: 10.2190/vl1b-7yee-91j5-mwra. [DOI] [PubMed] [Google Scholar]
- 5.Brady KT, Lydiard RB. Bipolar affective disorder and substance abuse. J Clin Psychopharmacol. 1992;12:17S–22S. doi: 10.1097/00004714-199202001-00004. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin FK. From the Alcohol, Drug Abuse, and Mental Health Administration. JAMA. 1990;264:2495. [PubMed] [Google Scholar]
- 7.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarate CA, Jr, Brutsche NE, Ibrahim L, et al. Replication of ketamine's antidepressant efficacy in bipolar depression: A randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermann D, Weber-Fahr W, Sartorius A, et al. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry. 2012;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 10.McCool BA, Christian DT, Diaz MR, Läck AK. Glutamate plasticity in the drunken amygdala: the making of an anxious synapse. Int Rev Neurobiol. 2010;91:205–233. doi: 10.1016/S0074-7742(10)91007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krystal JH, Petrakis IL, Limoncelli D, et al. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology. 2003;28:2020–2028. doi: 10.1038/sj.npp.1300252. [DOI] [PubMed] [Google Scholar]
- 12.Nery FG, Stanley JA, Chen HH, et al. Bipolar disorder comorbid with alcoholism: a 1H magnetic resonance spectroscopy study. J Psychiatr Res. 2010;44:278–285. doi: 10.1016/j.jpsychires.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarate CA, Jr, Charney DS, Manji HK. Modulation of the NMDA receptor complex could lead to the development of novel antidepressants. Arch Gen Psychiatry. in press. [Google Scholar]
- 14.Zarate CA, Jr, Quiroz JA, Singh JB, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry. 2005;57:430–432. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Brennan BP, Hudson JI, Jensen JE, et al. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology. 2010;35:834–846. doi: 10.1038/npp.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarate C, Jr, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18:293–303. doi: 10.3109/10673229.2010.511059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mann K, Kiefer F, Spanagel R, Littleton J. Acamprosate: recent findings and future research directions. Alcohol Clin Exp Res. 2008;32:1105–1110. doi: 10.1111/j.1530-0277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 18.Krystal JH, Petrakis IL, Krupitsky E, Schutz C, Trevisan L, D'Souza DC. NMDA receptor antagonism and the ethanol intoxication signal: from alcoholism risk to pharmacotherapy. Ann NY Acad Sci. 2003;1003:176–184. doi: 10.1196/annals.1300.010. [DOI] [PubMed] [Google Scholar]
- 19.Krystal JH, Petrakis IL, Webb E, et al. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch Gen Psychiatry. 1998;55:354–360. doi: 10.1001/archpsyc.55.4.354. [DOI] [PubMed] [Google Scholar]
- 20.Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- 21.Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- 23.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overall JE, Gorham DR. The Brief Psychiatric Rating-Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- 25.Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 26.Schumann G, Johann M, Frank J, et al. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65:826–838. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
- 27.Petrenko AB, Yamakura T, Fujiwara N, Askalany AR, Baba H, Sakimura K. Reduced sensitivity to ketamine and pentobarbital in mice lacking the N-methyl-D-aspartate receptor GluRepsilon1 subunit. Anesth Analg. 2004;99:1136–1140. doi: 10.1213/01.ANE.0000131729.54986.30. [DOI] [PubMed] [Google Scholar]
- 28.Anton RF, Oroszi G, O'Malley S, et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]


