Abstract
IL-9 producing Th9 cells have been associated with autoimmune diseases, such as experimental autoimmune encephalitis. However, the factors that negatively regulate Th9 cells during autoimmune inflammation are unclear. Here we show that IFN-γ inhibits Th9 differentiation both in vitro and in vivo. This suppressive activity was dependent on the transcription factor STAT-1. In addition to its direct inhibitory effect on Th9 differentiation, IFN-γ suppressed Th9 cells through the induction of IL-27 from dendritic cells. In vitro, treatment of naive CD4+ T cells with IL-27 suppressed the development of Th9 cells, which was partially dependent on the transcription factors STAT-1 and T-bet. Moreover, IL-27-treatment completely abrogated the encephalitogenicity of Th9 cells in the EAE model. Thus our results identify a previously unknown mechanism by which IFN-γ limits Th9 mediated autoimmune inflammation through dendritic cell modulation of IL-27.
Keywords: Th9 cells, IFN-γ, IL-27, EAE/MS, Inflammation
Introduction
Experimental autoimmune encephalomyelitis (EAE), induced by immunization of mice with MOG peptide 35–55 (MOG 35–55) in complete Freund's adjuvant, is a well-established model for the human autoimmune disease multiple sclerosis (MS). Evidence indicates the involvement of a specific type of T helper cell, termed Th9 cells in the pathogenesis of EAE (1–4). Th9 cells are characterized by the expression of IL-9. Th9 cells have been established as a separate lineage of T helper cells distinct from conventional Th1, Th2 and Th17 cells (5–7). It has been demonstrated that mice deficient for IL-9 or IL-9R are protected from EAE, and the suppression of EAE in IL-9−/− mice could be reversed when treated with recombinant IL-9 (1–4). In addition altered IL-9 or IL-9R expression has been linked to other autoimmune diseases such as asthma and arthritis (7–9). Although Th9 cells have been suggested to be mediators of the inflammation associated with autoimmune diseases, the factors that negatively regulate Th9 cells during autoimmune inflammation are not known.
IL-27 is a potent anti-inflammatory cytokine, which belongs to the IL-12 family and is comprised of an IL-12p40-related protein, encoded by the EBV-induced gene 3 (EBI3, also known as IL27), and a unique IL-12p35-like protein, IL-27p28v (10). Initial studies on the biology of IL-27 suggested a role for IL-27 in the initiation of the Th1 response (11, 12). However, recent studies have indicated that IL-27 has broad inhibitory effects on Th1 and Th2 subsets of T cells as well as on APC function (13,14). In addition, we and others have shown that IL-27 is capable of inducing IL-10 producing regulatory Tr1 cells while inhibiting IL-17 producing Th17 cells both from humans and mice and acts as a negative feedback mechanism against proinflammatory immune responses (15–19). Although IL-27 has been shown to negatively regulate other inflammatory T cell subsets, the role of IL-27 in the regulation of Th9 cells remain unknown.
Here we show that IFN-γ inhibits Th9 differentiation both in vitro and in vivo. In addition to its direct inhibitory effect on Th9 differentiation, IFN-γ suppressed Th9 cells through the induction of IL-27 from DCs. IL-27 suppressed the development of Th9 cells, which was partially dependent on the transcription factors STAT-1 and T-bet. Furthermore, IL-27 treatment completely abrogated the encephalitogenicity of Th9 cells in the EAE model. Taken together, our results identify a previously unknown mechanism by which IFN-γ limits Th9 mediated autoimmune inflammation through DC modulation of IL-27.
Materials and Methods
Mice
C57BL/6 WT, IFN-γ−/−, IFN-γR−/−, IL-10−/−, T-bet−/− and IL-21R−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). 129S6/SvEv STAT-1−/− and 129S6/SvEv control mice were obtained from Taconic. MOG specific TCR transgenic mice - 2D2 and IL-27R−/− mice were obtained from Dr. Vijay Kuchroo (Harvard University). STAT-3−/− mice were obtained from David E. Levy (New York University Medical Center). All mice were used at 6–8 weeks of age. Animals were maintained in a specific pathogen-free condition in the animal facility of Harvard Institutes of Medicine. All experiments were in accordance with guidelines from the committee on animals at Harvard Medical School.
Reagents
Cells were maintained with IMDM medium (Invitrogen). Neutralizing anti-IL-9 was obtained from BD Biosciences and anti-IL-27Ab was obtained from R&D systems. FACS antibodies to IL-9 and IFN-γ were obtained from Biolegend. Recombinant mouse TGF-β, IL-4 and IL-27 were obtained from R&D Systems. All RT-PCR primers and reagents were obtained from Applied Biosystems (CA, USA).
Induction and evaluation of EAE
Mice were injected subcutaneously in both flanks with 100 μg of MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK) dissolved in PBS emulsified in an equal volume of complete Freund's adjuvant- CFA (Difco) supplemented with 5mg/ml Mycobacterium tuberculosis H37Ra and injected twice intravenously with 200 ng of pertussis toxin (List Biological Laboratories) administered on the day of immunization and 48 h later. Clinical assessment of EAE was performed daily after disease induction according to the following criteria: 0, no disease; 1, tail paralysis; 2, hindlimb weakness or partial paralysis; 3, complete hindlimb paralysis; 4, forelimb and hindlimb paralysis; 5, moribund state. Mean clinical scores on separate days were calculated by adding scores of individual mice and dividing total number of mice in each group, including mice that did not develop signs of EAE.
Anti-IL-9 treatment
Mice (n =8) received 50μgm of IL-9 antibody (BD Biosciences) or IgG control intraperitoneally on every other day starting on day -1 post immunization.
RNA isolation, cDNA synthesis and real-time PCR
Total RNA was isolated from cell pellets using RNA easy Micro Kit (QIAGEN). RNA was stored at −80°C. First strand cDNA synthesis was performed for each RNA sample from 0.5–1 μgm of total RNA using Taqman reverse transcription reagents. cDNA was amplified using sequence specific primers (the following were from Applied Biosystems: IL-27, Mm00461164_ml; IL-10, Mm99999062_m1; IFN-γ, Mm01168134_m1; IL-21, Mm00517640_m1 and IL-9, Mm00434305_m1 real-time PCR mix (Applied Biosystems) on ABI7500 cycler. GAPDH gene was used as an endogenous control to normalize for differences in the amount of total RNA in each sample. All values were expressed as fold increase or decrease relative to the expression of GAPDH.
Cytokine analysis
Spleens or draining lymph nodes (inguinal regions) were harvested and pooled from EAE mice and single-cell suspensions were prepared. Cells were cultured at 5×105/well in 96-well U-bottom plates with 20 μg/ml of MOG35–55 peptide in RPMI 1640 medium supplemented with 10% FCS. For ELISA, supernatants were harvested at 72 h of culture. The concentrations of indicated cytokines were measured by quantitative capture ELISA according to the guidelines of the manufacturer (BD Biosciences).
Preparation and evaluation of CNS cells
Animals were perfused with cold PBS. Brains and spinal cords were dissected and incubated in 2.5mg/ml colleganase D for 30 minutes at 37°C. Single-cell suspensions were prepared by passing through 70μm strainer. Cells were washed in RPMI 1640 medium and mononuclear cells were isolated using a discontinuous Percoll gradient (Pharmacia, Piscataway, NJ). Cells were washed twice and CD4+ T cells were isolated from this suspension by magnetic separation using microbeads (Miltenyi Biotec).
Generation of DCs
DCs were derived from bone marrow progenitor cells. In brief, the femoral and tibial cells were harvested in DC culture medium (RPMI 1640 medium, 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 20 ng/ml GM-CSF, and 10 ng/ml IL-4) and seeded in 24-well plates at a density of 1 × 106 cells/ml/well. Culture medium was replaced with fresh medium every 3 days. At day 6, dislodged cells were used as bone marrow-derived DCs. Splenic DCs were isolated using CD11c beads (Miltenyi Biotec).
IFN-γ treatment of DCs
DCs were stimulated with IFN-γ in the presence or absence of LPS for 48 hours. Supernatants were collected as CM and stored at −70°C. The amount of IL-27 was measured using ELISA.
T Cell culture
Naive CD4+ T (CD4+ CD44lo CD62L+) cells were cultured in RPMI medium (Sigma). Medium was supplemented with 5% FCS, 1% penicillin/streptomycin, 1% L-glutamine and Na-pyruvate and 50 μM β-mercaptoethanol. Cells were stimulated with plate-bound anti-CD3 (2 μg/ml) and anti-CD28 (2 μg/ml). For Th9 cell differentiation, cells were stimulated in the presence of the following cytokines. 20 ng/ml IL-4, and 3 ng/ml TGF-β.
In some culture condition recombinant mouse IFN-γ (100 ng/ml) or IL-27 (100 ng/ml) were added. For adoptive transfer of EAE, 2D2 CD4+ T cells were stimulated under Th9 differentiation condition in the presence of absence of IL-27. Restimulated cells were collected and extensively washed with PBS. 5 × 106 cells were injected i.v. into Rag-1−/− mice. Recipient mice were injected i.p. with 200 ng of pertussis toxin (PT) (List Biological Laboratories) on day 0 and day 2 after T cell transfer.
Statistical Analysis
Statistical analysis was performed using the unpaired t test. A value of P < 0.05 was considered significant. Data are presented as mean S.E.M. For EAE, groups were compared using linear regression analysis.
Results
IFN-γ inhibits Th9 differentiation
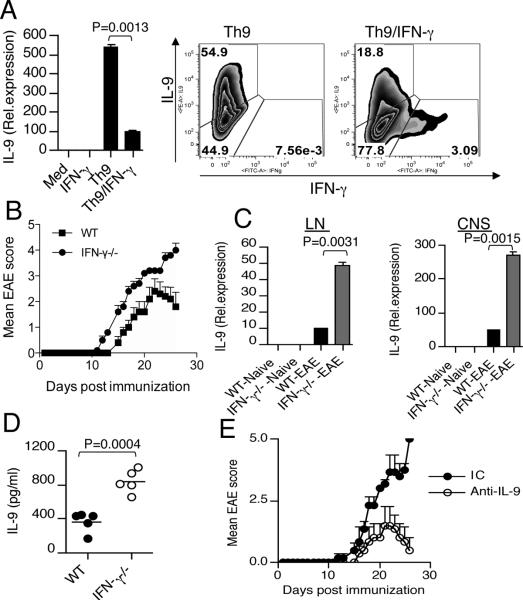
We first examined the effect of IFN-γ on the production of IL-9 from Th9 cells. We found that IFN-γ stimulation significantly inhibited IL-9 production from Th9 cells (Fig. 1A). Activation of the intracellular signaling molecule STAT-1 is a common feature of IFN-γ signaling (20). We therefore tested whether the inhibition of IL-9 production by IFN-γ was dependent on STAT-1 using STAT-1−/− T cells. We found that IFN-γ did not suppress Th9 development in STAT-1−/− T cells (Supplementary Fig. 1A). These data indicate that IFN-γ mediated suppression of Th9 development requires activation of STAT-1. To test whether IFN-γ regulates IL-9 expression in vivo, we induced EAE in WT and IFN-γ−/− mice. Consistent with previous studies, we found that IFN-γ−/− mice developed severe EAE (Fig. 1B). We observed altered IL-9 expression in T cells isolated from WT and IFN-γ−/− mice with EAE (Fig. 1C). In addition we found that IFN-γ−/− mice had high serum levels of IL-9 compared to WT mice (Fig. 1D). To investigate whether IL-9 blockade in vivo could reverse the severe EAE phenotype observed in IFNγ−/− mice, we induced EAE in IFNγ−/− mice and administered neutralizing anti-IL-9 antibody. We found that anti-IL-9 antibody treatment delayed the onset of clinical disease and ameliorated the severity of EAE (Fig. 1E). These results suggest that IFN-γ plays an important role in the regulation of IL-9 production from T cells and that neutralization of IL-9 suppresses the production of encephalitogenic T cells.
Figure 1. IFN-γ inhibits Th9 differentiation both in vitro and in vivo.
(A) Real-time PCR and flow cytometry analysis of naïve CD4+ T cells stimulated under Th9 conditions in the presence or absence of IFN-γ (100 ng/ml). (B) Clinical scores of WT and IFN-γ−/− mice at various times after immunization with MOG (33–55) in CFA. (C) Real-time RTPCR analysis of IL-9 in LN and CNS derived CD4+ T cells isolated from WT and IFN-γ−/− mice with or without EAE. (D) ELISA of serum IL-9 from WT and IFN-γ−/− mice with EAE. (E) Clinical scores of EAE in IFN-γ−/− mice (n=8/group) treated with anti-IL-9 antibody or isotype control. Results are representative of three independent experiments.
IFN-γ limits Th9 differentiation through dendritic cell modulation of IL-27
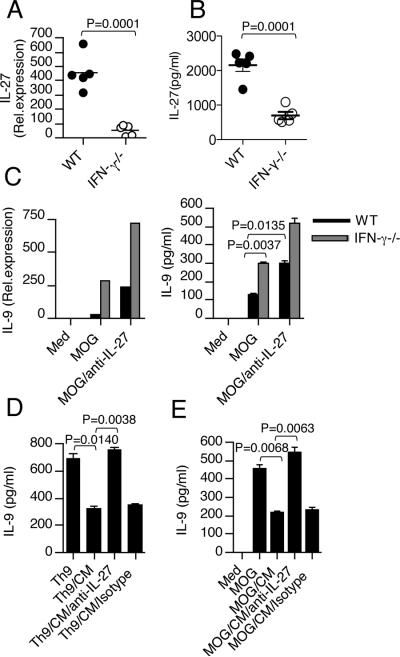
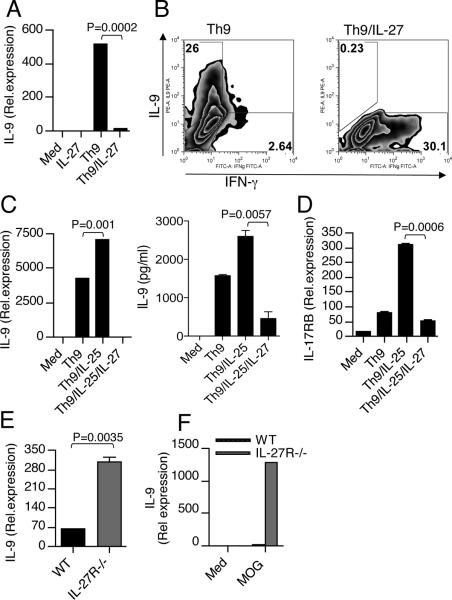
We have previously shown that IFN-γ induces expression of IL-27 by DCs (21). To test whether IFN-γ regulates DC expression of IL-27 in vivo, we induced EAE in WT and IFN-γ−/− mice. We found that expression of IL-27 was upregulated at both the mRNA and protein levels in WT-DCs of mice with EAE and that DCs from IFN-γ−/− mice with EAE had much less IL-27 expression compared with WT mice (Fig. 2A & B). Because we observed that IFN-γ deficiency led to reduced IL-27 expression in DCs and increased IL-9 in T cells during EAE, we investigated whether DC-derived IL-27 had a role in Th9 cell differentiation, specifically T cell production of IL-9. For this, we immunized WT and IFN-γ−/− mice with MOG and isolated splenocytes on day 10 post-immunization. Cells were then cultured with MOG in vitro in the presence or absence of anti-IL-27 antibody and MOG Ag-specific IL-9 production was measured. We found that T cells from IFN-γ−/− mice showed increased IL-9 levels compared with T cells from WT mice (Fig. 2C). In addition we found that the addition of neutralizing antibody to IL-27 significantly increased IL-9 production both from WT and IFN-γ−/− mice (Fig. 2C). Next, we tested whether conditioned medium (CM), from DCs stimulated with IFN-γ has an inhibitory effect on IL-9 production from T cells. We found that the addition of CM from IFN-γ treated DCs inhibited IL-9 production from Th9 culture (Fig. 2D). To determine whether IL-27 contributes to the inhibitory effect of the IFN-γ pathway, we used an anti-IL-27 antibody to block IL-27 activity. When anti-IL-27 antibody was added to block the IL-27 activity in the CM from IFN-γ treated DCs, the IFN-γ mediated inhibitory effect on Th9 cells was reversed (Fig. 2D). To determine whether IL-27 also contributes to IFN-γ mediated inhibition of IL-9 producing encephalitogenic T cells lymphocytes isolated from mice immunized with MOG/CFA were restimulated with MOG peptide in the presence of CM from IFN-γ treated DCs with or without IL-27 antibody. As shown in Fig. 2E, CM from IFN-γ treated DCs suppressed IL-9 production from antigen-specific T cells, and anti-IL-27 antibody inhibited this suppression. Moreover, the inhibition of Th9 was not due to the presence of IFN-γ in the culture supernatants as we used IFN-γR−/− T cells. Taken together, our results demonstrate that IFN-γ signaling events in DCs play a major role in negative regulation of Th9 development. These data suggested that IL-27 negatively regulates IL-9 production from T cells and prompted us to examine the function of IL-27 in the Th9 differentiation pathway in vitro. To directly investigate the effect of IL-27 on Th9 differentiation, we cultured naïve CD4+ T cells under Th9 polarizing condition in the presence or absence of rIL-27. We found that addition of IL-27 significantly inhibited IL-9 from Th9 cells (Fig. 3A & B). IL-27 blocked Th9 cell differentiation even more than IFN-γ. It has been recently shown that IL-25 is involved in the amplification of Th9 cells and expression of the IL-25 receptor on Th9 cells has been described (22). Although addition of IL-25 to Th9 differentiation conditions increased IL-9 expression, even under these strong Th9 polarizing conditions IL-27 markedly inhibited IL-9 secretion and IL-25R expression (Fig. 3C & D). Collectively, these data indicate that IL-27 inhibits the differentiation of Th9 cells. To confirm that IL-27 is indeed a negative regulator of IL-9 expression in vivo, we isolated lymphocytes from WT and IL-27R−/− mice with EAE and found that IL-27R−/− mice had elevated levels of IL-9 after MOG stimulation compared to cells from wild-type mice. In agreement with previous studies, we found that IL-27R−/− mice developed severe EAE (Supplementary Fig. 1B). Consistent with clinical scores, ex vivo CD4+ T cells isolated from the CNS of IL-27R−/− mice expressed higher levels of IL-9 in comparison with CD4+ T cells from WT mice (Fig. 3E). Moreover, we observed higher IL-9 expression by ex vivo re-stimulated lymph node cells from IL-27R−/− mice than by cells from WT mice (Fig. 3F).
Figure 2. IFN-γ limits IL-9 production through induction of IL-27 from DCs.
(A) (A) Real-time RT-PCR analysis of IL-27p28 in DCs isolated from spleen of EAE bearing WT and IFN-γ−/− mice (n =5 per group). (B) DCs from EAE bearing WT and IFN-γ−/− mice (n =5 per group) were stimulated with LPS (100ng/ml). IL-27 production was measured by ELISA. Results are representative of three independent experiments. (C) Splenocytes isolated from immunized WT and IFN-γ−/− mice on day 10 were restimulated with MOG peptide in the presence or absence of anti-IL-27 antibody. IL-9 levels were determined by real-time PCR and ELISA. (D) WT-DCs were stimulated with IFN-γ for 24 -48 hours. Supernatants from IFN-γ stimulated DCs were used as CM; they were added to Th9 culture in the presence or absence of anti-IL-27 antibody and cells were incubated for 72 hours. IL-9 production by CD4+ T cells was measured by ELISA. (E) IL-27 contributes to IFN-γ mediated inhibition of encephalitogenic T cells. Splenocytes isolated from immunized IFN-γR−/− mice were restimulated with MOG peptide for 72 hours in the presence of CM from IFN-γ treated DCs plus anti-IL-27 antibody or an isotype control. IL-9 levels were measured by ELISA. Data shown are representative of 3 independent experiments.
Figure 3. IL-27 inhibits Th9 differentiation both in vitro and in vivo.
(A) Real-time PCR and (B) flow cytometry analysis of naïve CD4+ T cells activated with anti-CD3 and anti-CD28 under Th9-inducing conditions in the presence or absence of IL-27. Results are representative of five independent experiments. (C) Real-time PCR and ELISA of IL-9 in naïve CD4+ T cells cultured under Th9 conditions together with IL-25 in the presence or absence of IL-27. Results are representative of three independent experiments. (D) Real-time PCR analysis of IL-25R on Th9 cells stimulated with or without IL-27. (E) Real-time PCR for IL-9 mRNA in CD4+ T cells isolated from the CNS of WT and IL-27R−/− mice with EAE. (F) Splenocytes from WT and IL-27R−/− mice were harvested 10 d after immunization and were stimulated ex vivo with MOG peptide 35–55. IL-9 levels were measured by real-time PCR. Results are representative of three independent experiments.
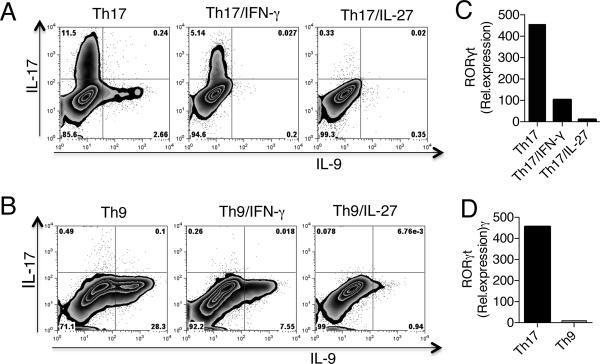
In addition to Th9 cells, Th17 cells can also produce IL-9. It has been demonstrated that IL-9 together with TGF-β can induce the differentiation of naive CD4+ T cells into Th17 cells in vitro, and that IL-9 produced by Th17 cells themselves amplifies Th17 development in an autocrine manner (23). IFN-γ and IL-27 have been shown to inhibit Th17 differentiation both in humans and mice (16, 24). Moreover, T cells from IFN-γ and IL-27R−/− mice had elevated levels of IL-17 during the course of EAE (18, 21). Thus we tested whether IFN-γ and IL-27 had suppressive function on IL-9 production by Th17 cells. We found that both IFN-γ and IL-27 inhibited IL-9 expression from Th17 cells (Fig. 4A). Consistent with previous reports, we also found that IFN-γ and IL-27 inhibited IL-17 and RORγt from these cells (Fig. 4A & B). However, in vitro polarized Th9 cells produced no IL-17 and these cells did not express RORγt (Fig. 4C & D). Thus our data indicate that the decrease in IL-9 production that we observed during EAE in WT mice can be attributed to the suppressive effect of IFN-γ/IL-27 on development of both Th9 and Th17 cells.
Figure 4. IFN-γ and IL-27 inhibits Th17 produced IL-9.
(A) Flow cytometry analysis of naïve CD4+ T cells stimulated under Th17 condition in the presence or absence of IFN-γ (100 ng/ml) and IL-27 (100 ng/ml). (B) Real-time PCR analysis of RORγt in Th17 cells in the presence or absence of IFN-γ and IL-27. (C) Flow cytometry analysis of naïve CD4+ T cells stimulated under Th9 condition in the presence or absence of IFN-γ (100 ng/ml) and IL-27 (100 ng/ml). (D) Real-time PCR analysis of RORγt in Th17 and Th9 cells.
IL-27 reciprocally regulates Th9 and Tr1 cells
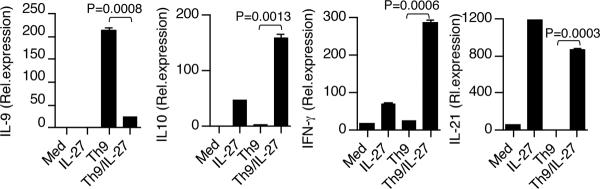
We and others have shown that IL-27 induces IFN-γ+ IL-10+ regulatory type 1 (Tr1) cells both from humans and mice (15, 16, 21). Thus we asked whether activation of Th9 cells in the presence of IL-27 induced Tr1 cells. We found that addition of IL-27 inhibited Th9 differentiation while inducing regulatory type 1 (Tr1) cytokines IL-10, IFN-γ and IL-21 (Fig. 5). Following this, we tested whether the inhibition of IL-9 production by IL-27 was dependent on any of these induced cytokines. We found that the addition of IL-27 to Th9 cells blocked IL-9 expression at RNA and protein levels in both IFN-γ−/− (Supplementary Fig. 2A) and IL-10−/− cells (Supplementary Fig. 2B). Thus, IFN-γ and IL-10 alone were not responsible for the suppression of IL-9 expression by IL-27. IL-27 drives the expansion of Tr1 cells by inducing the expression of IL-21, a member of the IL-2 family of cytokines, which acts as an autocrine growth factor for Tr1 cells (25). Thus, we asked whether the inhibition of IL-9 production by IL-27 was dependent on IL-21. We found that IL-27-mediated Th9 cell suppression was not secondary to the induction of IL-21, as addition of IL-27 to IL-21R−/− Th9 cells completely inhibited IL-9 expression both at RNA and protein levels (Supplementary Fig. 3). Collectively, these data indicated that IL-27 inhibits Th9 differentiation while inducing Tr1 cytokines and that the inhibition of Th9 cells is not mediated through any of the induced cytokines. Thus, our results demonstrate that there is a reciprocity in the regulation of the pathogenic cytokine IL-9 and the protective cytokine IL-10 in the immune system depending on IL-27 secretion by the innate immune system.
Figure 5. IL-27 inhibits Th9 differentiation while inducing Tr1 cells.
Real-time PCR and flow cytometry analysis of IL-9, IFN-γ, IL-10 and IL-21 in naïve CD4+ T cells activated with anti-CD3 and anti-CD28 under Th9-inducing conditions in the presence or absence of IL-27. Results are representative of three independent experiments.
IL-27 inhibits IL-9 production through STAT1 and T-bet dependent pathway
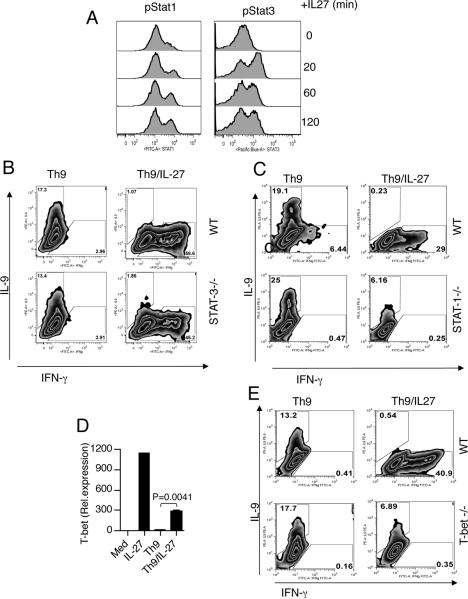
IL-27 induces the phosphorylation of STAT1 and STAT3 proteins both of which play an important role in T cell function (26, 27). To analyze whether the differentiation of Th9 cells in the presence of IL-27 alters their phosphorylation profile, we stimulated naïve CD4+ T cells for 24 h in the presence of Th9 differentiation conditions. After 24h IL-27 was added and phosphorylation of STAT1 and STAT3 was analyzed. We found that both STAT proteins were phosphorylated at the same level in the presence of Th9 conditions (Fig. 6A). Thus, IL-27 induces efficient phosphorylation of STAT1 and STAT3 after differentiation of CD4+ T cells under Th9 conditions, suggesting that both proteins could be involved in IL-27-mediated suppression of Th9 development. Thus, to determine if the ability of IL-27 to inhibit IL-9 production involved these transcription factors, we stimulated naïve CD4+ T cells from STAT-1−/− or STAT-3−/− mice and stimulated the cells under Th9 inducing conditions in the presence or absence of IL-27. The addition of IL-27 resulted in substantial inhibition of IL-9 production by WT, and STAT-3−/− CD4+T cells, but the effect was partially compromised in the absence of STAT1 (Fig. 6B and C). Thus, STAT3 was not responsible for the suppression of IL-9 expression by IL-27. Previous studies have shown that signaling through IL-27R leads to the activation of the transcription factor T-bet (12, 27, 28). We therefore tested whether the differentiation of Th9 cells in the presence of IL-27 leads to T-bet induction. We found that the addition of IL-27 induced T-bet expression in Th9 cells (Fig. 6D). To determine if the ability of IL-27 to inhibit IL-9 production is dependent on T-bet, we cultured naïve CD4+ T cells from WT and T-bet−/− mice under Th9 inducing conditions in the presence of IL-27. The addition of IL-27 resulted in complete inhibition of IL-9 production by WT CD4+T cells, but the effect was partially compromised in the absence of T-bet (Fig. 6E). Taken together our results suggest that IL-27 mediated inhibition of Th9 cells is mediated partially through the transcription factors T-bet and STAT-1.
Figure 6. Suppression of Th9 differentiation by IL-27 is partially dependent on STAT- 1and T-bet.
(A) IL-27 induces STAT1 and STAT3 phosphorylation in Th9 cells. Naïve CD4+ T cells were stimulated with CD3/CD28 for 24 h in the presence of TGF-β and IL-4 (Th9 conditions). Thereafter, the cells were harvested, rested in 37°C for 2 h in culture medium without cytokines and subsequently stimulated with IL-27 for 20, 60 and 120 min and subsequently the cells were analysed by flow cytometry for STAT1 and STAT3 phosphorylation. (B) Flow cytometry of naïve CD4+ T cells from WT and STAT-3−/− mice stimulated under Th9 polarizing condition in the presence or absence of IL-27. (C) Flow cytometry of naïve CD4+ T cells from WT and STAT-1−/− mice stimulated under Th9 polarizing condition in the presence or absence of IL-27. (D) Real-time PCR and flow cytometry analysis of T-bet in Th9 cells in the presence or absence of IL-27. (E) Flow cytometry of naïve CD4+ T cells from WT and T-bet−/− mice were stimulated under Th9 polarizing condition in the presence or absence of IL-27. Data are presented are representative of three separate experiments.
IL-27 inhibits encephalitogenity of Th9 cells
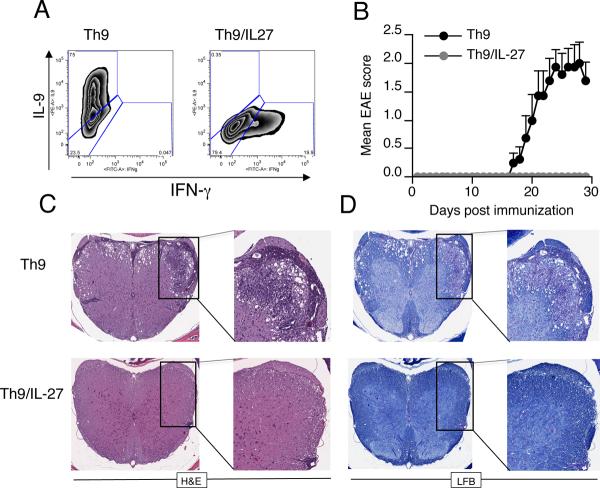
Because in vitro treatment of T cells with IL-27 inhibited the differentiation of Th9 cells, we next investigated whether IL-27 treatment inhibited encephalitogenity of Th9 cells in vivo. For this we stimulated MOG specific 2D2 T cells under Th9 polarizing conditions in the presence or absence of IL-27 (Fig. 7A) and transferred the resulting T cells into Rag-1−/− mice. We found all Th9 cell recipients developed EAE after transfer. However, IL-27 treatment completely suppressed EAE induced by adoptive transfer of Th9 cells (Fig. 7B). Histopathological analysis of the spinal cords revealed that Th9 cells reconstituted Rag-1−/− EAE mice had increased amounts of infiltrating inflammatory cells and increased demyelination. In contrast, IL-27 treated Th9 cells failed to induce CNS inflammation, which correlated with the absence of clinical EAE (Fig. 7C and D). These data demonstrate that IL-27 controls the adoptive induction of EAE by IL-9 producing cells and that the disease inhibitory effect of IL-27 correlates with the inhibition of IL-9. Taken together our findings identify IL-27 as an antagonist of Th9 development and indicate a possible therapeutic target for treating inflammatory diseases associated with Th9 cells.
Figure 7. IL-27 inhibits encephalitogenity of Th9 cells.
(A) Flow cytometry analysis of IL-9 expression in naïve CD4+ T cells from 2D2 mice differentiated into Th9 cells in the presence or absence of IL-27. (B) IL-27 inhibits encephalitogenity of Th9 cells. Clinical scores of mice that received Th9 cells treated with or wihout IL-27. Mice were monitored for the development of disease. Results are representative of two independent experiments. (C and D) Spinal cord sections were stained with H&E and luxol fast blue to assess inflammation and demyelination.
Discussion
It was initially thought that CD4+ T cells mediating autoimmunity in EAE had a Th1 phenotype characterized by the production of IFN-γ (29). However, this view has been challenged by studies describing more severe EAE in IFN-γ deficient animals. In fact, mice deficient in IL-12p35 or IFN-γR develop more severe EAE than wild-type mice (30, 31). In addition, IFN-γ−/− mice immunized with collagen in CFA develop severe arthritis (32). More over, a disease ameliorating effect of IFN-γ has been observed in lethal autoimmune myocarditis (33) suggesting that IFN-γ may actually have a protective function. Nonetheless, although endogenous IFN-γ is protective in animal models of arthritis and in EAE, the mechanisms that orchestrate the anti-inflammatory effects of IFN-γ in controlling autoimmune inflammation are poorly understood. Here we show that IFN-γ inhibits Th9 differentiation both in vitro and in vivo and that anti-IL-9 antibody treatment delays the onset of clinical disease and ameliorate the severity of EAE in IFN-γ−/− mice. Our finding that IFN-γ mediated inhibition of Th9 development provides a mechanistic basis for the severe EAE phenotype observed in IFN-γ−/− mice. Thus, through its antagonism of Th9 development, IFN-γ prevents the development of the pathogenic CD4+ T cell that is necessary for disease.
In addition to its direct inhibitory effect on Th9 differentiation, IFN-γ could also suppress Th9 cells through the induction of IL-27 from DCs. Indeed, we found that IFN-γ induces IL-27 expression in DCs which in turn leads to suppression of IL-9 from T cells. T cells cultured with supernatants from IFN-γ treated DCs produced lower IL-9, whereas addition of anti-IL-27 to these supernatants restored IL-9 production. In accordance with these in vitro results, DCs from IFN-γ−/− mice expressed low levels of IL-27 in comparison to DC from WT mice. The increased expression of IL-27 in WT vs. IFN-γ deficient mice correlates with decreased IL-9 expression in T cells during EAE, which suggests that in this setting IL-27 contributes to dampening inflammatory responses and driving the resolution of autoimmune pathology. In addition to Th9 cells, Th17 cells can also produce IL-9, which in turn amplifies and expands differentiated Th17 cells. Previous studies have suggested a pathogenic role of IL-9 as a Th17 derived cytokine that can contribute to inflammatory disease (2). In a recent study, it has been shown that IL-9 induces CCL20 production by astrocytes in vitro, thereby establishing a link between IL-9 and the infiltration of Th17 cells into the central nervous system (34). IFN-γ and IL-27 have been shown to inhibit Th17 differentiation in vitro. In addition, T cells from IFN-γ and IL-27R deficient mice had elevated levels of IL-17 during the course of EAE (18, 21). We found that IFN-γ and IL-27 inhibited IL-9 expression not only from Th9 cells but also from Th17 cells. Thus our data indicate that the decrease in IL-9 production that we observed during EAE in WT mice can be attributed to the suppressive effect of IFN-γ/IL-27 on development of both Th9 and Th17 cells.
IL-27 was first characterized as a pro-inflammatory cytokine associated with Th1 differentiation (12). However, subsequent studies with mice deficient in IL-27R showed exacerbated inflammatory responses to a variety of challenges, suggesting that IL-27 has important immunoregulatory functions in vivo. It has been shown that IL-27R−/− mice develop exacerbated EAE (18). Consistent with these findings, we observed increased IL-9 expression in T cells isolated from IL-27R−/− mice in comparison to WT mice. IL-27 has been shown to have suppressive effects on Th1, Th2 and Th17 cell differentiation. Here we found that IL-27 inhibits Th9 cell differentiation while inducing IL-10 producing Tr1 cells. In our experiments, IL-27 was more efficient than IFN-γ in suppressing IL-9 production. IL-27 induces both IL-10 and IFN-γ in T cells. Although IL-27 induced IFN-γ and IL-10 in Th9 cells, IL-27-mediated suppression of Th9 development occurred even in IFN-γ−/− and IL-10−/− T cells. IL-27 has been shown to induce the phosphorylation of STAT1 and STAT3 proteins and transcription factor T-bet. Our data demonstrate that IL-27 induced suppression of IL-9 production by Th9 cells is mediated by STAT-1 and T-bet. Although the activation of STAT1 and T-bet by IFN-γ or IL-27 has been associated primarily with the development of Th1 responses, our results underscore that this signaling pathway also mediates anti-inflammatory activities.
The role of IFN-γ in antagonizing the function of IL-9, a critical pathogenic cytokine in autoimmune conditions may be viewed as paradoxical since IFN-γ is considered a pro-inflammatory cytokine (20, 35). However, it is now recognized that IFN-γ has regulatory function in limiting inflammation. At the height of inflammation during the course of autoimmune pathology, the immune system is mobilized to restrict excess inflammation and it appears that IFN-γ acts as a master upstream regulator of both inflammatory and regulatory pathways. In summary, as shown in Supplementary Fig. 4 Our study identifies an important IFN-γ dependent regulatory process that serves to control IL-9 mediated autoimmune inflammation through its direct effect on T cells or by inducing IL-27 from DCs.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants (NS038037, AI043458, and NS23132). G.M. is the recipient of the NMSS post-doctoral fellowship and Junior Faculty award from Nancy Davis Foundation for Multiple Sclerosis. N.J. is supported by the Swiss MS Society.
References
- 1.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, Noelle RJ. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Nourbakhsh B, Ciric B, Zhang GX, A, Rostami Neutralization of IL-9 ameliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. J Immunol. 2010;185:4095–5100. doi: 10.4049/jimmunol.1000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Nourbakhsh B, Cullimore M, Zhang GX, Rostami A. IL-9 is important for T-cell activation and differentiation in autoimmune inflammation of the central nervous system. Eur J Immunol. 2011;41:2197–2206. doi: 10.1002/eji.201041125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 7.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, Nguyen ET, Robertson MJ, Perumal NB, Tepper RS, Nutt SL, Kaplan MH. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh CK, Raible D, Geba GP, Molfino NA. Biology of the interleukin-9 pathway and its therapeutic potential for the treatment of asthma. Inflamm Allergy Drug Targets. 2011;10:180–186. doi: 10.2174/187152811795564073. [DOI] [PubMed] [Google Scholar]
- 9.Burkhardt J, Petit-Teixeira E, Teixeira VH, Kirsten H, Garnier S, Ruehle S, Oeser C, Wolfram G, Scholz M, Migliorini P, Balsa A, Westhovens R, Barrera P, Alves H, Pascual-Salcedo D, Bombardieri S, Dequeker J, Radstake TR, Van Riel P, van de Putte L, Bardin T, Prum B, Buchegger-Podbielski U, Emmrich F, Melchers I, Cornelis F, Ahnert P. Association of the X-chromosomal genes TIMP1 and IL9R with rheumatoid arthritis. J Rheumatol. 2009;36:2149–2157. doi: 10.3899/jrheum.090059. [DOI] [PubMed] [Google Scholar]
- 10.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 11.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 12.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179:4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Miyazaki Y, Shinozaki Y, H, Yoshida Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179:6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 15.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 16.Murugaiyan G, Mittal A, Lopez-Diego R, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production from human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 18.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 20.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nat Immunol. 2010;11:250–256. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, Kuchroo VK, Khoury SJ. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 25.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 27.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173:3871–3877. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- 29.Sospedra M, Martin R. Immunology of multiple sclerosis. Annual review of immunology. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 30.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 31.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 32.Chu CQ, Swart D, Alcorn D, Tocker J, Elkon KB. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007;56:1145–1151. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- 33.Afanasyeva M, Georgakopoulos D, Belardi DF, Bedja D, Fairweather D, Wang Y, Kaya Z, Gabrielson KL, Rodriguez ER, Caturegli P, Kass DA, Rose NR. Impaired up-regulation of CD25 on CD4+ T cells in IFN-gamma knockout mice is associated with progression of myocarditis to heart failure. Proc Natl Acad Sci U S A. 2005;102:180–185. doi: 10.1073/pnas.0408241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Sonobe Y, Akahori T, Jin S, Kawanokuchi J, Noda M, Iwakura Y, Mizuno T, Suzumura A. IL-9 promotes Th17 cell migration into the central nervous system via CC chemokine ligand-20 produced by astrocytes. J Immunol. 2011;186:4415–21. doi: 10.4049/jimmunol.1003307. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J. Yin and yang interplay of IFN-gamma in inflammation and autoimmune disease. J Clin Invest. 2007;117:871–3. doi: 10.1172/JCI31860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.