Abstract
Plasmacytoid dendritic cells (pDCs) play a central role in innate and adaptive immune responses to viral infections, including human immunodeficiency virus type 1 (HIV-1). pDCs produce substantial quantities of type I interferon (IFN) and proinflammatory cytokines upon stimulation via Toll-like receptors (TLRs), specifically TLR7 or TLR9. The HIV-1 envelope glycoproteins (Env), exemplified by the gp120 monomer, are the focus of vaccines aimed at inducing B cell responses. We have studied how the interactions of gp120 with various receptors on human pDCs affect the activation of these cells via TLR9, and their subsequent ability to stimulate B cells. We observed that IFN-α production by pDCs in response to TLR9, but not TLR7 stimulation, was reduced by exposure to gp120. Specifically, gp120 inhibited the CpG-induced maturation of pDCs and their expression of TNF-α, IL-6, TLR9, IRF-7 and B cell-activating factor of the TNF family (BAFF). Receptor-blocking and cross-linking studies showed that these inhibitory effects of gp120 were mediated by interactions with CD4 and MCLRs, but not with the chemokine receptors CCR5 and CXCR4. Of note is that gp120 inhibited the activation of B cells by TLR9-stimulated pDCs. Taken together, our data show that HIV-1 gp120 impairs pDC functions, including activation of B cell responses, and imply that TLR9 ligands may not be good adjuvants to use in combination with Env vaccines.
Keywords: plasmacytoid dendritic cells, B cells, gp120, Toll-like receptor
Introduction
The human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein complex (Env) is a focus of vaccine design, particularly for strategies aimed at the induction of neutralizing antibodies (NAbs). As HIV-1 Env proteins can be poorly immunogenic, substantial efforts are being made to increase their abilities to induce broadly active NAbs. Much of this work is aimed at altering the topology of the Env proteins to increase the immunogenicity of key NAb epitopes (1–3). However, strategies to increase the overall Ab response to Env might also be valuable, for example by raising the NAb titer as a component of an overall increased Ab response, or by allowing smaller quantities of Env immunogens to be used. One area of research is whether gp120, a biologically active protein, might limit its own immunogenicity by directly affecting immune system cells when delivered in large amounts (50–500 µg) to localized injection sites (3).
The gp120 glycoprotein binds to several surface receptors on T and B cells, monocytes/macrophages and dendritic cells, including CD4, chemokine receptors and mannose-binding C-type lectin receptors (MCLRs) such as DC-SIGN, DCIR and BDCA-2 (4–6). Interactions between gp120 and MCLRs may modulate immune cell functions. For example, gp120 binding to DC-SIGN modulates Toll-like receptor (TLR) signaling and activates the Raf-1 kinase-dependent acetylation of transcription factor NFκB, which prolongs and enhances IL-10 transcription by DCs (7, 8). HIV-1 uses DC-SIGN, via gp120 binding, to facilitate the productive infection of DCs, and also triggers TLR8 signaling via its single-stranded RNA content (9). The mannose-dependent binding of gp120 to MCLRs can also trigger IL-10 production and impair the maturation of monocyte-derived dendritic cells (MDDCs) (10). Preventing gp120-MCLR interactions by removing mannose moieties from gp120 or by using the blocking ligand griffithsin (GRFT) enhances anti-gp120 Ab responses in mice (11, 12). Finally, gp120 activates B cells to undergo Ig class switching through a CD40-independent pathway involving BAFF and MCLRs (13).
Plasmacytoid dendritic cells (pDCs) serve as a link between innate and adaptive immunity (14, 15). pDCs express the gp120 receptors CD4, CCR5 and CXCR4, as well as MCLRs including BDCA-2 and DCIR (5, 6). They also express TLR7 and TLR9, which recognize single-stranded RNA and unmethylated DNA/synthetic CpG oligodeoxynucleotides (ODNs), respectively (15–18). TLR ligation activates pDCs to produce high levels of type I interferon (IFN), which has anti-viral activity, as well as other cytokines such as TNF-α and IL-6 that activate NK cells, monocytes, B cells and T cells (15). The roles played by pDCs during HIV-1 infection have been studied extensively; progressive infection is associated with a reduction in the number of pDCs and an impairment of their functions, such as a decrease in type I IFN secretion (19–22). In vitro studies have shown that pDCs produce substantial quantities of IFN-α when exposed to HIV-1, an effect mediated by TLR7 recognition of viral ssRNA (23–26). Conversely, the exposure of pDCs to gp120 inhibits TLR9-mediated IFN-α secretion and pDC-driven NK cell cytotoxicity (5, 27). The consequences of such pDC dysfunctions for other immune cells, and some aspects of the mechanisms, remain to be fully understood.
pDCs regulate B cell differentiation and immunoglobulin production via IFN and IL-6 secretion. Thus, the release of IFN from pDCs triggers naive B cells to develop into plasmablasts, which become differentiated to antibody-secreting B cells in response to IL-6 (28). pDCs also promote the proliferation and differentiation of B cells that are stimulated by BCR crosslinking and TLR9 ligation, in processes involving both soluble factors and cell-to-cell contact (29, 30). Other pDC-boosted events include the enhanced differentiation of TLR7/8-stimulated memory B cells, the IFN-α-mediated, T cell-dependent differentiation of naïve B cells, and the TLR7-dependent, IFN-independent activation of naïve B cells (31–33). At a mechanistic level, interactions between CD70 on pDCs and CD27 on memory B cells are what drive B cell growth and differentiation (34). Furthermore, pDCs and myeloid DCs (mDCs) both upregulate B Cell-Activating Factor (BAFF) and A Proliferation-Inducing Ligand (APRIL) expression, via an IFN-mediated pathway (35, 36). The production of these two cytokines by pDCs is involved in the T cell-independent induction of IgA by B cells in gut-associated lymphoid tissue (GALT) (36). Similarly, mDCs trigger CD40-independent Ig class switching in B cells through BAFF and APRIL (35). However, little is known about how exposure to gp120 affects pDC-mediated B cell growth and differentiation, and hence how these cells respond to Env-based vaccines.
CpG oligonucleotides (ODNs) have adjuvant and immune-stimulatory properties that make them of interest for treating, or vaccinating against, allergy, cancer and viral infections (37, 38). Here, we have examined how gp120 affects the response of pDCs to CpG ODNs, and the ability of the treated pDCs to subsequently stimulate B cell differentiation. We observed that CpG-induced pDC maturation, cytokine secretion and TLR9, interferon regulatory factor (IRF)-7 and BAFF mRNA expression were all reduced by exposure to gp120. Furthermore, the addition of gp120 to co-cultures of CpG-stimulated pDCs and B cells suppressed B cell proliferation, plasma cell differentiation and Ig secretion. A better understanding of the various interactions between gp120, TLR activators, pDCs and B cells may therefore guide improvements to adjuvant strategies for HIV-1 Env vaccines.
Materials and Methods
Isolation of pDCs and B cells
pDCs and B cells were isolated from buffy coats obtained from the New York Blood Center. pDCs were purified from human peripheral blood mononuclear cells (PBMC) using a CD304 (BDCA-4) Microbead Kit (Miltenyi Biotec). The purity of the enriched pDC population was >97%, as assessed by CD123 and BDCA-2 staining. Total primary B cells were isolated from PBMC using the B cell Isolation Kit II (Miltenyi Biotec). pDCs and all B cell subsets were cultured in RPMI 1640 containing 10% fetal bovine serum, 2mM L-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, 1mM sodium pyruvate and 10 mM HEPES (all from Invitrogen).
Treatment of pDCs with recombinant HIV-1 gp120 and CpG ODNs
Freshly isolated pDCs (5×104 - 2×105 cells in 300 µl per well of a 48-well plate) were treated for 2 days with CpG-B (ODN 2006; Invivogen) at 7 µg/ml in the presence or absence of endotoxin-free recombinant gp120 (1 µg/ml). JR-FL gp120 (Progenics Pharmaceuticals) was used in all experiments unless otherwise stated. MN gp120 or IIIB gp120 (both from ImmunoDiagnostics) were used when specified. A recombinant HIV-1 p24 protein (ImmunoDiagnostics Inc) served as a comparison with gp120 in certain experiments. In some studies, pDCs (2.5×104 - 5×104 cells in 200 µl per well of a 96-well plate) were activated for 18 h with CpG-A (ODN 2216; Invivogen) at 500 ng/ml, and with or without gp120, as previously described (5). Note that the number of pDCs that could be isolated varied among donors, but the same number of cells was used in all experiments from any single donor. IFN-α, IL-6 and TNF-α concentrations in culture supernatants were analyzed by ELISA according to the manufacturers’ instructions (IFN-α kit from PBL Biomedical Laboratories; TNF-α and IL-6 kits from BD Bioscience). For phenotypic analysis, cells were stained with monoclonal antibodies (MAbs) against CD40 (clone 61D3), CD83 (clone HB15e), HLA-DR (clone L243) and CCR7 (clone 3D12) (all from BD Biosciences), and analyzed using an LSR flow cytometer (BD Biosciences).
Receptor-blocking and cross-linking experiments
To inhibit gp120 binding to CD4, CCR5, CXCR4 and MCLRs on pDCs, we used various MAbs and ligands. The cells were incubated for 30 min at 37°C with the anti-CD4 MAb (clone RPA-T4; BD Biosciences) at 10 µg/ml, the small molecule CCR5 ligand vicriviroc (VVC; Schering-Plough Research Institute) at 1 µM, or the small molecule CXCR4 ligand AMD3100 (Genzyme) at 1 µM, prior to addition of gp120 and CpG-B. Alternatively, gp120 was mixed with soluble CD4 (sCD4; Progenics Pharmaceuticals) at 10 µg/ml, or the carbohydrate binding protein griffithsin (GRFT; Intrucept Biomedicine) at 20 nM, for 1 h at room temperature before addition to the pDC culture. In cross-linking experiments, pDCs were incubated with an anti-BDCA-2 antibody (clone AC144; Miltenyi Biotec), or a matched isotype control (mouse IgG1 from eBioscience), at 5 µg/ml for 30 min at 37°C, washed and then cultured with CpG-B for 2 days. ELISAs were used to quantify the cytokine contents of culture supernatants.
Real-time PCR
Total RNA was isolated from pDC using the Qiagen RNAeasy Kit (Qiagen). On-column DNase digestion was performed to remove contaminating genomic DNA according to the manufacturer’s instructions. Total RNA was reverse transcribed into cDNA. Briefly, secondary RNA structure was removed by heating for 5 min at 70°C. First-strand cDNA synthesis was carried out for 1 h at 37°C in 20 µl of a solution containing 25 µg/ml random primers, 0.5 mM dNTPs, 5 mM DTT, 40 U RNase Inhibitor and 200 U Murine Moloney Leukemia Virus (M-MLV) reverse transcriptase in 1x M-MLV buffer (Promega), followed by denaturation for 10 min at 70°C. cDNA templates were serially diluted and used for real-time PCR reactions to quantify TLR9 (assay ID: Hs00370913_s1), IRF-7 (assay ID: Hs00185375_m1), BAFF (assay ID: Hs00198106_m1) and APRIL (assay ID: Hs00601664_g1) in a 384-well plate. Each reaction was carried out in 10 µl of a solution containing 2x Taqman® reaction mix and 0.5 µl Taqman® FAM dye-labeled MGB probe, using a 7900HT Fast real-time PCR machine (Applied Biosystems). Target gene expression was normalized against the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH; assay no. Hs02758991_g1). The threshold cycle (CT) values for ΔCT (ΔCT = CT of target gene − CT of GAPDH) were calculated and converted into arbitrary units using the formula [(2−ΔCt) × 1,000].
pDC and B cell co-cultures
To examine how gp120-treated pDCs affected the regulation of B cell responses, an in vitro pDC-B cell co-culture system was established. Here, CpG-B was used because preliminary studies showed it to be the most potent B cell stimulator in this experimental system (Supplementary Fig. 1). Freshly isolated pDCs were treated with CpG-B (7 µg/ml) and gp120 (1 µg/ml) for 2 days before washing with complete medium to remove residual CpG-B and gp120. For co-culture, 2×104 B cells were added to 1×104 pDCs in 96-well round bottom plates in the presence of soluble Mega CD40 ligand (200 ng/ml; Enzo Life Sciences), IL-2 (50 U/ml; NIH AIDS and Reference Reagent Program) and IL-10 (10 ng/ml; R&D Systems). On days 0, 3, 5 and 7 of the co-culture, the viability of the B cells was examined using 7-amino-actinomycin D (7–AAD; BD Biosciences) and CD19 staining, followed by flow cytometry. The number of viable (7–AAD−) B cells was quantified as a percentage of the total. To analyze B cell proliferation, the cells were pulsed with BrdU for 6 h on day 5 before BrdU incorporation was measured using a cell proliferation ELISA kit according to the manufacturer’s instructions (Roche Diagnostics). On day 7, cells from the co-culture were collected, stained with CD20 (clone 2H7) and CD38 (clone HIT3), and analyzed by a LSR II flow cytometer (BD Biosciences). For analysis of IgG and IgM secretion by ELISA (Bethyl Laboratories), the cells were co-cultured for 14 days before culture supernatants were collected.
Statistical analysis
Data are presented as the means ± SEM of independent experiments carried out using pDCs from different donors. We used non-parametric statistics throughout because too few data points were available to allow testing for Gaussian distribution. Blood-donor and other inter-experimental variation meant that pairing of the data points might be effective. When pairing was effective (the correlation coefficient, r, ranged from 0.81 to 1, and the p values for the significance of the pairing ranged from 0.001 to 0.042), we performed Wilcoxon's matched pairs test (WMPT). In all other cases, we used the Mann-Whitney U test. Because all observed differences were in the predicted direction and variation in the opposite direction beyond control values could be considered random, we used one-tailed tests. The alpha level was set to 0.05. Statistical tests were performed in Prism 5.0d (GraphPad Software).
Results
HIV-1 gp120 impairs CpG-induced IFN-α production by pDCs
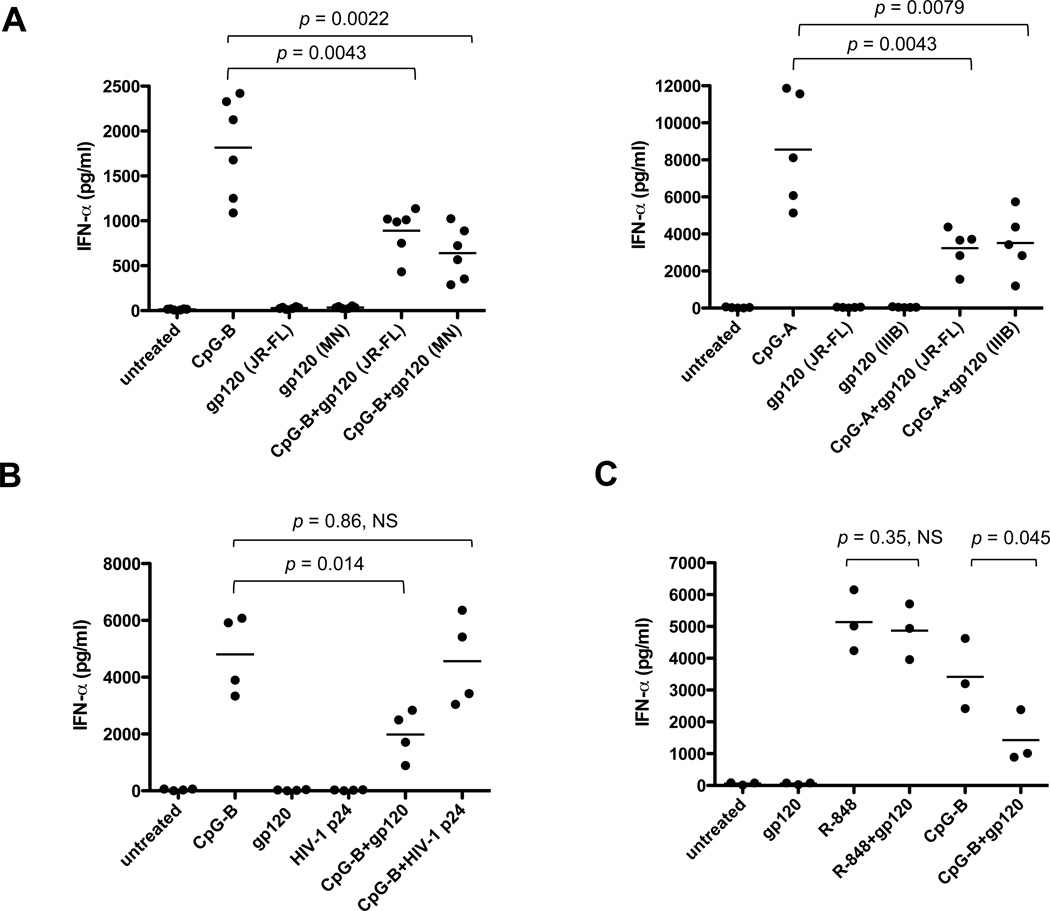
To examine the effects of gp120 on pDC function, the cells were treated with endotoxin-free recombinant proteins derived from the R5 strain JR-FL, the X4 strain MN or the X4 strain IIIB in the presence of TLR9 agonists (CpG-B or CpG-A). In all these studies, gp120 (JR-FL unless specified) was used at 1 µg/ml (8.3 nM); preliminary experiments showed that this concentration was sufficient to exert biological effects and we wished to avoid possible toxic effects of higher amounts. By themselves, neither JR-FL nor MN gp120 induced IFN-α secretion from pDCs but both inhibited, by 30-73%, IFN-α secretion in response to CpG-B (JR-FL: p = 0.0043; MN: p = 0.0022) (Fig. 1A). The IFN-α response to CpG-A was also inhibited (JR-FL: p = 0.0043; IIIB: p = 0.0079) (Fig. 1A). In contrast, the HIV-1 p24 Gag protein had no inhibitory effect (p = 0.86, not significant, NS) (Fig. 1B). Furthermore, gp120 did not inhibit IFN-α production in response to TLR7 activation by R-848 at 1 µg/ml (p = 0.35, NS) (Fig. 1C). Hence the gp120-mediated suppression of IFN-α production is specific for the TLR9 pathway. These observations are consistent with a prior report on how gp120 affects pDCs (5).
FIGURE 1.
(A) Inhibition of CpG-induced IFN-α production by HIV-1 gp120. Freshly isolated pDCs were treated with CpG-B or CpG-A (as indicated) in the presence or absence of recombinant gp120 (JR-FL, MN or IIIB as indicated). The culture supernatants were collected after 2 days (CpG-B) or 18 h (CpG-A) for IFN-α quantification by ELISA. The black horizontal lines indicate the mean values of data derived using pDCs from six (CpG-B) or five (CpG-A) individual donors. (B) HIV-1 gp120, but not HIV-1 p24, impaired CpG-B-induced IFN-α production by pDCs. The cells were treated with CpG-B in the presence or absence of JR-FL gp120. Culture supernatants were collected after 2 days for IFN-α ELISA. The results shown are the means of duplicate measurements using pDCs from four individual donors. (C) JR-FL gp120 decreases TLR9-mediated, but not TLR7-mediated, IFN-α production. The experimental conditions were similar to those for panel B. The results shown are the means of duplicate measurements using pDCs from three individual donors. NS, not significant.
gp120 inhibits CpG-B-induced pDC maturation
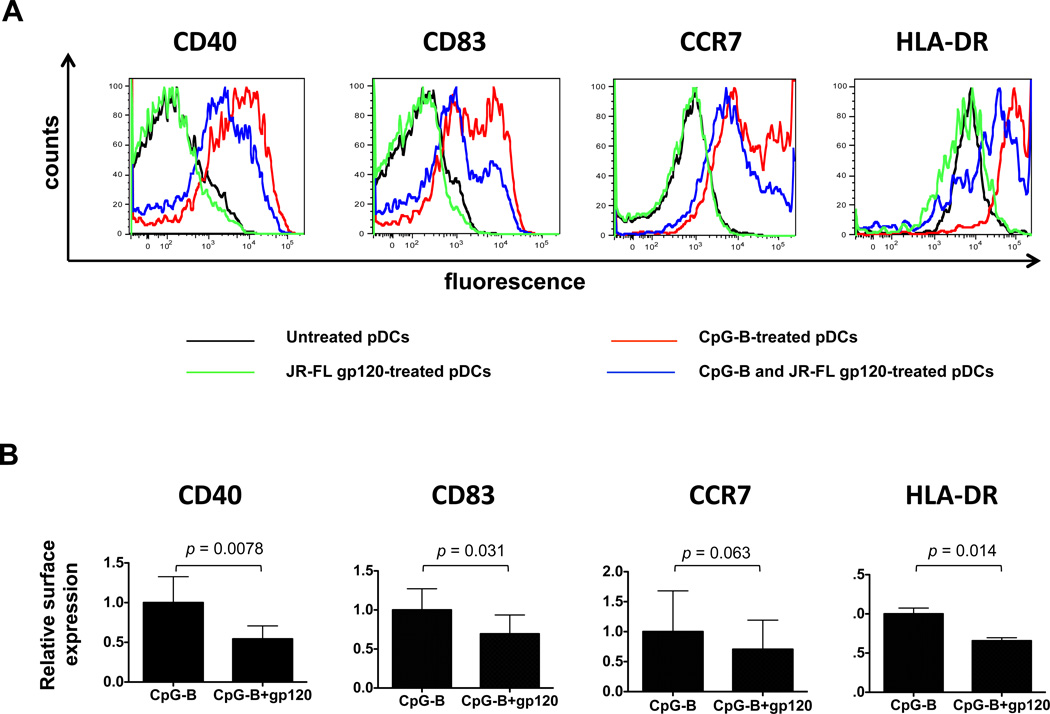
Next, we examined how gp120 affected the TLR9-stimulated maturation of pDCs, as determined by upregulation of maturation markers (Fig. 2A). As expected, CpG-B induced pDCs to mature, but the presence of gp120 reduced the expression levels of various maturation markers: CD40 (by 46%, p = 0.0078, WMPT), CD83 (by 31%, p = 0.031, WMPT), HLA-DR (by 34%, p = 0.014), all compared to stimulation with CpG-B alone (Fig. 2B). The reduction in CCR7 expression was of borderline significance (p = 0.063). By itself, gp120 did not affect the baseline expression level of any of the maturation markers (Fig. 2A). Similar results were obtained using CpG-A in place of CpG-B (Supplementary Fig. 2).
FIGURE 2.
HIV-1 gp120 inhibits CpG-B-induced pDC maturation. (A) pDCs were treated with CpG-B in the presence or absence of JR-FL gp120 and stained with MAbs against CD40, CD83, CCR7 and HLA-DR. The black curve represents untreated pDCs; green curve, JR-FL gp120-treated pDCs; red curve, CpG-B-treated pDCs; blue curve, CpG-B and JR-FL gp120-treated pDCs. (B) Expression of CD40, CD83, CCR7 and HLA-DR in CpG-B and JR-FL gp120-treated pDCs relative to CpG-B-treated pDCs. The data shown represent the mean values ± SEM obtained using pDCs from at least four individual donors.
gp120-mediated inhibition of IFN-α production involves CD4 and MCLRs
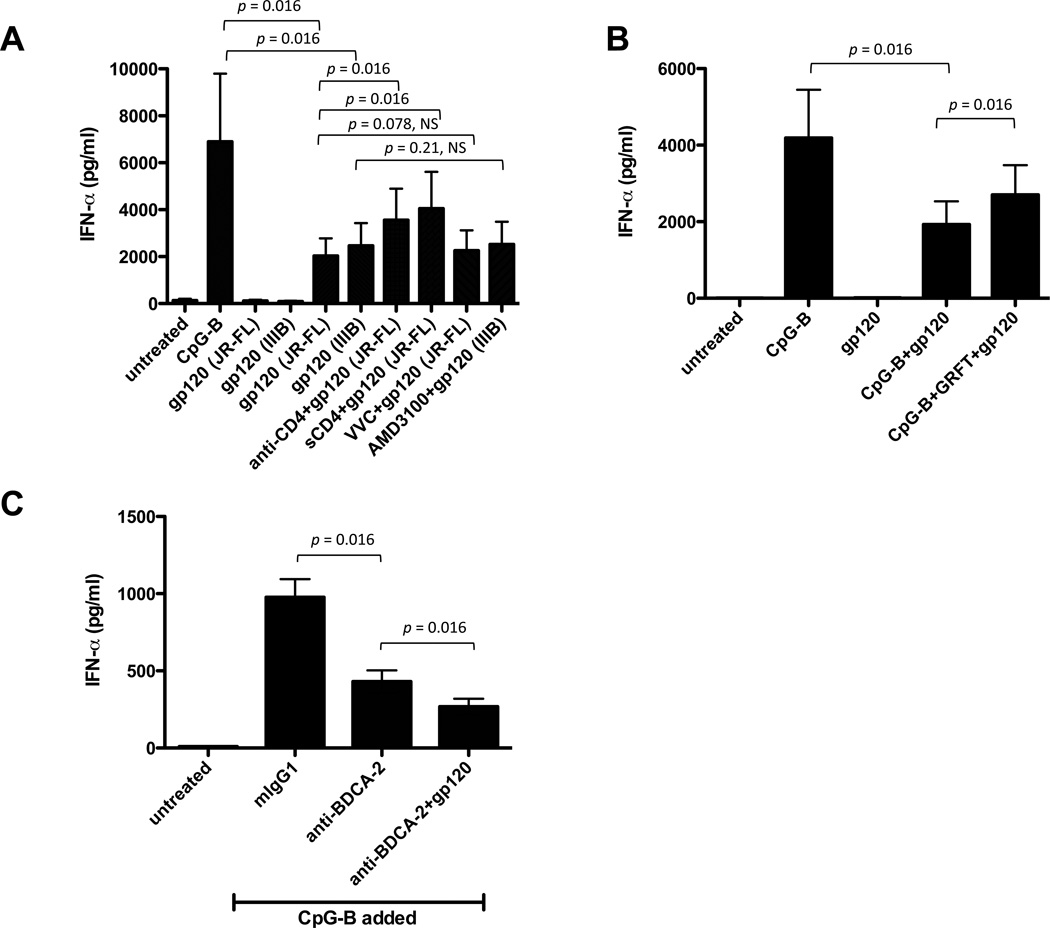
We used saturating concentrations of inhibitors of gp120-receptor interactions to study the underlying mechanisms. Treating the pDCs with an anti-CD4 MAb or ligating gp120 with sCD4 both partially reversed (by 19-38%, p = 0.016, WMPT) the JR-FL gp120-mediated inhibition of IFN-α production, implying the gp120-CD4 interaction was involved. In contrast, the CCR5 and CXCR4 ligands VCV and AMD3100 did not reverse the inhibitory actions of JR-FL and IIIB gp120s, respectively (Fig. 3A).
FIGURE 3.
gp120 inhibition of IFN-α production is mediated via CD4 and MCLRs. (A) pDCs were incubated with anti-CD4 MAb (clone RPA-T4) or VVC prior to addition of JR-FL gp120 (or with AMD3100 before addition of IIIB gp120). Alternatively, JR-FL gp120 was incubated with or without sCD4 before addition to pDCs. (B) JR-FL gp120 was incubated with or without GRFT before addition to pDCs. In both panels, the cells were stimulated with CpG-B and the culture supernatants were collected after 2 days for quantification of IFN-α by ELISA. The data shown are the mean values ± SEM from three independent experiments. (C) Combining JR-FL gp120 and an anti-BDCA-2 MAb (clone AC144) further inhibits IFN-α production by pDCs. The bar represents the mean values ± SEM from three independent experiments.
GRFT binds to the mannose moieties on gp120 and inhibits various interactions between this protein and MCLRs such as DC-SIGN, but it has no effect on gp120-CD4 binding (12, 39–41). GRFT also blocks HIV-1 from binding to DC-SIGN and the subsequent DC-SIGN-mediated transfer of the virus to CD4+ cells (42). We found that GRFT partially antagonized, by 16-28% (p = 0.016, WMPT), the inhibitory effect of gp120 on IFN-α production (Fig. 3B), suggesting that a gp120-MCLR interaction is also involved in these events.
It has been shown that gp120 binds to BDCA-2, an MCLR expressed on pDCs (5). To study if BDCA-2 signaling triggers IFN-α production, we used a specific MAb (clone AC144) that activates this receptor by a cross-linking mechanism. The ligation of BDCA-2 by this MAb inhibited CpG-B-induced IFN-α production by 56% (p = 0.016, WMPT) (Fig. 3C). One possible implication is that the suppressive effects of gp120 on pDC function are mediated, at least in part, via binding to BDCA-2. We could not test this hypothesis directly as although the BDCA-2 MAb blocks gp120 binding, it also itself activates signaling via this receptor. When the anti-BDCA-2 MAb was combined with gp120, there was a further suppression of the IFN-α response to CpG-B (p = 0.016, WMPT) (Fig. 3C). These findings suggest that the dual ligation of CD4 and BDCA-2 may have a synergistic inhibitory effect.
Together, our data suggest that gp120 binding to CD4 and MCLRs such as, but not limited to, BDCA-2 impairs the IFN-α response of pDCs to the TLR9 ligand, CpG-B.
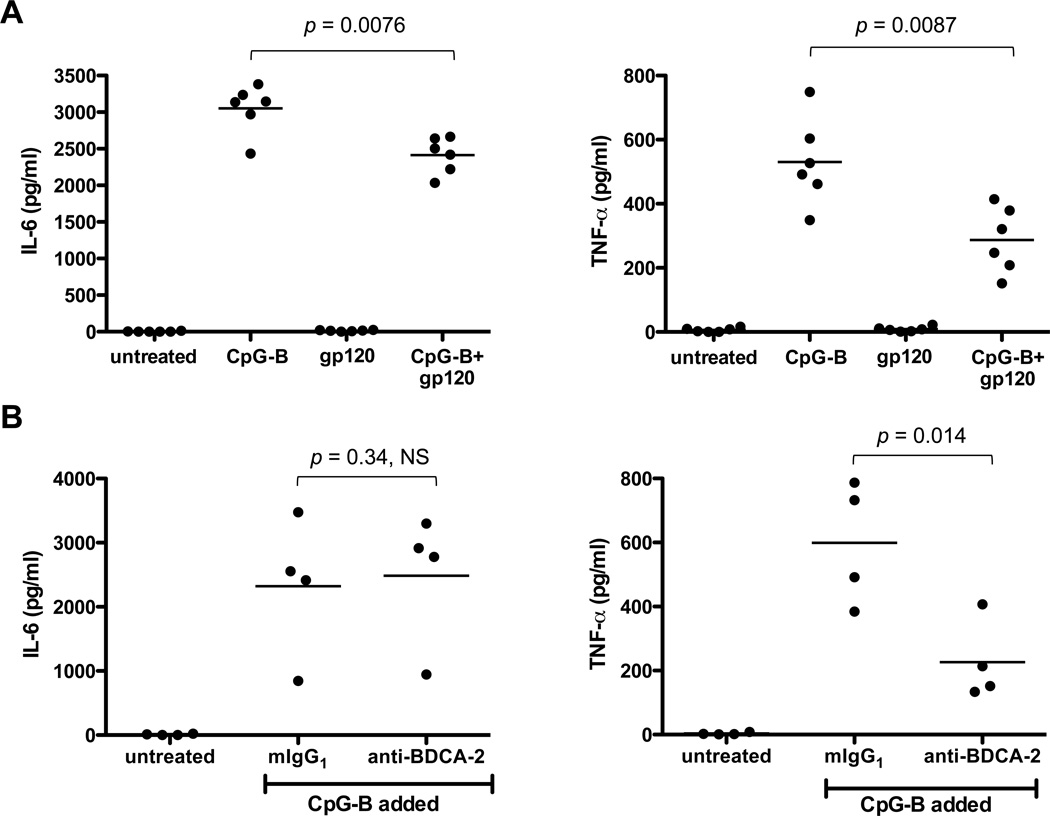
gp120 and BDCA-2 cross-linking downregulate IL-6 and TNF-α production in CpG-B-treated pDCs
In addition to IFN-α, pDCs produce other cytokines with antiviral or immune-stimulatory activities, including IL-6 and TNF-α. Both cytokines were induced by CpG-B stimulation and both responses were inhibited by gp120, albeit to different extents (IL-6 by 21%, p = 0.0076 and TNF-α by 46%, p = 0.0087; Fig. 4A). The anti-BDCA-2 MAb reduced CpG-B-stimulated production of TNF-α by 64% (p = 0.014), but had no effect on IL-6 (Fig. 4B). The receptor interactions of gp120 and the ligation of BDCA-2 by a MAb therefore cause similar, but not identical, impairments of pDC function. Moreover, different cytokines are affected in different ways.
FIGURE 4.
gp120 and BDCA-2 cross-linking each inhibit IL-6 and TNF-α production by CpG-B-treated pDCs. (A) pDCs were treated with CpG-B in the presence or absence of JR-FL gp120. (B) pDCs were incubated with an anti-BDCA-2 MAb (clone AC144), washed and then stimulated with CpG-B. In both experiments, IL-6 and TNF-α concentrations in the culture supernatants were measured after 48 h. The results represent the mean values derived from six (gp120) and four (anti-BDCA-2 MAb) independent experiments.
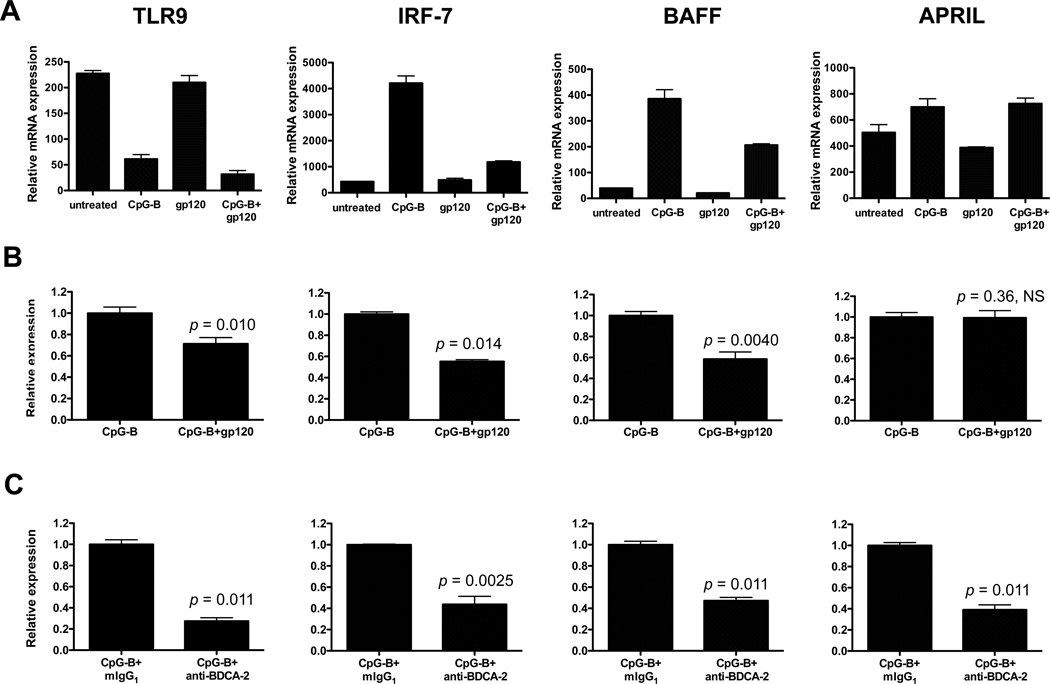
gp120 and BDCA-2 cross-linking inhibit TLR9, IRF-7 and BAFF mRNA expression in CpG-B-treated pDCs
TLR9 ligation on pDCs triggers an intracellular signaling cascade that activates IRF-7, which in turn regulates IFN gene transcription (43–45). Moreover, BAFF and APRIL expression in myeloid DCs and pDCs are induced via the type I IFN signaling pathway (35, 36). In light of the suppressive effects of gp120 and the anti-BDCA-2 MAb described above, we used qPCR to quantify TLR9, IRF-7, APRIL and BAFF mRNA expression under similar conditions of CpG-B stimulation, both in the presence and absence of gp120 (Fig. 5A and 5B). TLR9 expression was significantly reduced in response to CpG-B, and the addition of gp120 further lowered its expression (by 28% compared to CpG-B alone; p = 0.010). A different data pattern was seen with IRF-7 and BAFF mRNA in that CpG-B markedly upregulated their expression, but markedly less so when gp120 was also present. Thus, the gp120-mediated reductions in IRF-7 and BAFF mRNAs were 45% (p = 0.014) and 42% (p = 0.0040) respectively (Fig. 5A and 5B). In contrast, CpG-B induced APRIL expression, but gp120 had no suppressive effect (Fig. 5A and 5B). Finally, BDCA-2 cross-linking inhibited the expression of TLR9 (by 68%, p = 0.011), IRF-7 (by 56%, p = 0.0025), BAFF (by 51%, p = 0.0011) and APRIL (by 61%, p = 0.0011) in CpG-B-treated pDCs (Fig. 5C). The responses to BDCA-2 cross-linking and gp120 were similar with the exception that APRIL mRNA was significantly reduced only by the former (Fig. 5B and 5C).
FIGURE 5.
gp120 and BDCA-2 cross-linking each suppress TLR9, IRF-7 and BAFF mRNA expression in CpG-B-treated pDCs. (A) pDCs were treated with CpG-B in the presence or absence of JR-FL gp120 for 4 h before TLR9, IRF-7, BAFF and APRIL mRNA levels were quantified by qPCR. One experiment representative of three is shown. (B) The relative expression of TLR9, IRF-7, BAFF and APRIL mRNA is presented, for comparison with CpG-B. The results shown are the mean values ± SEM derived from three independent experiments. (C) pDCs were incubated with anti-BDCA-2 MAb (clone AC144) for 30 min at 37°C, washed and then stimulated with CpG-B for 4 h. The results shown are the mean values ± SEM derived using pDCs from three individual donors. NS, not significant.
gp120 suppresses the B cell stimulatory capacity of CpG-B-treated pDCs
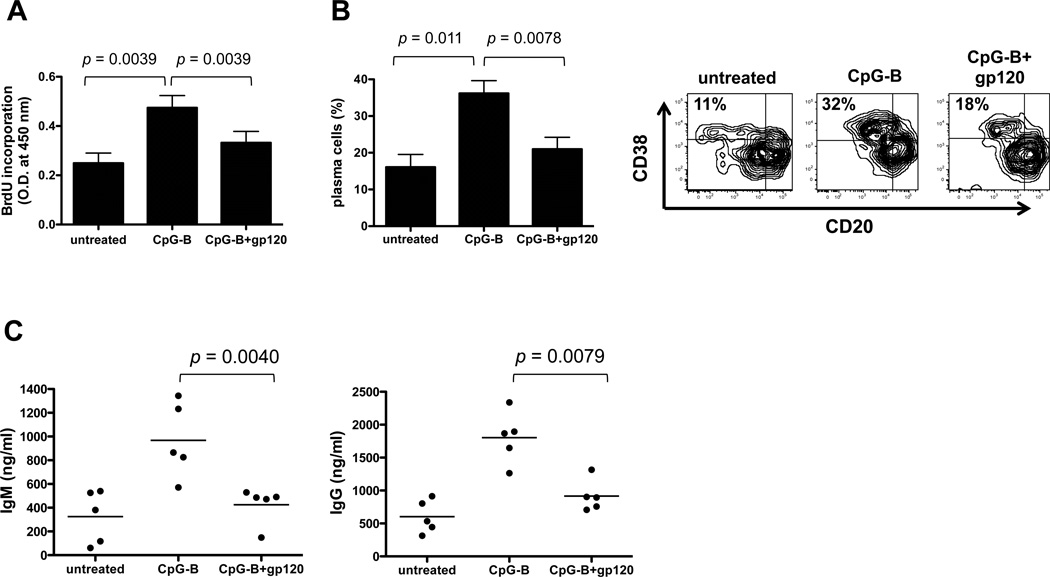
As pDCs are potent stimulators of B cells, we examined how gp120 treatment affected the ability of TLR9-activated pDCs to drive B cell proliferation and differentiation. We used CpG-B as the TLR9 ligand, as it is more potent than CpG-A for B cell stimulation. Freshly isolated pDCs were treated with CpG-B, with and without gp120, for 2 days and washed thoroughly prior to co-culture with primary B cells; to mimic T cell help, CD40L, IL-2 and IL-10 were added to the cultures. The viability of CD19+ B cells in co-cultures with pDCs was examined by 7-AAD staining on days 0, 3, 5 and 7. Cell viability (untreated vs. CpG-B) was 91.3% vs. 94.5% on day 0, 84.75% vs. 91.8% on day 3, 80.2% vs. 83.6% on day 5 and 76.1% vs. 86.1% on day 7 (Supplementary Fig. 3). B cell viability in co-cultures treated with CpG-B and gp120 was comparable to that seen in co-cultures given only CpG-B. B cell proliferation was assessed on day 5 by measuring BrdU incorporation. CpG-B-treatment significantly increased the ability of pDCs to drive B cell proliferation, relative to untreated pDCs, but the boosting effect was substantially inhibited (by 63%, p = 0.0039, WMPT) when the pDCs were also exposed to gp120 (Fig. 6A).
FIGURE 6.
gp120-treated pDCs have a reduced capacity to stimulate B cell growth and differentiation. Freshly isolated pDCs were incubated with CpG-B and with or without JR-FL gp120 for 2 days, and then washed to remove residual ligands. A co-culture involving 2 × 104 B cells and 1 × 104 pDCs was established in 96-well round bottom plates. (A) After 5 days of co-culture, the cells were pulsed with BrdU for 6 h before measurement of cellular BrdU incorporation. The bars represent the mean values ± SEM derived from four individual experiments. (B) After 7 days of co-culture, the cells were collected, stained with anti-CD20 and anti-CD38 and analyzed by flow cytometry. One representative dot plot showing plasma cell population in the upper left quadrant is shown. The bars represent the mean values ± SEM derived from four individual experiments. (C) After 14 days of co-culture, IgM and IgG concentrations in the culture supernatants were quantified by ELISA. The results shown are the mean values derived from experiments on cells from five individual donors.
To study differentiation to plasma cells, B cells were harvested from the co-culture on day 7 and examined for plasma cell markers, as defined by a CD20loCD38hi phenotype. The presence of CpG-B-treated pDCs in the co-culture significantly increased the percentage of CD20lo CD38hi plasma cells, compared to untreated pDCs (32% vs. 11% shown in the upper left quadrant of dot plot). The additional presence of gp120 reduced the plasma cell percentage by 76% (p = 0.0039, WMPT) (Fig. 6B).
Supernatants were collected from the co-cultures on day 14 for quantification of IgG and IgM secretion. CpG-B treatment of the pDCs increased by 3-fold the subsequent secretion of IgM and IgG by the B cells, as compared to untreated pDCs (967 ng/ml vs. 325 ng/ml for IgM; 1802 ng/ml vs. 622 ng/ml for IgG). The levels of both IgG and IgM were significantly reduced (by 84%, p = 0.0040 and 75%, p = 0.0079, respectively) when gp120 was also present (Fig. 6C). Overall, these experiments show that gp120 inhibits the capacity of TLR9-triggered pDCs to activate B cells and drive their differentiation into Ig-secreting plasma cells.
Discussion
pDCs play an important role in the induction of innate and adaptive immune responses; they are potent producers of type I interferon, a cytokine with strong anti-viral activity against viral infections (15). TLR7 and TLR9, which recognize single-stranded RNA and unmethylated DNA motifs respectively, are the principal triggers of type I IFN production by pDCs (17, 18). A previous study found that HIV-1 gp120 inhibits TLR9-mediated pDC activation and cytokine production (5). Here, we have further investigated how gp120 affects the functions of pDCs and the ability of these cells to drive B cell responses in vitro. We found that gp120 suppresses CpG-induced pDC maturation and cytokine production, which impairs the subsequent growth and differentiation of B cells.
The gp120 glycoprotein can bind to pDCs via the CD4, CCR5 and CXCR4 receptors, as well as via MCLRs such as BDCA-2 and DCIR (5, 6). We found that impeding gp120 binding to either CD4 or MCLRs partially reversed its inhibitory effect on IFN-α production, suggesting that both types of receptor are involved. Indeed, ligating both BDCA-2 and CD4 at the same time inhibited the IFN-α response to CpG-B synergistically. There are several reports that receptor cross-linking regulates IFN-α production by pDCs. For example, cross-linking CD4, BDCA-2 and BDCA-4 reduces their IFN response to Herpes Simplex Virus (46). Similarly, ligating BDCA-2 or DCIR inhibits CpG-induced IFN-α and TNF-α production (47–49). TLR9 ligands trigger the MyD88-IRF-7 signaling cascade, which leads to IFN secretion, a process in which IRF-7 plays an important role by initiating IFN gene transcription (45). Here, we showed that the inhibitory effect of gp120 on the IFN-α response was associated with a reduction in IRF-7 mRNA expression. Overall, our observations suggest that gp120 binding to MCLRs and CD4 antagonizes TLR9-mediated signaling events that normally upregulate IFN-α production.
Several other viral proteins appear to have properties similar to HIV-1 gp120 in this regard. Thus, Hepatitis B virus and its surface antigen (HBsAg), and Hepatitis C virus, all inhibit IFN production in response to TLR9, but not TLR7, agonists (50–55). The IFN response of primary breast and ovarian tumor-associated pDCs to TLR9, but not TLR7, ligands was also impaired (56). Furthermore, pDCs from HIV-1-infected individuals express lower levels of IFN-α and IRF-7 mRNAs after exposure to TLR9 agonists, when compared to cells from healthy people (57). The implication, again, is that TLR9 signaling is defective in these cells. A suggested explanation is that the interactions of viral proteins/components with immunoregulatory receptors such as MCLRs cause a reduction in TLR9 expression (56). Consistent with this hypothesis, we observed that gp120 and BDCA-2 ligation each reduced TLR9 mRNA expression in CpG-treated pDCs.
It has been well documented that pDCs regulate B cell differentiation and Ig secretion, both via soluble factors such as IFN-α, IL-6 and BAFF (28, 32, 33, 35) and through cell-to-cell contact (29, 30, 34). The production of IFN-α by pDCs enhances interactions between B cells and T cells, leading to the T cell-dependent differentiation of naïve B cells into Ab-secreting cells (33). In addition, pDC-derived IFN-α enhances a T cell-independent B cell response to TLR7/8 stimulation (32). Here, we found that, gp120 suppressed IFN-α, IL-6 and TNF-α production in CpG-B-treated pDCs, impairments that may be sufficient to account for the reductions in B cell proliferation, differentiation and Ig secretion in the corresponding pDC and B cell co-cultures. Type I IFN also upregulates the expression of two potent B cell stimulatory cytokines, BAFF and APRIL, in both pDCs and mDCs (35, 36). These two cytokines play an important role in B cell survival, activation, differentiation and Ig class switching (58–60). We observed that CpG-B stimulated BAFF expression in pDCs and that this response was significantly reduced by gp120, partly contributing to the diminished capacity of the gp120-treated cells to activate B cell differentiation and Ig secretion.
There have been several studies on whether HIV-1 infection affects TLR agonist responsiveness. Thus, pDCs isolated from acutely and chronically infected individuals respond poorly to TLR9 ligands (61). An impaired responsiveness to TLR9 activation has also been observed in memory and naïve B cells from infected people (62). Whether these various observations are attributable to suppression by gp120, either as a soluble or a virion- or cell-associated protein, is unknown (63). Overall, HIV-1 infection perturbs many components of the immune system, including B-cells (e.g., hypergammaglobulinemia and polyclonal B-cell hyperactivation (64–67). B-cell dysregulation during HIV-1 infection involves the induction of activation-induced cytidine deaminase (AID), which is essential for Ig-class switch recombination (CSR) and somatic hypermutation (68). Moreover, there are indirect effects of HIV-1 infection that perturb the B-cell compartment. For example, viral RNA can trigger changes in pDC functions via TLR7/8, inducing high levels of IFN-α that stimulate B-cell differentiation (26). The key point is that HIV-1 infection is a much more complex situation involving more stimulatory or suppressive factors than arises when Env proteins are used as vaccines, even when these proteins are mixed with adjuvants that interact with the TLR system (e.g., CpG ODNs that act via TLR9). Our focus in this study was to better understand what might happen in the vaccine context, rather than considering HIV-1 infection. Our central conclusion is that gp120 binding to CD4 and MCLR on pDCs could impair TLR9 signaling, leading to reduced production of IFN and BAFF and an impaired B-cell response.
Our findings are consistent with several reports that gp120 can have immunosuppressive effects on immune cells such as DCs (10, 69, 70), and T cells (71–74). Paradoxically, gp120 has also been reported to be a superantigen initiating polyclonal Ab responses in VH3 B cells (75–78). Finally, the binding of gp120 to MCLRs triggers B cells to undergo polyclonal Ig class switching in the presence of BAFF (13). It remains to be understood which, if any, of these various suppressive or stimulatory effects of gp120 are relevant after vaccination, when local Env concentrations may be particularly high (63)
In summary, we have shown that gp120 can suppress TLR9-mediated pDC functions, including their ability to stimulate B cell responses. Hence, CpG ODNs may be unsuitable adjuvants for use in an HIV-1 Env vaccine, with other TLR activators being a better choice. The quality of T cell responses was improved when mice were immunized with HIV-1 Env peptides in combination with TLR 2/6, TLR3 and TLR9 ligands (79). However, B cell responses were not assessed in that study. Although we have only tested gp120 monomers in the present study, the receptor interactions of soluble, trimeric forms of Env are gp120-dominated and may be at least qualitatively similar to those seen with the monomer.
Supplementary Material
Acknowledgments
This research was supported in part by NIH grant AI36082 to JPM. RWS is a recipient of Vidi grant from the Netherlands Organization for Scientific Research (NWO) and a Starting Investigator Grant from the European Research Council (ERC-StG-2011-280829-SHEV).
Abbreviations
- APRIL
a proliferation-inducing ligand
- BDCA-2
blood dendritic cell antigen-2
- BAFF
B cell-activating factor of the TNF family
- DCIR
dendritic cell immunoreceptor
- DC-SIGN
dendritic cell-specific ICAM-3-grabbing non-integrin
- Env
Envelope glycoprotein
- GRFT
griffithsin
- IRF-7
interferon regulatory factor 7
- MCLRs
mannose-binding C-type lectin receptors
- MDDCs
monocyte-derived dendritic cells
- ODN
oligodeoxynucleotide
- pDCs
plasmacytoid dendritic cells
- R-848
resiquimod
- VVC
vicriviroc
Footnotes
Disclosures
The authors have no financial conflicts of interests.
References
- 1.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 2004;5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 2.Pantophlet R, Burton DR. GP120: target for neutralizing HIV-1 antibodies. Annu. Rev. Immunol. 2006;24:739–769. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- 3.Klasse PJ, Sanders RW, Cerutti A, Moore JP. How can HIV-type-1-Env immunogenicity be improved to facilitate antibody-based vaccine development? AIDS Res. Hum. Retroviruses. 2012;28:1–15. doi: 10.1089/aid.2011.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 5.Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, Arthos J, Fauci AS. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-α secretion in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA. 2007;104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert AA, Gilbert C, Richard M, Beaulieu AD, Tremblay MJ. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood. 2008;112:1299–1307. doi: 10.1182/blood-2008-01-136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat. Immunol. 2009;10:1081–1088. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- 9.Gringhuis SI, van der Vlist, M M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TB. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat. Immunol. 2010;11:419–426. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- 10.Shan M, Klasse PJ, Banerjee K, Dey AK, Iyer SP, Dionisio R, Charles D, Campbell-Gardener L, Olson WC, Sanders RW, Moore JP. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Patho. 2007;3:e169. doi: 10.1371/journal.ppat.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee K, Andjelic S, Klasse PJ, Kang, Y Y, Sanders RW, Michael E, Durso RJ, Ketas TJ, Olson WC, Moore JP. Enzymatic removal of mannose moieties can increase the immune response to HIV-1 gp120 in vivo. Virology. 2009;389:108–121. doi: 10.1016/j.virol.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee K, Michael E, Eggink D, van Montfort T, Lasnik AB, Palmer KE, Sanders RW, Moore JP, Klasse PJ. Occluding the mannose moieties on human immunodeficiency virus type 1 gp120 with griffithsin improves the antibody responses to both proteins in mice. AIDS Res. Hum. Retroviruses. 2012;28:206–214. doi: 10.1089/aid.2011.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, Moore JP, Cerutti A. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J. Immunol. 2006;176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 14.Kadowaki N, Liu YJ. Natural type I interferon-producing cells as a link between innate and adaptive immunity. Hum. Immunol. 2002;63:1126–1132. doi: 10.1016/s0198-8859(02)00751-6. [DOI] [PubMed] [Google Scholar]
- 15.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 16.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan, F F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, Patterson S. Loss of blood CD11c(+) myeloid and CD11c(-) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 20.Pacanowski J, Kahi S, Baillet M, Lebon P, Deveau C, Goujard C, Meyer L, Oksenhendler E, Sinet M, Hosmalin A. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 21.Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, Huang L, Levy JA, Liu YJ. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 22.Kamga I, Kahi S, Develioglu L, Lichtner M, Maranon C, Deveau C, Meyer L, Goujard C, Lebon P, Sinet M, Hosmalin A. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J. Infect. Dis. 2005;192:303–310. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 23.Patterson S, Rae A, Hockey N, Gilmour J, Gotch F. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 2001;75:6710–6713. doi: 10.1128/JVI.75.14.6710-6713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yonezawa A, Morita R, Takaori-Kondo A, Kadowaki N, Kitawaki T, Hori T, Uchiyama T. Natural alpha interferon-producing cells respond to human immunodeficiency virus type 1 with alpha interferon production and maturation into dendritic cells. J. Virol. 2003;77:3777–3784. doi: 10.1128/JVI.77.6.3777-3784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fonteneau JF, Larsson M, Beignon AS, McKenna K, Dasilva I, Amara A, Liu YJ, Lifson JD, Littman DR, Bhardwaj N. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. . J. Clin. Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reitano KN, Kottilil S, Gille CM, Zhang X, Yan M, O'Shea MA, Roby G, Hallahan CW, Yang J, Lempicki RA, et al. Defective plasmacytoid dendritic cell-NK cell cross-talk in HIV infection. AIDS Res. Hum. Retroviruses. 2009;25:1029–1037. doi: 10.1089/aid.2008.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual, and Banchereau, J V. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 29.Poeck H, Wagner M, Battiany J, Rothenfusser S, Wellisch D, Hornung V, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–3064. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 30.Ding C, Cai Y, Marroquin J, Ildstad ST, Yan J. Plasmacytoid dendritic cells regulate autoreactive B cell activation via soluble factors and in a cell-to-cell contact manner. J. Immunol. 2009;183:7140–7149. doi: 10.4049/jimmunol.0901175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, Hartmann G. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J. Immunol. 2005;174:4043–4050. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 32.Douagi I, Gujer C, Sundling C, Adams WC, Smed-Sorensen A, Seder RA, Karlsson Hedestam GB, Lore K. Human B cell responses to TLR ligands are differentially modulated by myeloid and plasmacytoid dendritic cells. J. Immunol. 2009;182:1991–2001. doi: 10.4049/jimmunol.0802257. [DOI] [PubMed] [Google Scholar]
- 33.Gujer C, Sandgren KJ, Douagi I, Adams WC, Sundling C, Smed-Sorensen A, Seder RA, Karlsson Hedestam GB, Lore K. IFN-alpha produced by human plasmacytoid dendritic cells enhances T cell-dependent naive B cell differentiation. J. Leukoc. Biol. 2011;89:811–821. doi: 10.1189/jlb.0810460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw J, Wang YH, Ito T, Arima K, Liu YJ. Plasmacytoid dendritic cells regulate B-cell growth and differentiation via CD70. Blood. 2010;115:3051–3057. doi: 10.1182/blood-2009-08-239145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M, Ohteki T. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity. 2011;34:247–257. doi: 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 38.Hennessy EJ, Parker AE, O'Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat. Rev. Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 39.Mori T, O'Keefe BR, Sowder RC, 2nd, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Jr. Buckheit RW, McMahon JB, Boyd MR. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 40.O'Keefe BR, Vojdani F, Buffa V, Shattock RJ, Montefiori DC, Bakke J, Mirsalis J, d'Andrea AL, Hume SD, Bratcher B, et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. USA. 2009;106:6099–6104. doi: 10.1073/pnas.0901506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moulaei T, Shenoy SR, Giomarelli B, Thomas C, McMahon JB, Dauter Z, O'Keefe BR, Wlodawer A. Monomerization of viral entry inhibitor griffithsin elucidates the relationship between multivalent binding to carbohydrates and anti-HIV activity. Structure. 2010;18:1104–1115. doi: 10.1016/j.str.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexandre KB, Gray ES, Mufhandu H, McMahon JB, Chakauya E, O'Keefe BR, Chikwamba R, Morris, L L. The lectins griffithsin, cyanovirin-N and scytovirin inhibit HIV-1 binding to the DC-SIGN receptor and transfer to CD4(+) cells. Virology. 2012;423:175–186. doi: 10.1016/j.virol.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai J, Megjugorac NJ, Amrute SB, Fitzgerald-Bocarsly P. Regulation of IFN regulatory factor-7 and IFN-alpha production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J. Immunol. 2004;173:1535–1548. doi: 10.4049/jimmunol.173.3.1535. [DOI] [PubMed] [Google Scholar]
- 44.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba, A.Takaoka Y, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 45.Cao W, Liu YJ. Innate immune functions of plasmacytoid dendritic cells. Curr. Opin. Immunol. 2007;19:24–30. doi: 10.1016/j.coi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Fanning SL, George TC, Feng D, Feldman SB, Megjugorac NJ, Izaguirre AG, Fitzgerald-Bocarsly P. Receptor cross-linking on human plasmacytoid dendritic cells leads to the regulation of IFN-alpha production. J. Immunol. 2006;177:5829–5839. doi: 10.4049/jimmunol.177.9.5829. [DOI] [PubMed] [Google Scholar]
- 47.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka, T T, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J. Exp. Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, Sarris A, Giese T, Endres S, Hartmann G. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J. Immunol. 2003;170:4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 49.Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ, Adema GJ. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 50.Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T, et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J. Immunol. 2007;178:3186–3197. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 51.Shiina M, Rehermann B. Cell culture-produced hepatitis C virus I mpairs plasmacytoid dendritic cell function. Hepatology. 2008;47:385–395. doi: 10.1002/hep.21996. [DOI] [PubMed] [Google Scholar]
- 52.Gondois-Rey F, Dental C, Halfon P, Baumert TF, Olive D, Hirsch I. Hepatitis C virus is a weak inducer of interferon alpha in plasmacytoid dendritic cells in comparison with influenza and human herpesvirus type-1. PLoS. One. 2009;4:e4319. doi: 10.1371/journal.pone.0004319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie Q, Shen HC, Jia NN, Wang H, Lin LY, An BY, Gui HL, Guo SM, Cai W, Yu H, et al. Patients with chronic hepatitis B infection display deficiency of plasmacytoid dendritic cells with reduced expression of TLR9. Microbes Infect. 2009;11:515–523. doi: 10.1016/j.micinf.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Xu Y, Hu Y, Shi B, Zhang X, Wang J, Zhang Z, Shen F, Zhang Q, Sun S, Yuan Z. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Mol. Immunol. 2009;46:2640–2646. doi: 10.1016/j.molimm.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 55.Woltman AM, L.Op den Brouw M, Biesta PJ, Shi CC, Janssen HL. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One. 2011;6:e15324. doi: 10.1371/journal.pone.0015324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirsch I, Caux C, Hasan U, Bendriss-Vermare N, Olive D. Impaired Toll-like receptor 7 and 9 signaling: from chronic viral infections to cancer. Trends Immunol. 2010;31:391–397. doi: 10.1016/j.it.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Sachdeva N, Asthana V, Brewer TH, Garcia D, Asthana D. Impaired restoration of plasmacytoid dendritic cells in HIV-1-infected patients with poor CD4 T cell reconstitution is associated with decrease in capacity to produce IFN-alpha but not proinflammatory cytokines. J. Immunol. 2008;181:2887–2897. doi: 10.4049/jimmunol.181.4.2887. [DOI] [PubMed] [Google Scholar]
- 58.Cerutti A, Qiao X, He B. Plasmacytoid dendritic cells and the regulation of immunoglobulin heavy chain class switching. Immunol. Cell Biol. 2005;83:554–562. doi: 10.1111/j.1440-1711.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- 59.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr. Opin. Immunol. 2005;17:282–289. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Cerutti A, Puga I, Cols M. Innate control of B cell responses. Trends Immunol. 2011;32:202–211. doi: 10.1016/j.it.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang JJ, Lacas A, Lindsay RJ, Doyle EH, Axten KL, Pereyra F, Rosenberg ES, Walker BD, Allen TM, Altfeld M. Differential regulation of toll-like receptor pathways in acute and chronic HIV-1 infection. AIDS. 2012;26:533–541. doi: 10.1097/QAD.0b013e32834f3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang W, Lederman MM, Mohner RJ, Rodriguez B, Nedrich TM, Harding SF, Sieg CV. Impaired naive and memory B-cell responsiveness to TLR9 stimulation in human immunodeficiency virus infection. J. Virol. 2008;82:7837–7845. doi: 10.1128/JVI.00660-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klasse PJ, Moore JP. Is there enough gp120 in the body fluids of HIV-1-infected individuals to have biologically significant effects? Virology. 2004;323:1–8. doi: 10.1016/j.virol.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 65.De Milito A. B lymphocyte dysfunctions in HIV infection. Curr. HIV Res. 2004;2:11–21. doi: 10.2174/1570162043485068. [DOI] [PubMed] [Google Scholar]
- 66.De Milito A, Nilsson A, Titanji K, Thorstensson R, Reizenstein E, Narita M, Grutzmeier S, Sonnerborg A, Chiodi F. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103:2180–2186. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 67.Moir S, Fauci AS. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perise-Barrios AJ, Munoz-Fernandez MA, Pion M. Direct Phenotypical and functional dysregulation of primary human B cells by human immunodeficiency virus (HIV) type 1 in vitro. PLoS One. 2012;7:e39472. doi: 10.1371/journal.pone.0039472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fantuzzi L, Purificato C, Donato K, Belardelli, F F, Gessani S. Human immunodeficiency virus type 1 gp120 induces abnormal maturation and functional alterations of dendritic cells: a novel mechanism for AIDS pathogenesis. J. Virol. 2004;78:9763–9772. doi: 10.1128/JVI.78.18.9763-9772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chougnet C, Gessani S. Role of gp120 in dendritic cell dysfunction in HIV infection. J. Leukoc. Biol. 2006;80:994–1000. doi: 10.1189/jlb.0306135. [DOI] [PubMed] [Google Scholar]
- 71.Weinhold KJ, Lyerly HK, Stanley SD, Austin AA, Matthews TJ, Bolognesi DP. HIV-1 GP120-mediated immune suppression and lymphocyte destruction in the absence of viral infection. J. Immunol. 1989;142:3091–3097. [PubMed] [Google Scholar]
- 72.Diamond DC, Sleckman BP, Gregory T, Lasky LA, Greenstein JL, Burakoff SJ. Inhibition of CD4+ T cell function by the HIV envelope protein, gp120. J. Immunol. 1988;141:3715–3717. [PubMed] [Google Scholar]
- 73.Oyaizu N, Chirmule N, Kalyanaraman VS, Hall WW, Pahwa R, Shuster M, Pahwa S. Human immunodeficiency virus type 1 envelope glycoprotein gp120 produces immune defects in CD4+ T lymphocytes by inhibiting interleukin 2 mRNA. Proc. Natl. Acad. Sci. USA. 1990;87:2379–2383. doi: 10.1073/pnas.87.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schols D, De Clercq E. Human immunodeficiency virus type 1 gp120 induces anergy in human peripheral blood lymphocytes by inducing interleukin-10 production. J. Virol. 1996;70:4953–4960. doi: 10.1128/jvi.70.8.4953-4960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berberian L, Goodglick L, Kipps TJ, Braun J. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science. 1993;261:1588–1591. doi: 10.1126/science.7690497. [DOI] [PubMed] [Google Scholar]
- 76.Zouali M. B-cell superantigens: implications for selection of the human antibody repertoire. Immunol. Today. 1995;16:399–405. doi: 10.1016/0167-5699(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 77.Muller S, Kohler H. B cell superantigens in HIV-1 infection. Int. Rev. Immunol. 1997;14:339–349. doi: 10.3109/08830189709116524. [DOI] [PubMed] [Google Scholar]
- 78.Townsley-Fuchs J, Neshat MS, Margolin DH, Braun J, Goodglick L. HIV-1 gp120: a novel viral B cell superantigen. Int. Rev. Immunol. 1997;14:325–338. doi: 10.3109/08830189709116523. [DOI] [PubMed] [Google Scholar]
- 79.Zhu Q, Egelston C, Gagnon S, Sui Y, Belyakov IM, Klinman DM, Berzofsky JA. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J. Clin. Invest. 2010;120:607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.