Abstract
AIMS
The purpose of this study was to determine the association between the nicotine metabolic rate and smoking behavior, including addiction, in adolescent smokers.
DESIGN
Baseline data from a prospective study of adolescent smoking behaviors and nicotine metabolism.
SETTING
The setting was an outpatient university hospital in San Francisco.
PARTICIPANTS
Adolescent smokers (n=164) aged 13–17 years old.
MEASUREMENTS
Participants completed self-report measures of smoking behavior and nicotine dependence (modified Fagerström Tolerance Questionnaire, mFTQ). The nicotine metabolite ratio (NMR), a phenotypic marker of the rate of nicotine metabolism, was calculated using the ratio of concentrations of deuterium-labeled 3’-hydroxycotinine to cotinine-d4.
FINDINGS
Participants reported smoking a mean of 2.86 cigarettes per day (CPD) (median= 1.78, SD=3.35) for 1.37 years (median= 1.0, SD=1.36). Results from multivariate analyses accounting for age, race/ethnicity, gender and duration of smoking indicated that slower metabolizers smoked more CPD than faster metabolizers (the NMR was inversely related to CPD; p=.02). Slower metabolizers also showed greater dependence on the mFTQ (NMR was negatively associated with the mFTQ; p=.02).
CONCLUSIONS
In adolescence, slower clearance of nicotine may be associated with greater levels of addiction, perhaps mediated by a greater number of cigarettes smoked.
INTRODUCTION
Many adolescents experiment with cigarette smoking, yet only some become addicted (1). It is likely that a combination of biologic and social factors underlie why some adolescents are more susceptible to addiction than others. The finding that genetic factors account for roughly 70% of the variance in nicotine dependence in adults supports some type of biologic vulnerability (2). One factor that may account for a portion of the variability in vulnerability to nicotine addiction is the rate of nicotine metabolism in the individual.
Addicted adult smokers smoke cigarettes to achieve levels of nicotine adequate to produce desired psychopharmacologic effects, such as fending off nicotine withdrawal (3); they adjust their smoking behavior to compensate for changes in the availability of nicotine or in the rate of elimination of nicotine from the body (4, 5). Thus, one would predict that those who metabolize nicotine more quickly will need to smoke more and will develop greater dependence. Indeed, in some (6–9) but not all (10–12) studies of adult smokers, genetically slower metabolizers of nicotine smoke fewer cigarettes per day. In addition, since the proportion of slower metabolizers decreases with increasing age of smoking, slower metabolizers appear to be more likely to quit than individuals with faster metabolism (9). Furthermore, because smokers with slow metabolism are under-represented among addicted smokers, some researchers have suggested that slower metabolizers are less likely to become addicted to nicotine (6–8). However, although it seems plausible that nicotine metabolism may affect smoking behaviors in adult smokers, the influence of nicotine metabolism during adolescence, when the development of nicotine dependence is most likely to occur, is less clear.
There have been few studies looking at the rate of nicotine metabolism as a mediator of dependence during adolescence, and these studies have yielded conflicting results (13–17). Nicotine is metabolized primarily by the liver enzyme CYP2A6. When looking at the effects of nicotine metabolism on the development of addiction in adolescent smokers, O’Loughlin and colleagues (14) reported that adolescents with CYP2A6 alleles associated with slower metabolism of nicotine were at increased risk of becoming nicotine dependent. In contrast, Audrain-McGovern and colleagues (13) found that adolescents with a genetic profile consistent with normal nicotine metabolism (wild type CYP2A6) progressed to nicotine dependence (defined by the mFTQ) more quickly than slower metabolizers.
Several possible reasons exist for the discrepant results, including differing phenotypic definitions of addiction across studies, different genotypic markers of metabolism, and different measures of addiction (18). The majority of studies of adolescent nicotine metabolism focused on polymorphisms of the CYP2A6 gene, which, when present, are associated with slower nicotine metabolism (13, 14, 19). A limitation of using genotypic markers for metabolic rate is that nicotine metabolism is affected differently by the many functionally significant polymorphisms of the CYP2A6 gene (20). Moreover, nicotine metabolism can be affected by other factors, including other genes (e.g., CYP2B6 and CYP2E1) and environmental factors, such as medications (e.g., oral contraceptives) (21, 22). Thus, even smokers with wild type CYP2A6genes, who are nominally normal metabolizers, demonstrate more than four-fold variability in the rate of nicotine metabolism (9). Measurement of the rate of nicotine metabolism using phenotypic measures rather than genetic markers will capture the integrated contribution from these other influences (23, 24).
One way of measuring nicotine metabolism is to use the nicotine metabolite ratio (NMR). The NMR is the ratio of the nicotine metabolites 3’-hydroxycotinine (3HC) to cotinine. The metabolism of cotinine to 3HC is mediated largely or entirely by CYP2A6. Since nicotine is also primarily metabolized by CYP2A6, the NMR reflects the rate of nicotine metabolism (25, 26). Cotinine levels are relatively stable throughout the day in daily smokers, and because concentrations of 3HC are formation-limited, the ratio of 3HC/cotinine also remains stable (9). The NMR reflects the rate of in-vivo nicotine clearance (27), such that the higher the NMR, the greater the nicotine clearance (28). A limitation of NMR based on cotinine and 3HC derived from nicotine in tobacco is its interpretation in intermittent smokers, who do not have stable levels of cotinine. Adolescent smokers are often opportunistic smokers and smoke intermittently. Consequently, their levels of cotinine can fluctuate considerably throughout the week and even within the day. Therefore, other methods of assessing nicotine metabolism are needed. In this study, we used administration of deuterium-labeled cotinine to more directly assess metabolism.
Determining how adolescent smoking is affected by the rate of nicotine metabolism may help to identify individuals who are more susceptible to developing nicotine addiction. In addition, studying these effects in adolescent smokers can offer insight into the effects of nicotine metabolism on this crucial stage on the smoking continuum. For example, one might expect slower metabolizers to be less likely to progress to heavier, more dependent smoking because they retain nicotine longer, resulting in higher levels and potentially more adverse effects of nicotine. Conversely, faster metabolizers who clear nicotine quickly might be expected to smoke more cigarettes per day and to accelerate their smoking more quickly. The purpose of this study was to assess the effects of nicotine metabolism on cigarettes smoked per day and nicotine dependence in adolescent smokers. Based on adult smoking data, we hypothesized that faster metabolizers would smoke a greater numbers of cigarettes per day and would report higher levels of addiction.
METHODS
Participants
As part of an ongoing longitudinal study of the effects of nicotine metabolism on smoking trajectory, 164 adolescent (aged 13–7) smokers (smoking at least once a month, but less than 5 cigarettes a day) were recruited from San Francisco Bay area high schools and pediatric clinics using fliers and online advertising. Individuals interested in joining the study contacted study personnel via phone, e-mail, text messaging, or social media. Study staff screened interested individuals for admission criteria. We chose to focus on adolescents who smoked fewer than 5 cigarettes per day in order to capture potential increases in smoking over the duration of the three-year study.
Potential participants needed be able to attend a 9-hour outpatient hospital visit, needed to be healthy (i.e., no chronic diseases), and had to tell their parent/legal guardian about the study. To help ensure confidentiality for the participants, a small number of “non-smoker” spaces were available to allow participants to join the study without disclosing their smoking status to the parent or legal guardian. Exclusion criteria included using any type of nicotine replacement therapy (NRT) in the past week and being, or attempting to become, pregnant. No participants were excluded due to NRT use or pregnancy. Participants were paid $100 for the 9-hour hospital visit.
Informed Consent
The research design and procedures were reviewed and approved by the University of California Institutional Review Board. Informed, written assent from the adolescent subject and consent from one parent were obtained for each subject before data collection. Study staff reviewed the study procedures with eligible individuals, as well as with the person’s parent/legal guardian. Initial discussion regarding study requirements took place over the telephone. At this time study procedures, including saliva and urine collection, survey administration, and expectations for the outpatient hospital stay (participant must refrain from smoking, must remain in the hospital unit, etc.) were clarified. Finally, any questions the participant or the parent/legal guardian had were answered and a baseline appointment was set up. On the day of the baseline appointment, all study procedures were reviewed again, additional questions answered, and all consent/assent forms signed.
Experimental Procedures
Participants were instructed to present to the Pediatric Clinical Research Center at UCSF at approximately 9 A.M. for a 9-hour outpatient hospital visit. Participants were allowed to smoke up until their visit but were instructed that smoking would not be permitted during the visit.
Female participants were screened for pregnancy with urine pregnancy testing. Participants completed the baseline survey, which included questions about demographics, including ethnicity (classified categorically as white, African-American, Hispanic, Asian, and Other), and smoking behaviors, including the frequency and quantity of cigarette smoking and the types of cigarettes smoked. They were asked when they first tried smoking, when they first smoked a whole cigarette, and when they began smoking regularly. Participants completed the modified Fagerström Tolerance Questionnaire (mFTQ)(29) scale to measure nicotine dependence.
To avoid the potential variability in NMR related to non steady-state levels of cotinine derived from natural nicotine in light and intermittent smokers, deuterium-labeled cotinine (cotinine-d4) was administered (30). Using this technique, it was possible to reliably capture the rate of nicotine clearance in very light intermittent smokers. Importantly, there is no difference in the metabolic breakdown of natural cotinine and labeled cotinine (e.g., cotinine-d4) and neither is affected by the presence or absence of nicotine (31). The use of deuterium-labeled cotinine allowed similar comparisons across all levels of smoking since we only measured the labeled cotinine and its labeled metabolite.
Participants were given 2 mg of orally administered deuterium-labeled cotinine (cotinine-d4) solution (30). Eight hours post cotinine administration, a saliva sample was obtained from the participants for measurement of the cotinine-d4 and deuterium-labeled 3’-hydroxycotinine (3HC-d4). Cotinine-d4 and 3HC-d4 were measured using liquid chromatography-tandem mass spectrometry (32). The NMR was then calculated from the 8-hour salivary sample using the deuterium-labeled 3HC (3HC-d4) to cotinine-d4 ratio (27).
Data Analyses
Mean cigarettes smoked per day was calculated using the sum of usual number of cigarettes smoked during each day of the week and dividing by seven. Descriptive univariate analyses of all variables were performed using means and standard deviations. We analyzed the relationship of NMR, CPD and mFTQ to demographic factors, including age, gender, race/ethnicity.
Finally, we conducted separate regression analyses predicting cigarettes per day (CPD) and mFTQ score (each square-root transformed to reduce skewness) from nicotine metabolism as measured by the NMR. The models also included the potential confounding variables of age, gender, race/ethnicity, and duration of smoking. We then performed backward selection using the Akaike information criterion (AIC) to drop non-contributing variables from the models; the resulting models are reported as the adjusted models. Because the mFTQ also contains information about smoking rate (CPD), we also re-ran the equation predicting mFTQ while removing CPD from the mFTQ scoring.
RESULTS
Two hundred three adolescents were screened, 170 were found to be eligible and 164 participated. The participating sample had a mean age of 16.1 years (SD=0.96) and was 66.5% female (N=109; see Table 1). The participants’ race/ethnicity was diverse with 44 (26.8%) participants reporting their race as White, 32 (19.5%) as African American, 31 (18.9%) as Hispanic, 10 (6.1%) as Asian, 5 (3%) as “Other” and 42 (25.6%) as more than one race.
TABLE 1.
Participant Characteristics
| Participant Characteristic | Smokers (n=164) |
|---|---|
| Age (years) | 16.1 ±.955 |
| % Female | 66.5% |
| Duration of smoking at least weekly (years) | 1.37 ±1.36 |
| Median cigarettes smoked per day | 1.78 |
| NMR (3HC-d4/cot-d4 ratio) | .174 ±.094 |
| Median mFTQ1 score | 2.00 |
modified Fagerström Tolerance Questionnaire (29) (0–2= no dependence).
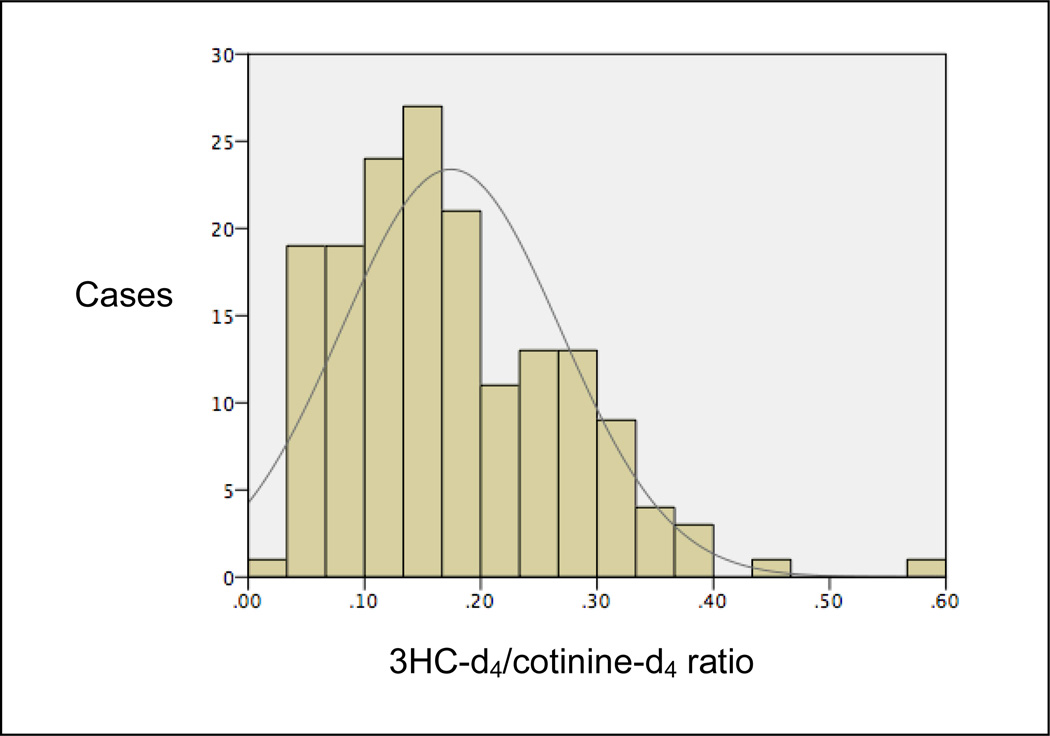
On average, participants reported smoking 2.86 cigarettes per day (median= 1.78, SD=3.35) and smoked for a mean of 1.37 years (median= 1.0, SD=1.36). Although on telephone screening we used inclusion criteria of smoking at least 1 cigarette per month but fewer than 5 CPD, for eligibility, 30 (18%) adolescents reported smoking 5–10 CPD and 7 (4%) reported smoking 10–20 CPD on the self-administered questionnaire. Forty-three (26%) participants reported smoking daily. Mean score on the mFTQ was 2.61 (median= 2.0, range= 0–7, SD=1.49). Duration of smoking (e.g., years since smoking first cigarette) was also correlated with CPD (r=.24, p=.01) and the mFTQ (r=36, p=<.001). Mean NMR value was 0.17 (SD=.094, median= .16, range= .03–.57; see Figure 1). There were no significant differences in the mean number of cigarettes smoked per day (p= .92) or scores on the mFTQ (p= .71) when comparing racial or ethnic groups.
Figure 1.
Saliva 3HC-d4/cotinine-d4 ratios in the study sample.
There was no difference in NMR between males and females (p=.70). Whites had faster rates of metabolism as measured by the NMR than did Blacks/African Americans (p<.01) and Asians. No differences were seen between Whites and Hispanics (p=.44) or adolescents of mixed race (p=.10). Hispanics also had faster rates of metabolism than African Americans (p=.03) and Asians (p=.01).
NMR and Cigarettes per Day
There was no significant difference in NMR between the daily and the non-daily smokers. However, there was a trend toward daily smokers metabolizing more slowly than non-daily smokers (12 versus .18, p=.07). In the multivariate analyses, NMR was inversely related to CPD, such that faster metabolizers smoked fewer CPD (see Table 2). That is, every 1-point increase in NMR was associated with a decrease of 3.61 (1.92) cigarettes per day, after adjusting for gender and duration of smoking.
Table 2.
The association of cigarettes per day (CPD)a with the nicotine metabolite ratio (NMR).
| Univariate | Multivariate | Trimmed Multivariatec | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Each variable alone | All variables together | Contributing variables | |||||||
| Estimate | Std. Error | p value | Estimate | Std. Error | p value | Estimate | Std. Error | p value | |
| NMR | −1.4 | ± .80 | .08 | −1.9 | ± .78 | .02 | −1.9 | ± .78 | .02 |
| Genderb | −.28 | ± .16 | .08 | −.41 | ± .15 | .01 | −.37 | ±.15 | .02 |
| Duration of Smoking | .15 | ± .05 | <.01 | .16 | ± .06 | <.01 | .15 | ±.05 | <.01 |
| Age | −.04 | ± .08 | .57 | −.01 | ± .08 | .99 | |||
| Raced | −.01 | ± .03 | .70 | −.03 | ± .03 | .18 | |||
| African American | −.26 | ± .23 | .25 | −.10 | ± .23 | .66 | |||
| Hispanic | −.15 | ± .23 | .50 | −.01 | ± .22 | .96 | |||
| Asian | .12 | ± .37 | .75 | .10 | ± .36 | .77 | |||
| Other | .22 | ± .47 | .64 | .15 | ± .44 | .73 | |||
| Mixed race | −.24 | ± .21 | .25 | −.30 | ± .20 | .14 | |||
CPD analyzed as square root transformed.
Female gender was used as the reference group.
The models were reduced by backwards selection, dropping variables that did not contribute to prediction, based on the Akaike information criterion (AIC). The column shows only the variables retained in the reduced model.
White race was used as the reference race.
NMR and Addiction
NMR was negatively associated with the mFTQ such that the faster the rate of metabolism, the lower the score on the mFTQ (see Table 3). When the mFTQ analyses were recalculated without including CPD in the mFTQ scoring, the associations with NMR remained similar (Estimate=−1.01, p=.01).
Table 3.
The association of the Fagerström Tolerance Questionnaire modified for use in adolescents (mFTQ)a with the nicotine metabolite ratio (NMR).
| Univariate | Multivariate | Trimmed Multivariatec | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Each variable alone | All variables together | Contributing variables | |||||||
| Estimate | Std. Error | p value | Estimate | Std. Error | p value | Estimate | Std. Error | p value | |
| NMR | −.88 | ± .43 | .04 | −.97 | ± .41 | .02 | −.96 | ± .41 | .02 |
| Genderb | −.01 | ± .08 | .88 | −.04 | ± .08 | .64 | |||
| Duration of Smoking | .12 | ± .03 | <.01 | .13 | ± .03 | <.01 | .12 | ± .03 | <.01 |
| Age | −.01 | ± .04 | .92 | −.07 | ± .04 | .10 | |||
| Raced | −.01 | ± .01 | .54 | −.01 | ± .01 | .34 | |||
| African American | −.06 | ± .12 | .65 | −.01 | ± .13 | .94 | |||
| Hispanic | −.05 | ± .12 | .69 | −.03 | ± .12 | .79 | |||
| Asian | .12 | ± .19 | .52 | .01 | ± .18 | .95 | |||
| Other | −.19 | ± .23 | .40 | −.28 | ± .23 | .22 | |||
| Mixed race | −.07 | ± .11 | .54 | −.08 | ± .11 | .46 | |||
mFTQ analyzed as square root transformed.
Female gender was used as the reference group.
The models were reduced by backwards selection, dropping variables that did not contribute to prediction, based on the Akaike information criterion (AIC). The column shows only the variables retained in the reduced model.
White race was used as the reference race.
DISCUSSION
Contrary to our hypothesis, slower metabolizers actually smoked more cigarettes per day and had higher addiction scores than did faster metabolizers. The finding of increased cigarettes per day among slower metabolizers is the opposite of findings in some other studies of adolescent smokers (13, 19) and most studies of adult smokers (28, 33, 34). Specifically, in other studies, faster rates of nicotine clearance are generally associated with greater numbers of cigarettes smoked per day (13, 19, 35). Our finding of an association between slower metabolism using the NMR and higher addiction scores is also novel. To the best of our knowledge, no studies of smokers have reported significant associations between the NMR and addiction scores. It is worth noting that most of the participants had relatively low scores on the mFTQ. However, evidence suggests that similar measures are valid down to extremely low levels of dependence, as demonstrated by Shiffman and colleagues (36, 37), who found meaningful and measurable variations in dependence even among very light and intermittent smokers.
Our findings suggest that among adolescent smokers very early in their smoking career, those with faster metabolism have not yet begun to compensate for their increased nicotine clearance by increasing the number of cigarettes smoked per day, as has been observed among older addicted smokers. Furthermore, given that this group is clearing nicotine more quickly, it is likely that their brains are being exposed to smaller amounts of nicotine per cigarette compared to slower metabolizers. Conversely, the brains of slower metabolizers are exposed to greater amounts of nicotine for a longer period of time, and thus may be more likely to develop symptoms of addiction at this early stage of smoking. In other words, the slower inactivation and resultant increased levels of nicotine may increase susceptibility to dependence by increasing nicotine exposure and nicotine effects on the developing brain in teens.
The fact that gender remained in the model for the association of CPD with NMR was not surprising since males smoked more CPD in our sample. However, there were no gender differences in NMR. Duration of smoking remained in the models for both associations of NMR with CPD and mFTQ. This makes conceptual sense since, in general, the longer an adolescent has been smoking the greater the likelihood of increasing the quantity of cigarettes smoked and the level of addiction reported.
There are no immediate comparison studies since, to the best of our knowledge, this is the first study to employ labeled cotinine as a means of measuring the NMR in adolescent smokers. However, when looking at the effect of metabolism on CPD, other studies in adolescent smokers had results similar to the adult findings, in that slower metabolizers reported smoking fewer CPD (13, 14, 16). Because these studies used different methods from the current study (e.g., two used genotyping and one used already addicted adolescent smokers), it is not possible to accurately compare these groups with the participants in the current study.
An explanation for the disparity in findings presented in some of these studies and our study is our use of the NMR rather than genotype. Using this method, we captured a more complete picture of the nicotine metabolic rate by encompassing more polymorphic alleles and other environmental factors that may contribute to nicotine metabolism, but that are not measured by genotyping alone. By using the NMR rather than focusing on specific genotypes, we were able to account for these differences and thus may have a wider range of faster and slower metabolizers.
The discrepancy between our findings and those among adults cannot be explained by the fact that adults smoke more cigarettes per day and have been smoking for longer; since neither the duration of adolescents’ smoking nor how many cigarettes they smoked influenced the direction of our findings. Further, existing research demonstrates that the half-life of cotinine is similar across age groups (38, 39), suggesting that early adolescents are unlikely to have different rates of nicotine metabolism compared with adults. As indicated by a growing body of literature in both human and rodent models (40–44), it is possible that there is something unique about this period of brain development that influences an adolescent’s response to nicotine exposure.
CONCLUSION
While it is likely that the rate of nicotine metabolism influences smoking patterns and the severity of addiction, it may impact differently at different stages (e.g., adolescent versus adult) of smoking. Our results suggest that during adolescence, slower clearance of nicotine may promote rather than impede the development of addiction, perhaps mediated by higher levels of nicotine exposure per cigarette. More studies are needed to confirm these findings. The current findings highlight the importance of studying smokers at various stages of smoking progression.
Acknowledgment
The work was performed at the University of California, San Francisco, San Francisco, CA.
This study was supported by NIH/NCI R01 CA140216, NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131 and NIDA P30 - DA12393.
Footnotes
Declaration of Interest: None of the authors have sources of funding, direct or indirect, and/or any connection with the tobacco, alcohol, or gaming industries or any body substantially funded by one of these organizations. Dr. Benowitz has consulted for Pfizer and GlaxoSmithKline, and has been a paid expert in litigation against tobacco companies, including providing testimony of tobacco addiction in adolescents. Dr. Shiffman has consulted for GlaxoSmithKline, and Dr. Moscicki has consulted for Merck Pharmaceuticals. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Contributor Information
Mark L. Rubinstein, Division of Adolescent Medicine, University of California, San Francisco, San Francisco, CA
Saul Shiffman, Department of Psychology, University of Pittsburgh, Pittsburgh, PA
Anna-Barbara Moscicki, Division of Adolescent Medicine, University of California, San Francisco, San Francisco, CA
Michelle A. Rait, Division of Adolescent Medicine, University of California, San Francisco, San Francisco, CA
Saunak Sen, Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, CA
Neal L. Benowitz, Division of Clinical Pharmacology and Experimental Therapeutics, Departments of Medicine, and Bioengineering & Therapeutic Sciences, University of California, San Francisco, San Francisco, CA
REFERENCES
- 1.Administration SAAMHS. Results from the 2009 National Survey on Drug Use and Health: Volume I. In: Studies OoA., editor. Summary of National Findings. Rockville, MD: HHS Publication; 2010. [Google Scholar]
- 2.Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1(Suppl 2):S51–S57. doi: 10.1080/14622299050011811. discussion S69-70. [DOI] [PubMed] [Google Scholar]
- 3.Benowitz NL. Nicotine addiction. Prim Care. 1999;26:611–631. doi: 10.1016/s0095-4543(05)70120-2. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz NL, Jacob P., 3rd Nicotine renal excretion rate influences nicotine intake during cigarette smoking. J Pharmacol Exp Ther. 1985;234:153–155. [PubMed] [Google Scholar]
- 5.Benowitz NL. Compensatory smoking of low-yield cigarettes; Paper presented at the Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine; Bethesda, MD. 2001. [Google Scholar]
- 6.Pianezza ML, Sellers EM, Tyndale RF. Nicotine metabolism defect reduces smoking. Nature. 1998;393:750. doi: 10.1038/31623. [DOI] [PubMed] [Google Scholar]
- 7.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Gu DF, Hinks LJ, Morton NE, Day IN. The use of long PCR to confirm three common alleles at the CYP2A6 locus and the relationship between genotype and smoking habit. Ann Hum Genet. 2000;64:383–390. doi: 10.1046/j.1469-1809.2000.6450383.x. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- 10.Berlin I, Gasior MJ, Moolchan ET. Sex-based and hormonal contraception effects on the metabolism of nicotine among adolescent tobacco-dependent smokers. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2007;9:493–498. doi: 10.1080/14622200701243193. [DOI] [PubMed] [Google Scholar]
- 11.Lea RA, Dickson S, Benowitz NL. Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. J Anal Toxicol. 2006;30:386–389. doi: 10.1093/jat/30.6.386. [DOI] [PubMed] [Google Scholar]
- 12.Malaiyandi V, Goodz SD, Sellers EM, Tyndale RF. CYP2A6 genotype, phenotype, and the use of nicotine metabolites as biomarkers during ad libitum smoking. Cancer Epidemiol Biomarkers Prev. 2006;15:1812–1819. doi: 10.1158/1055-9965.EPI-05-0723. [DOI] [PubMed] [Google Scholar]
- 13.Audrain-McGovern J, Al Koudsi N, Rodriguez D, et al. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:e264–e274. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- 14.O'Loughlin J, Paradis G, Kim W, et al. Genetically decreased CYP2A6 and the risk of tobacco dependence: a prospective study of novice smokers. Tob Control. 2004;13:422–428. doi: 10.1136/tc.2003.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubinstein ML, Benowitz NL, Auerback GM, Moscicki AB. Rate of nicotine metabolism and withdrawal symptoms in adolescent light smokers. Pediatrics. 2008;122:e643–e647. doi: 10.1542/peds.2007-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moolchan ET, Franken FH, Jaszyna-Gasior M. Adolescent nicotine metabolism: ethnoracial differences among dependent smokers. Ethn Dis. 2006;16:239–243. [PubMed] [Google Scholar]
- 17.Moolchan ET, Parzynski CS, Jaszyna-Gasior M, et al. A link between adolescent nicotine metabolism and smoking topography. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:1578–1583. doi: 10.1158/1055-9965.EPI-08-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koudsi NA, O’Loughlin J, Rodriguez D, Audrain-McGovern J, Tyndale RF. The genetic aspects of nicotine metabolism and their impact on adolescent nicotine dependence. Journal of Pediatric Biochemistry. 2010;1:105–123. [Google Scholar]
- 19.Huang S, Cook DG, Hinks LJ, et al. CYP2A6, MAOA, DBH, DRD4, and 5HT2A genotypes, smoking behaviour and cotinine levels in 1518 UK adolescents. Pharmacogenetics and genomics. 2005;15:839–850. doi: 10.1097/01213011-200512000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Malaiyandi V, Sellers EM, Tyndale RF. Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clin Pharmacol Ther. 2005;77:145–158. doi: 10.1016/j.clpt.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Mwenifumbo JC, Tyndale RF. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics. 2007;8:1385–1402. doi: 10.2217/14622416.8.10.1385. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of experimental pharmacology. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther. 1997;282:1608–1614. [PubMed] [Google Scholar]
- 26.Nakajima M, Yamamoto T, Nunoya K, et al. Role of human cytochrome P4502A6 in C-oxidation of nicotine. Drug Metab Dispos. 1996;24:1212–1217. [PubMed] [Google Scholar]
- 27.Dempsey D, Tutka P, Jacob P, 3rd, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5:621–624. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- 29.Prokhorov AV, De Moor C, Pallonen UE, et al. Validation of the modified Fagerström tolerance questionnaire with salivary cotinine among adolescents. Addictive Behaviors. 2000;25:429–433. doi: 10.1016/s0306-4603(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 30.Jacob P, 3rd, Benowitz NL, Shulgin A. Synthesis of optically pure deuterium-labelled nicotine, nornicotine and cotinine. Journal of Labelled Compounds and Radiopharmaceuticals. 1988;25:1117–1128. [Google Scholar]
- 31.Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56:483–493. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 32.Jacob P, 3rd, Yu L, Duan M, et al. Determination of the nicotine metabolites cotinine and trans-3'-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2011;879:267–276. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnstone E, Benowitz N, Cargill A, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther. 2006;80:319–330. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Lerman C, Tyndale R, Patterson F, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Benowitz NL, Perez-Stable EJ, Herrera B, Jacob P., 3rd Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. J Natl Cancer Inst. 2002;94:108–115. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]
- 36.Shiffman S, Ferguson SG, Dunbar MS, Scholl S. Tobacco dependence among intermittent smokers. Nicotine & Tobacco Research. 2012 doi: 10.1093/ntr/nts097. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiffman S, Sayette MA. Validation of the nicotine dependence syndrome scale (NDSS): a criterion-group design contrasting chippers and regular smokers. Drug Alcohol Depend. 2005;79:45–52. doi: 10.1016/j.drugalcdep.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leong JW, Dore ND, Shelley K, et al. The elimination half-life of urinary cotinine in children of tobacco-smoking mothers. Pulm Pharmacol Ther. 1998;11:287–290. doi: 10.1006/pupt.1998.0153. [DOI] [PubMed] [Google Scholar]
- 39.Dempsey D, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Ther. 2000;67:458–465. doi: 10.1067/mcp.2000.106129. [DOI] [PubMed] [Google Scholar]
- 40.Rubinstein ML, Luks TL, Moscicki AB, et al. Smoking-related cue-induced brain activation in adolescent light smokers. J Adolesc Health. 2011;48:7–12. doi: 10.1016/j.jadohealth.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 42.Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892:269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- 43.Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- 44.DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescents. Tobacco Control. 2000;9:313–319. doi: 10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]