Abstract
When excessively activated or deregulated, complement becomes a major link between infection and inflammatory pathology including periodontitis. This oral inflammatory disease is associated with a dysbiotic microbiota, leads to the destruction of bone and other tooth-supporting structures, and exerts an adverse impact on systemic health. We have previously shown that mice deficient either in complement C5a receptor (C5aR; CD88) or TLR2 are highly and similarly resistant to periodontitis, suggesting that a crosstalk between the two receptors may be involved in the disease process. Here we show that C5aR and TLR2 indeed synergize for maximal inflammatory responses in the periodontal tissue and uncover a novel pharmacological target to abrogate periodontitis. Using two different mouse models of periodontitis, we show that local treatments with a C5aR antagonist inhibited periodontal inflammation through downregulation of TNF, IL-1β, IL-6, and IL-17 and further protected against bone loss, regardless of the presence of TLR2. These findings not only reveal a crucial co-operation between C5aR and TLR2 in periodontal inflammation, but provide proof-of-concept for local targeting of C5aR as a powerful candidate for the treatment of human periodontitis.
Keywords: rodent (models), bacterial (infections), complement, cytokines, inflammation, periodontitis
Introduction
Periodontitis is a prevalent chronic inflammatory disease that causes destruction of the bone and soft tissues that surround and support the teeth (periodontium) (1). Disease is initiated by dysbiotic bacterial communities forming on subgingival tooth surfaces when periodontal tissue homeostasis is disrupted (2, 3). A major mechanism of homeostatic breakdown involves the action of “keystone pathogen” species capable of compromising immune surveillance in the periodontium (4-6). The actual damage upon the periodontium is inflicted primarily by the host inflammatory response to chronic challenge by the dysbiotic periodontal microbiota (7, 8).
In its severe form, periodontitis affects 10-15% of the adult population and may lead to tooth loss and/or adversely impact systemic health (1, 9). In this regard, periodontitis is a risk factor for certain systemic inflammatory diseases, such as atherosclerotic heart disease, rheumatoid arthritis, adverse pregnancy outcomes, diabetes, and aspiration pneumonia (10-15). The graveness of this oral disease and its economic burden (16, 17) underscore the importance of implementing new and cost-effective therapeutic interventions.
The complement system is centrally involved in immunity and inflammation through direct effects on immune cells or via crosstalk and regulation of other host signaling pathways (18). Complement activation proceeds via distinct cascade mechanisms (classical, lectin, or alternative) which converge at the third component (C3) and lead to the generation of effector molecules that mediate recruitment and activation of inflammatory cells (via the anaphylatoxins C3a and C5a), microbial opsonization and phagocytosis (via opsonins such as C3b), and direct lysis of susceptible microbes (through the C5b-9 membrane attack complex) (19). However, when excessively activated or deregulated, complement may lead to inflammatory pathology (19). In this regard, clinical observations and experimental studies suggest that complement is involved in periodontal inflammation (20-24).
Mice systemically co-treated with TLR agonists and cobra venom factor, a potent complement activator, elicit dramatically high plasma levels of proinflammatory cytokines and a similar outcome is seen in TLR agonist-treated mice lacking decay-accelerating factor, a membrane complement inhibitor (25). These findings indicate that complement can amplify inflammation through synergism with TLR signaling. Whether this “complement-TLR synergism” operates locally in the periodontal tissue is yet to be determined. A possible crosstalk between complement and TLRs in the periodontal tissue, as shown in systemic models of inflammation (24, 25), may have important implications for therapeutic approaches against periodontitis.
Our recent work implicates complement also as a target of microbial immune subversion. Specifically, the periodontal bacterium Porphyromonas gingivalis acts as a keystone pathogen which subverts C5a receptor (C5aR; CD88)3 and impairs host defense leading to the development of a dysbiotic microbiota (increased total counts and altered composition) (6). This altered microbiota, in turn, provokes complement-dependent inflammation and bone loss in a mouse periodontitis model (6). Taken together, our findings suggest that complement-targeted therapeutic approaches could confer combined anti-microbial and anti-inflammatory effects in periodontitis.
In this study, we showed that local administration of a C5aR antagonist (C5aRA) efficiently protected mice against periodontal inflammation and bone loss in both preventive and therapeutic modes of treatment. C5aRA abrogated the synergism between C5aR and TLR2, which was required for maximal inflammatory responses in the periodontium, consequently inhibiting local inflammation. Our new findings therefore provide proof-of-concept for the efficacy of C5aRA as a locally administered therapeutic agent against periodontitis.
Materials and Methods
Mice
All mouse experimental procedures described in this study have been reviewed and approved by the institutional animal care and use committee, in compliance with established federal and state policies. Specific-pathogen-free mice were maintained in individually ventilated cages and were used for experiments at the age of 8 to 12 weeks. The Tlr2-/- mice, originally on a C57BL/6 genetic background (The Jackson Laboratory), were backcrossed for nine generations onto a BALB/c background (24). The rationale was that BALB/c mice were considered to be a better model for periodontitis than C57BL/6, as the former genotype was shown to be more susceptible to inflammatory bone loss (26). BALB/c wild-type and C5ar-/- mice were obtained from the Jackson Laboratory.
C5aR (CD88) antagonist
A specific C5aR antagonist (C5aRA; PMX53), the cyclic hexapeptide Ac-F[OP(d)Cha-WR] (acetylated phenylalanine–[ornithyl-proline-(d)cyclohexylalanine-tryptophyl-arginine]), and an inactive analog (iC5aRA), Ac-F[OP(d)Cha-A(d)R] (acetylated phenylalanine–[ornithyl-proline-(d)cyclohexylalanine-alanine-(d)arginine]), were synthesized as previously described (27).
Microinjection of C5aR and TLR2 agonists in the gingiva
Mouse C5a (R&D Systems) and/or a prototypical TLR2 agonist, Pam3CSK4 (InVivogen), were microinjected locally into the palatal gingiva of wild-type, Tlr2-/-, and C5ar-/- mice (C5a, 50 ng per site; Pam3CSK4, 2 μg per site). Using a 28.5-gauge MicroFine needle (BD Biosciences), microinjections were performed on the mesial of the first molar and in the papillae between first and second and third molars on both sides of the maxilla (6). Where indicated, wild-type mice were microinjected with C5aRA or iC5aRA (5 μg per site) 1h earlier than a combined microinjection of the two agonists (C5a and Pam3CSK4) together. The concentrations used for the various reagents were determined in preliminary experiments or in previous publications (6, 24, 28). The mice were euthanized 24h later and gingiva were dissected to assess cytokine responses.
Cytokine responses
Gingival tissue was excised from around the maxillary molars and homogenized as previously described (29). Cytokine levels were determined in soluble extracts by ELISA using commercially available kits (eBioscience). Cytokine protein concentrations were normalized to the total protein concentrations in the tissue homogenates, as measured using the Coomassie Plus Bradford protein assay kit (Pierce). Alternatively, the excised gingival tissue was used to extract total RNA, using the PerfectPure RNA cell kit (5 Prime, Fisher), which was quantified by spectrometry at 260 and 280 nm. The RNA was reverse-transcribed using the High-Capacity cDNA Archive kit (Applied Biosystems) and real-time PCR with cDNA was performed using the ABI 7500 Fast System, according to the manufacturer's protocol (Applied Biosystems). TaqMan probes, sense primers, and antisense primers for detection and quantification of cytokine genes by qPCR were purchased from Applied Biosystems. To ensure adequate material for analyses, protein and RNA were extracted from the gingiva of identically treated groups of mice, one of which was used for protein analysis, whereas the other, replicate group was used for mRNA expression analysis.
P. gingivalis-induced periodontitis model
Periodontal inflammation and bone loss was induced in specific-pathogen-free mice by oral inoculation with P. gingivalis, essentially as originally described by Baker (26). Briefly, by means of a ball-ended feeding needle, mice were orally inoculated five times at 2-day intervals with 109 CFU P. gingivalis (ATCC 33277) suspended in 2% carboxy-methylcellulose vehicle. Sham-inoculated controls received vehicle alone. The mice were euthanized at various time points after the last oral inoculation, as specified in the figures. Assessment of periodontal bone loss in defleshed maxillae was performed under a dissecting microscope (x40) fitted with a video image marker measurement system (VIA-170K; Boeckeler Instruments). Specifically, the distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) was measured on 14 predetermined points on the buccal surfaces of the maxillary molars. To calculate bone loss, the 14-site total CEJ-ABC distance for each mouse was subtracted from the mean CEJ-ABC distance of sham-infected mice (26). The results were expressed in mm and negative values indicated bone loss relative to sham controls. In intervention experiments, C5aRA was administered into the palatal gingiva through 1-μl microinjections on the mesial of the first molar and in the papillae between first and second and third molars, on both sides of the maxilla.
The levels of P. gingivalis colonization in the periodontal tissue were determined using qPCR of the ISPg1 gene (6, 30). ISPg1 was selected to increase the sensitivity of P. gingivalis detection, as this gene is present in 31 copies in the genome of P. gingivalis ATCC 33277 (the gene copy numbers were therefore divided by 31 to obtain genome equivalents). For this purpose, genomic DNA was isolated from maxillary periodontal tissue (including both soft and hard tissue, that is, teeth and immediately surrounding bone) using the DNeasy kit (Qiagen) and was quantified by spectrometry at 260 and 280 nm. qPCR was performed using the ABI 7500 Fast System and TaqMan probes, sense primers, and antisense primers used were purchased from Applied Biosystem. The primer sets used to enumerate P. gingivalis copy number were published previously (30).
Ligature-induced periodontitis model
Periodontal inflammation and bone loss in this model is initiated by massive local accumulation of bacteria on ligated molar teeth (31). To this end, a 5-0 silk ligature was tied around the maxillary left second molar. The contralateral molar tooth in each mouse was left unligated (baseline control). Inflammatory bone loss was examined 5 days after placement of the ligatures, which remained in place in all mice during the experimental period. Bone measurements were performed on the ligated second molar (3 sites corresponding to mesial cusp, palatal groove, and distal cusp) and the affected adjacent regions (sites corresponding to distal cusp and distal groove of the first molar, and palatal cusp of the third molar). To calculate bone loss, the 6-site total CEJ-ABC distance for the ligated side of each mouse was subtracted from the 6-site total CEJ-ABC distance of the contralateral unligated side of the same mouse. In intervention experiments in this model, C5aRA microinjections were performed at one site per mouse corresponding to the palatal gingiva of the ligated molar.
Statistical analysis
Data were evaluated by analysis of variance and the Dunnett multiple-comparison test using the InStat program (GraphPad Software, San Diego, CA). Where appropriate (comparison of two groups only), two-tailed t tests were performed. A p value < 0.05 was taken as the level of significance.
Results
C5aR and TLR2 agonists synergize for periodontal inflammation
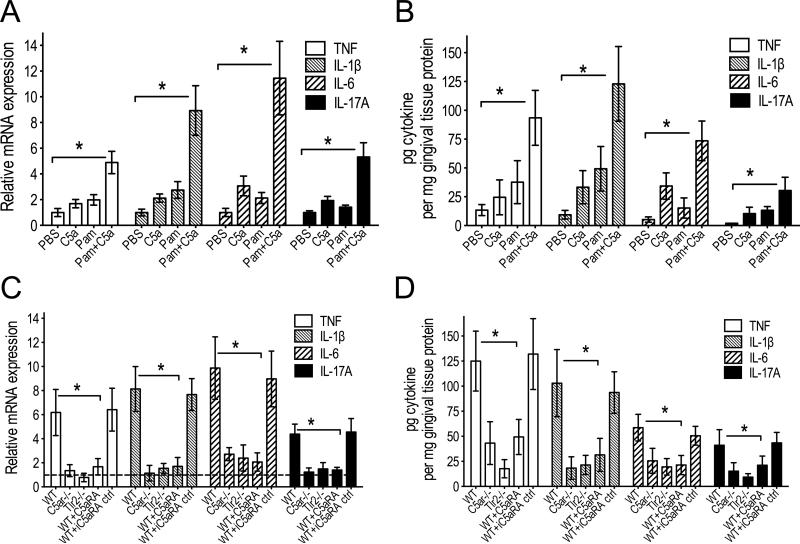
Both complement and TLRs are implicated in periodontal disease pathogenesis in mice and humans (6, 23, 32-35). Mice deficient in either C5aR or TLR2 are essentially resistant against inflammatory periodontal bone loss (24, 36). We therefore hypothesized that periodontal inflammation may depend on synergy between C5aR and TLR2 and that pharmacological blockade of a single receptor might be sufficient to inhibit the development of periodontitis. To address whether C5aR and TLR2 crosstalk in the periodontium, we microinjected C5a and/or Pam3CSK4 (a prototypical TLR2 agonist) locally into the gingiva of wild-type mice and assessed the inflammatory response 24h later. The co-injection of C5a and Pam3CSK4 induced significantly higher TNF, IL-1β, IL-6, and IL-17A mRNA and protein levels than each agonist alone (p < 0.01; Fig. 1, A and B). The magnitude of these responses induced by combined stimulation of C5aR and TLR2 pointed against a simple additive effect from each individual agonist, but rather suggested a synergism between C5aR and TLR2 to amplify the production of proinflammatory cytokines. To further examine and confirm this notion, we co-injected C5a and Pam3CSK4 into the gingiva of wild-type, C5ar-/- and Tlr2-/- mice. Increased mRNA expression and protein production for each cytokine were observed only when both receptors were functional (wild-type mice), whereas the cytokine responses were dramatically and similarly diminished in mice lacking either C5aR or TLR2 (p < 0.01; Fig. 1, C and D). Wild-type mice locally treated in the gingiva with a potent C5aRA (PMX-53), but not with an inactive control (iC5aRA), displayed diminished cytokine responses to combined challenge with C5a and Pam3CSK4, similarly to those seen in C5ar-/- or Tlr2-/- mice (Fig. 1, C and D). These data indicate that C5aR and TLR2 synergize for induction of periodontal proinflammatory cytokines, which could thus be controlled by pharmacological inhibition of just one of the receptors involved (C5aR).
FIGURE 1. C5aR and TLR2 synergize in periodontal inflammation.
(A, B) C5a and/or Pam3CSK4 (Pam), or PBS control, were microinjected into the gingiva of wild-type mice as outlined in Materials and Methods. (C, D) C5a and Pam in combination were microinjected into the gingiva of wild-type, Tlr2-/-, and C5ar-/- mice. The wild-type mice were additionally pretreated with C5aRA or iC5aRA control (ctrl) microinjections (each compound at 5 μg per site) 1h prior to the combined Pam and C5a treatment. All mice were euthanized 24h later and gingiva were dissected to assess the indicated cytokine responses at the mRNA (A and C) or protein (B and D) level. The cytokine mRNA expression levels were normalized against GAPDH mRNA and expressed as fold induction relative to the transcript levels of PBS-microinjected mice, which were assigned an average value of 1 (in C, the value for the PBS controls is indicated by a dashed line in lieu of bars, for clarity). Data are means ± SD (n = 6 mice per group) from one of two independent experiments with similar results. *p < 0.01 between the indicated groups.
C5aRA inhibits periodontal inflammation in P. gingivalis-inoculated mice
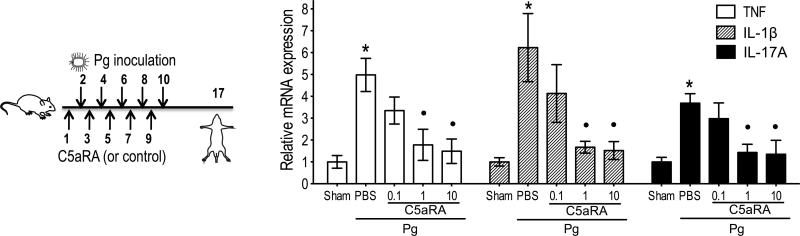
P. gingivalis is strongly associated with human periodontitis (1-3) and is thought to orchestrate periodontal inflammation by remodeling the periodontal microbiota into a dysbiotic state (6). We thus examined whether a preventive approach involving the pharmacological targeting of C5aR is capable to inhibit P. gingivalis-induced periodontal inflammation. To this end, C5aRA (0.1, 1, or 10 μg) or PBS control were microinjected into the mouse gingiva at two day-intervals for a total of five injections, and one day after each treatment the mice were inoculated orally with P. gingivalis in 2% carboxymethylcellulose vehicle or vehicle only (Fig. 2 left). One week after the last inoculation, the gingiva were dissected and analyzed by qPCR for mRNA expression of the proinflammatory cytokines TNF, IL-1β, and IL-17A involved in bone resorption. At a dose of 1 μg, C5aRA significantly (p <0.01) inhibited the induction of all three cytokines and its efficacy (≥ 80% inhibition) was not significantly different from a 10-fold higher dose (Fig. 2 right).
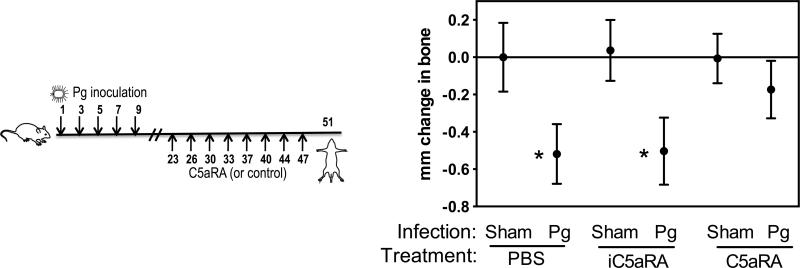
FIGURE 2. C5aRA prevents periodontal inflammation.
Groups of mice were microinjected in the gingiva with the indicated amount (μg) of C5aRA (or PBS control) five times at two-day intervals prior to oral inoculation with P. gingivalis (Pg), as indicated on the left panel (numbers indicate days). A group of mice was inoculated with vehicle alone (Sham) to serve as the baseline for the host response. One week after the last inoculation, the gingiva were dissected and analyzed by qPCR for mRNA expression of the indicated cytokines (normalized against GAPDH mRNA levels and presented as fold change relative to the transcript levels of sham-infected mice, which were assigned an average value of 1). Data are means ± SD (n = 3 mice per group) from one of two independent experiments yielding similar results. *, significant (p < 0.01) induction of cytokine expression in Pg-infected (without C5aRA) compared to sham-infected mice; •, significant (p < 0.01) inhibition of cytokine expression by C5aRA.
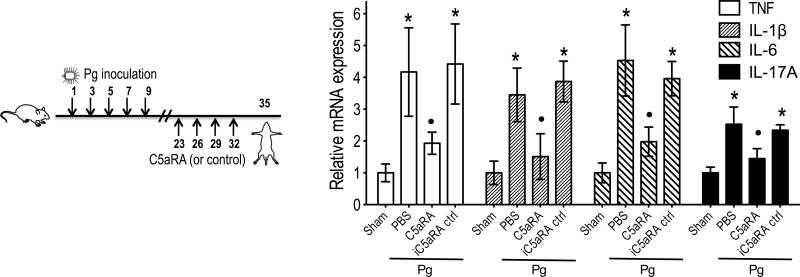
We then set out to determine if C5aRA can act in a therapeutic way (i.e., applied after P. gingivalis inoculation and inflammation). In this case, we first performed five oral inoculations with P. gingivalis, and the mice were not treated until 2 weeks later. This interval (i.e., 2 weeks) was established in a previous study and represents the minimum time required to observe significant P. gingivalis-induced bone loss (30). Therefore, two weeks after the last P. gingivalis inoculation, the mice were locally microinjected with 1 μg C5aRA or equal amount of iC5aRA control, or PBS, every three days for a total of four times (Fig. 3 left). The mice were euthanized three days after the last treatment. C5aRA (but not the inactive analog) significantly reversed inflammation by downregulating (by ≥70%) the expression levels of the pro-inflammatory mediators TNF, IL-1β, IL-6, and IL-17A (p < 0.01; Fig. 3 right). Taken together, these data (Figs. 2 and 3) indicate that local targeting of C5aR is efficient in preventing or reversing P. gingivalis-induced periodontal inflammation.
FIGURE 3. C5aRA reverses periodontal inflammation.
Groups of mice were first inoculated with P. gingivalis (Pg), as indicated on the left panel, and two weeks later were locally administered 1 μg of C5aRA or iC5aRA control (or PBS), every three days for a total of four times. Three days later, the mice were euthanized. Gingiva were dissected and analyzed by qPCR for mRNA expression of the indicated cytokines (normalized against GAPDH mRNA levels and presented as fold change relative to the transcript levels of sham-infected mice, which were assigned an average value of 1). Data are means ± SD (n = 3 mice per group) from one of two independent experiments with similar results. *, significant (p < 0.01) induction of cytokine expression in Pg-infected (without C5aRA) vs. sham-infected mice; •, significant (p < 0.01) inhibition of cytokine expression by C5aRA.
C5aRA protects against P. gingivalis-instigated periodontal bone loss
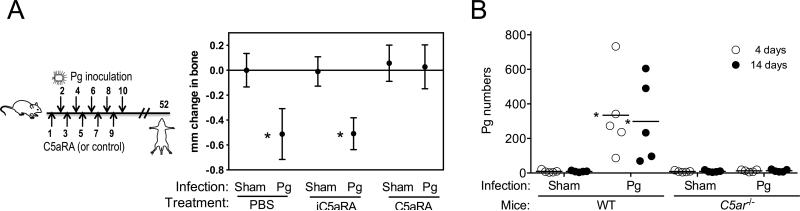
The capacity of C5aRA to inhibit periodontal inflammation suggested that this antagonist could additionally block bone loss. The protocols used for preventive or therapeutic intervention against inflammation (Figs. 2 and 3, left panels) were modified by extending the experimental period to allow development of extensive periodontal bone loss (specifically, mice were euthanized six weeks after the last P. gingivalis inoculation). When used in a preventive setup (Fig. 4A, left), C5aRA completely blocked the induction of periodontal bone loss in P. gingivalis-inoculated mice, as their bone levels were indistinguishable from those of sham-infected mice (Fig. 4A, right). In contrast, iC5aRA control had no effect (Fig. 4A, right). The protection conferred by C5aRA could be attributed to its ability to block a receptor (C5aR) required by P. gingivalis to evade immune surveillance and establish infection (6, 28). This notion was supported by our new findings that P. gingivalis failed to colonize the periodontium in the absence of C5aR. Indeed, P. gingivalis was hardly detectable in C5ar-/- mice four or fourteen days after the last inoculation, although it could readily colonize the periodontium of wild-type mice (Fig. 4B). Importantly, however, C5aRA could significantly inhibit periodontal bone loss (by 67%; p < 0.01) even when this antagonist was administered fourteen days after the last P. gingivalis inoculation (Fig. 5), a time interval sufficient for measurable periodontal bone loss to occur (30). Therefore, mice already colonized with P. gingivalis can also be protected by C5aRA administered after the onset of the disease.
FIGURE 4. C5aRA prevents induction of P. gingivalis-instigated bone loss.
(A) Groups of mice were microinjected in the gingiva with 1 μg of C5aRA or iC5aRA control followed by oral inoculation with P. gingivalis (Pg) or vehicle only (Sham), as indicated on the left panel (numbers indicate days), and were euthanized six weeks later. Bone loss measurements were performed in defleshed maxillae. Data are means ± SD (n = 6 mice per group) from one of two independent experiments with similar results; negative values indicate bone loss in Pg-inoculated mice relative to sham controls. (B) Quantitative detection of Pg by qPCR of the ISPg1 gene, in the periodontal tissue of Pg-inoculated or sham-inoculated wild-type (WT) or C5ar-/- mice, four or fourteen days after the last Pg inoculation. The Pg numbers are total per tissue (maxilla). Each symbol represents an individual mouse and small horizontal lines indicate the mean. The experiment was performed twice yielding similar results. *, p < 0.01 compared to corresponding sham control.
FIGURE 5. C5aRA therapeutically inhibits induction of P. gingivalis-instigated bone loss.
Groups of mice were orally inoculated with P. gingivalis (or vehicle only; Sham) followed by microinjection in the gingiva with 1 μg of C5aRA or iC5aRA control, as indicated on the left panel (numbers indicate days). Mice were euthanized at the specified time point and bone loss measurements were performed in defleshed maxillae. Data are means ± SD (n = 6 mice per group) from one of two independent experiments with similar results; negative values indicate bone loss in P. gingivalis (Pg)-inoculated mice relative to sham controls. *, p < 0.01 compared to corresponding sham control.
C5aRA inhibits ligature-induced periodontitis
Periodontitis fundamentally represents disruption of homeostasis between the host and the periodontal microbiota (2, 3) and is associated with multiple etiologies and disease modifiers (1, 11, 37, 38). Although P. gingivalis can disrupt periodontal homeostasis, host factors may also lead to similar dysbiotic effects. For instance, leukocyte adhesion deficiency leads to dysbiosis and periodontitis in both humans and mice (2, 4). We therefore set out to determine whether C5aRA could inhibit periodontal bone loss in a model where periodontitis was induced independently of P. gingivalis. For this purpose, we used the “ligature-induced periodontitis model”, where a silk ligature is placed around molar teeth resulting in massive local bacterial accumulation and rapid induction of severe bone loss in specific-pathogen-free (but not germ-free) animals (31).
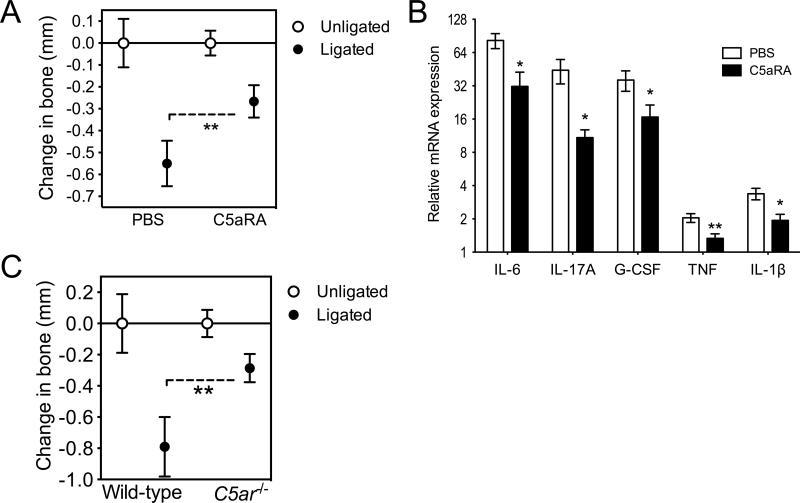
Periodontal bone loss was rapidly induced (within 5 days) at the ligated sites relative to the contralateral unligated sites (zero baseline) (Fig. 6A). Importantly, mice locally treated with C5aRA at the ligated sites displayed about 50% less bone loss compared to PBS-treated controls (p < 0.01; Fig. 6A). Consistent with the acute inflammatory nature of this model, high expression levels of IL-6 as well as IL-17A and G-CSF (which orchestrate neutrophil mobilization and recruitment (39)) were induced in the ligated sites of PBS-treated mice, whereas TNF and IL-1β were induced at relatively lower expression levels (Fig. 6B). The expression of all these proinflammatory cytokines was, however, significantly inhibited in mice treated with C5aRA (p < 0.05; Fig. 6B) in line with the protective effect of C5aRA on bone loss (Fig. 6A). These data suggest that C5aR signaling is crucial for triggering inflammation-mediated bone loss, even in models that do not involve microbial exploitation of C5aR for subversion of host defense (i.e., as opposed to the P. gingivalis-induced periodontitis model, where P. gingivalis exploits C5aR to subvert host defense, establish colonization, and cause dysbiosis and periodontal inflammation (6, 28) and this study)). This notion was independently confirmed by findings that C5ar-/- mice were protected against ligature-induced periodontitis relative to wild-type controls (p < 0.01; Fig. 6C).
FIGURE 6. Pharmacologic or genetic ablation of C5aR inhibits ligature-induced periodontitis.
(A and B) Periodontitis was induced by placing a silk ligature around the second maxillary molar of mice. C5aRA (or PBS control) was microinjected at 1 μg in the palatal gingiva of the ligated second maxillary molar, one day before placement of the ligature and every day thereafter until the day before sacrifice (day 5). (A) Bone loss was measured in defleshed maxillae. (B) Dissected gingiva were processed for qPCR to determine mRNA expression of the indicated molecules (normalized against GAPDH mRNA and expressed as fold change in the transcript levels in the ligated site relative to those of the contralateral unligated site, which were assigned an average value of 1). (C) Determination of ligature-induced periodontal bone loss in wild-type or C5ar-/- mice at day 5, performed as above. Data are means ± SD (n = 5 to 6 mice per group) from one of two independent experiments with similar results; negative values in A and C indicate bone loss relative to the unligated contralateral tooth. *, p < 0.05; **, p < 0.01 between the indicated groups (A, C) or as compared to PBS control (B).
Collectively, our data demonstrate a crosstalk between C5aR and TLR2 in periodontitis and provide proof-of-concept that local pharmacological inhibition of C5aR is a promising approach for the treatment of periodontal inflammation and bone loss.
Discussion
Currently, there is urgent need for developing anti-microbial or host-modulation strategies as adjuncts to existing periodontal therapy (40, 41). Conventional periodontal treatment is often not sufficient by itself to control destructive inflammation and many patients develop recurrent disease (42). New adjunctive therapeutic modalities are likely to be more effective if they are based on good understanding of the underlying immunopathology. In this regard, we have shown that C5aR signaling is centrally involved in inflammatory periodontal bone loss. The crucial involvement of C5aR in periodontitis is not surprising given the extensive interconnections of complement with a variety of inflammatory and immunological networks and the important role of C5aR in the modulation of immune responses (19). C5aR crosstalks and synergizes with TLR2 for periodontal inflammation (this study) and, moreover, the C5aR-TLR2 crosstalk is crucially involved in immune subversion by P. gingivalis (3, 28). The latter mechanism enables P. gingivalis to act as a keystone pathogen of the periodontal microbiota (5, 6). Taken together, these findings provide a mechanistic basis for the protective effects of C5aRA in periodontitis seen in this study.
When used preventively, C5aRA completely abrogated periodontal bone loss in P. gingivalis-inoculated mice. This is likely because P. gingivalis depends upon a functional C5aR in order to colonize the periodontal tissue (Fig. 4B), which in turn is required to cause dysbiosis and periodontitis (6). C5aRA was effective in inhibiting periodontitis even when used therapeutically, i.e., after the onset of the disease. This is important since periodontal intervention would normally be implemented in a therapeutic rather than a preventive manner. Nevertheless, adjunctive periodontal therapy could also be provided on a preventive basis to high-risk individuals for periodontitis, such as cigarette smokers, diabetic patients, or individuals with systemic diseases affecting neutrophil function (43).
As alluded to above, C5aRA may exhibit two distinct modes of action, anti-microbial and anti-inflammatory, against periodontitis. First, it can block C5aR access to P. gingivalis and thereby deprive the bacterium of a crucial immune subversion strategy (inhibition of the leukocyte killing capacity) (28), required for tissue persistence of P. gingivalis and promotion of its dysbiotic effects (6). It should be noted that, generally, complement inhibition is not an anti-microbial strategy but the specialized subversion mechanism of this keystone pathogen justify this approach as anti-microbial in the case of periodontitis. Second, C5aRA can abrogate the C5aR-TLR2 inflammatory synergism in the periodontal tissue, as shown in this study using purified agonists of the two receptors. Obviously, the capacity of C5aRA to protect against ligature-induced periodontitis could not be attributed to counteraction of P. gingivalis immune subversion, but instead to inhibition of several proinflammatory cytokines (see below). This mode of C5aRA action is important in that the breakdown of periodontal homeostasis may not always or inevitably involve P. gingivalis. For instance, disrupted periodontal homeostasis leading to severe inflammation and bone loss is a frequent finding in individuals with neutrophil dysfunctions (44). In this context, we have shown that neutrophil dysfunctions in mice (due to lack of the LFA-1 integrin or the CXC chemokine receptor 2) can lead to dysbiosis and periodontitis in the absence of P. gingivalis (6).
The role of TNF, IL-1β, and IL-6 in destructive periodontal inflammation in humans and animal models is well established (45, 46). Recent evidence also suggests a pathogenic role for IL-17. This cytokine has been implicated on the basis of its increased production levels in diseased gingiva and in gingival crevicular fluid of periodontitis patients (7, 47-49), and a causal link between IL-17 and periodontal bone loss was demonstrated in mice (50). C5aRA inhibited all four proinflammatory and bone resorptive cytokines investigated. This readily explains its protective action, which ranged from 52 to 100% inhibition of bone loss depending on the mode of intervention (preventive or therapeutic) and model employed. In a recent ligature-induced periodontitis study in rats, chemically similar C5aRA was administered in the drinking water, prior to and during the disease, and was clearly less effective (< 20% inhibition of bone loss) (51); this might be due to the different mode of administration, although the different animal species used might be another contributory factor. In the same study, the authors did not examine the effect of C5aRA treatment on periodontal inflammation or other possible mechanisms underlying their observation (51). Modes of local administration, i.e, which restrict the action of C5aRA to the periodontal tissue as in the present study, are likely to be safer since systemic inhibition of complement may predispose to increased susceptibility to microbial infections.
In summary, at least in preclinical models, the antagonistic blockade of C5aR prevents or arrests the development of periodontitis by counteracting microbial immune evasion and suppressing the induction of proinflammatory and bone-resorptive cytokines. C5aR antagonists are well tolerated and are in early clinical trials for the treatment of inflammatory diseases (52, 53). The findings of this study suggest that locally applied C5aR antagonists may potentially find application for the treatment of human periodontitis.
Footnotes
This work was supported by grants from the National Institutes of Health GM062134, AI030040, AI068730 (to J.D.L.) and DE015254, DE021580, and DE021685 (to G.H.).
Abbreviations used in this paper: ABC, alveolar bone crest; CEJ, cementoenamel junction; C5aR, C5a receptor (CD88); C5aRA, C5aR antagonist; iC5aRA, inactive C5aRA analog; qPCR, quantitative real-time PCR.
References
- 1.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 3.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: The Polymicrobial Synergy and Dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012;27 doi: 10.1111/j.2041-1014.2012.00663.x. Epub ahead of print: DOI: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 2012;91:816–820. doi: 10.1177/0022034512453589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajishengallis G, Darveau RP, Curtis MA. The keystone pathogen hypothesis. Nat. Rev. Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J. Dent. Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Dyke TE. The management of inflammation in periodontal disease. J. Periodontol. 2008;79:1601–1608. doi: 10.1902/jop.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol. 2000. 2010;53:28–44. doi: 10.1111/j.1600-0757.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genco RJ, Van Dyke TE. Prevention: Reducing the risk of CVD in patients with periodontitis. Nat. Rev. Cardiol. 2010;7:479–480. doi: 10.1038/nrcardio.2010.120. [DOI] [PubMed] [Google Scholar]
- 11.Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 12.Awano S, Ansai T, Takata Y, Soh I, Akifusa S, Hamasaki T, Yoshida A, Sonoki K, Fujisawa K, Takehara T. Oral health and mortality risk from pneumonia in the elderly. J. Dent. Res. 2008;87:334–339. doi: 10.1177/154405910808700418. [DOI] [PubMed] [Google Scholar]
- 13.Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 14.Jeffcoat M, Parry S, Sammel M, Clothier B, Catlin A, Macones G. Periodontal infection and preterm birth: successful periodontal therapy reduces the risk of preterm birth. BJOG. 2011;118:250–256. doi: 10.1111/j.1471-0528.2010.02713.x. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg K, Wegner N, Yucel-Lindberg T, Venables PJ. Periodontitis in RA-the citrullinated enolase connection. Nat. Rev. Rheumatol. 2010;6:727–730. doi: 10.1038/nrrheum.2010.139. [DOI] [PubMed] [Google Scholar]
- 16.Brown LJ, Johns BA, Wall TP. The economics of periodontal diseases. Periodontol. 2000. 2002;29:223–234. doi: 10.1034/j.1600-0757.2002.290111.x. [DOI] [PubMed] [Google Scholar]
- 17.Beikler T, Flemmig TF. Oral biofilm-associated diseases: trends and implications for quality of life, systemic health and expenditures. Periodontol. 2000. 2011;55:87–103. doi: 10.1111/j.1600-0757.2010.00360.x. [DOI] [PubMed] [Google Scholar]
- 18.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patters MR, Niekrash CE, Lang NP. Assessment of complement cleavage in gingival fluid during experimental gingivitis in man. J. Clin. Periodontol. 1989;16:33–37. doi: 10.1111/j.1600-051x.1989.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 21.Nikolopoulou-Papaconstantinou AA, Johannessen AC, Kristoffersen T. Deposits of immunoglobulins, complement, and immune complexes in inflamed human gingiva. Acta Odontol. Scand. 1987;45:187–193. doi: 10.3109/00016358709098858. [DOI] [PubMed] [Google Scholar]
- 22.Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. II. Evidence for cleavage of complement components C3, C3 proactivator (factor B) and C4 in gingival fluid. J. Periodontol. 1977;48:778–784. doi: 10.1902/jop.1977.48.12.778. [DOI] [PubMed] [Google Scholar]
- 23.Hajishengallis G. Complement and periodontitis. Biochem. Pharmacol. 2010;80:1992–2001. doi: 10.1016/j.bcp.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang S, Krauss JL, Domon H, McIntosh ML, Hosur KB, Qu H, Li F, Tzekou A, Lambris JD, Hajishengallis G. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J. Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker PJ, Dixon M, Roopenian DC. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect. Immun. 2000;68:5864–5868. doi: 10.1128/iai.68.10.5864-5868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J. Med. Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, Triantafilou M, Triantafilou K, Lambris JD, Hajishengallis G. Microbial hijacking of complement-toll-like receptor crosstalk. Sci. Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin X, Han X, Kawai T, Taubman MA. Antibody to receptor activator of NF-kB ligand ameliorates T cell-mediated periodontal bone resorption. Infect. Immun. 2011;79:911–917. doi: 10.1128/IAI.00944-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntosh ML, Hajishengallis G. Inhibition of Porphyromonas gingivalis-induced periodontal bone loss by CXCR4 antagonist treatment. Mol. Oral Microbiol. 2012;27 doi: 10.1111/j.2041-1014.2012.00657.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahanonda R, Pichyangkul S. Toll-like receptors and their role in periodontal health and disease. Periodontol. 2000. 2007;43:41–55. doi: 10.1111/j.1600-0757.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 33.Krauss JL, Potempa J, Lambris JD, Hajishengallis G. Complementary Tolls in the periodontium: how periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host. Periodontol. 2000. 2010;52:141–162. doi: 10.1111/j.1600-0757.2009.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P, Liu J, Xu Q, Harber G, Feng X, Michalek SM, Katz J. TLR2-dependent modulation of osteoclastogenesis by Porphyromonas gingivalis through differential induction of NFATc1 and NF-kappaB. J. Biol. Chem. 2011;286:24159–24169. doi: 10.1074/jbc.M110.198085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rojo-Botello NR, Garcia-Hernandez AL, Moreno-Fierros L. Expression of toll-like receptors 2, 4 and 9 is increased in gingival tissue from patients with type 2 diabetes and chronic periodontitis. J. Periodontal Res. 2012;47:62–73. doi: 10.1111/j.1600-0765.2011.01405.x. [DOI] [PubMed] [Google Scholar]
- 36.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: Activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 37.Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Mol. Oral Microbiol. 2010;25:25–37. doi: 10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kornman KS. Interleukin 1 genetics, inflammatory mechanisms, and nutrigenetic opportunities to modulate diseases of aging. Am. J. Clin. Nutr. 2006;83:475S–483S. doi: 10.1093/ajcn/83.2.475S. [DOI] [PubMed] [Google Scholar]
- 39.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Hajishengallis G. Toll gates to periodontal host modulation and vaccine therapy. Periodontol. 2000. 2009;51:181–207. doi: 10.1111/j.1600-0757.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasturk H, Kantarci A, Van Dyke TE. Paradigm shift in the pharmacological management of periodontal diseases. Front Oral Biol. 2012;15:160–176. doi: 10.1159/000329678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armitage GC. Classifying periodontal diseases--a long-standing dilemma. Periodontol. 2000. 2002;30:9–23. doi: 10.1034/j.1600-0757.2002.03002.x. [DOI] [PubMed] [Google Scholar]
- 43.Heitz-Mayfield LJ. Disease progression: identification of high-risk groups and individuals for periodontitis. J. Clin. Periodontol. 2005;32(Suppl 6):196–209. doi: 10.1111/j.1600-051X.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 44.Deas DE, Mackey SA, McDonnell HT. Systemic disease and periodontitis: manifestations of neutrophil dysfunction. Periodontol. 2000. 2003;32:82–104. doi: 10.1046/j.0906-6713.2003.03207.x. [DOI] [PubMed] [Google Scholar]
- 45.Graves D. Cytokines that promote periodontal tissue destruction. J. Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 46.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- 47.Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, Yamada N, Hata M, Yamane J, Terada N. The involvement of IL-23 and the Th17 pathway in periodontitis. J. Dent. Res. 2009;88:633–638. doi: 10.1177/0022034509339889. [DOI] [PubMed] [Google Scholar]
- 48.Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, Silva JS. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol. Immunol. 2009;24:1–6. doi: 10.1111/j.1399-302X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 49.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J. Clin. Periodontol. 2005;32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 50.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung KJ, Hashim A, Curtis MA, Chavakis T, Hajishengallis G. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Breivik T, Gundersen Y, Gjermo P, Taylor SM, Woodruff TM, Opstad PK. Oral treatment with complement factor C5a receptor (CD88) antagonists inhibits experimental periodontitis in rats. J. Periodontal Res. 2011;46:643–647. doi: 10.1111/j.1600-0765.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- 52.Qu H, Ricklin D, Lambris JD. Recent developments in low molecular weight complement inhibitors. Mol. Immunol. 2009;47:185–195. doi: 10.1016/j.molimm.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodruff TM, Nandakumar KS, Tedesco F. Inhibiting the C5-C5a receptor axis. Mol. Immunol. 2011;48:1631–1642. doi: 10.1016/j.molimm.2011.04.014. [DOI] [PubMed] [Google Scholar]