Abstract
HIV-1 is grouped phylogenetically into clades, which may impact rates of HIV-1 disease progression. Clade D infection in particular has been shown to be more pathogenic. Here we confirm in a Nairobi-based prospective female sex worker cohort (1985–2004) that Clade D (n = 54) is associated with a more rapid CD4 decline than clade A1 (n = 150, 20.6% vs 13.4% decline per year, 1.53-fold increase, p = 0.015). This was independent of “protective” HLA and country of origin (p = 0.053), which in turn were also independent predictors of the rate of CD4 decline (p = 0.026 and 0.005, respectively). These data confirm that clade D is more pathogenic than clade A1. The precise reason for this difference is currently unclear, and requires further study. This is first study to demonstrate difference in HIV-1 disease progression between clades while controlling for protective HLA alleles.
Introduction
HIV infection is characterized by a prolonged host-pathogen interaction that, in the absence of antiretroviral therapy, eventually leads to CD4 decline and AIDS. The rate of HIV disease progression is heterogeneous and driven by many factors [1]. A better understanding of the host, viral, and environmental factors that contribute to this heterogeneity would improve our understanding of HIV pathogenesis and therapeutic strategies. HIV exhibits extreme genetic diversity, in some cases differing by more than 30% amino acid homology in the Env protein. The HIV-1 main group (M), which accounts for most infections globally, has been classified into numerous clades (subtypes) and circulating recombinant forms (CRFs), with distinct geographical distributions [2]. In sub-Saharan Africa, where the pandemic originated [3], most countries have multiple clades and/or CRFs in circulation.
Several previous studies have assessed the impact of HIV subtype on the rate of disease progression. An early study in Kenya showed that clade C was associated with faster progression, higher viral loads, and lower CD4 counts than clades A1 or D [4]. Another study in Senegal demonstrated that infection by clades other than A1 was associated with an increased risk of disease progression during the period of follow-up [5]. Recent studies in Kenya, Tanzania, and Uganda suggest that clade D is associated with more rapid disease progression than clade A, ranging from approximately 1.3 to >6 times faster [6], [7], [8], [9]. It is unclear what immunological or virological mechanism(s) underlie these differences [10]. One report suggested that clade D was associated with CXCR4-tropism [11], which has previously been associated with faster progression to AIDS [12]. Therefore, while differences in progression between clades have been observed previously, it remains uncertain whether these are due to differences in viral replication. We sought to confirm the differences in CD4 decline between clades A1 and D at different stages of HIV-1 disease progression, and explore whether these were associated with differences in HIV viral loads in vivo.
Methods
Cohort Description
We enrolled participants from a large prospective female sex worker cohort in Nairobi, Kenya. All participants provided informed written consent, and Institutional Review Boards at Kenyatta National Hospital and University of Manitoba approved the study. HIV-1 clade was determined cross-sectionally by HIV-1 p24 proviral DNA sequencing, as described (n = 377) [13]. The region sequenced corresponds to HXB2 positions 1,508–2,198. Only clade A1 and D-infected participants were included; >90% of the cohort was infected by these clades. Class I HLA haplotypes were determined by DNA sequencing, as previously described [13]. HLA alleles were considered protective based on their ability to slow disease progression consistently in prior studies; protective alleles included HLA-B*1302, B*27, B*5101, B*5701/3, and B*8101 [14]. Participant characteristics and details of follow-up of those infected by clades A1 and D in the present study are shown in Table 1. CD4 counts were measured biannually in this cohort from 1990 onwards using Becton Dickinson Tritest reagents. The majority of the cohort (>80%) was HIV seropositive at enrollment. All participants were ART-naive for the period of follow-up in this study. Viral loads are not standard of care in this cohort and were measured, where available, using Roche bDNA viral load assay v. 3.0.
Table 1. Baseline characteristics of the study cohort.
| Variable | Clade A1 (n = 150) | Clade D (n = 54) | P value |
| Mean age, years (median, IQR) | 35 (30–39) | 32 (27–38) | 0.32 |
| Kenyan | 25.5% | 14.8% | 0.13 |
| Follow-up, days (median, IQR) | 2,438 (1,309–3,824) | 2,057 (972–3,230) | 0.159 |
| CD4 count at baseline (median, IQR) | 576 (453–740) | 516 (413–689) | 0.133 |
| No. of CD4 counts (median, IQR) | 11 (6–16) | 8.5 (4–15) | 0.077 |
| Protective HLA | 29.1% | 18.9% | 0.20 |
| % with VL data | 48.7% | 35.2% | 0.11 |
| Average log10 copies/ml (VL; median, IQR) | 3.74 (3.02–4.48) | 3.95 (3.53–4.91) | 0.186 |
Statistical Approach
Chi-square and Mann-Whitney tests were used to compare participant characteristics, where appropriate. Slopes of CD4 decline were analyzed using unstructured linear mixed models with random intercept and slope. In order to “linearize” rates of decline, CD4 counts were transformed using the natural log (lnCD4). Natural log-derived parameters are more easily interpreted than square or cube roots, and previous reports suggest these measures behave similar, in terms of linearization of rates of decline, over the ranges studied [15]. In the most basic model, decline of lnCD4 was a dependent variable, with follow-up and the interaction between clade and time as outcome variables. Potential confounders were then added as additional co-variables including protective HLA (interacting with time), and country of origin (interacting with time).
Results
Participant Characteristics
Of the 377 participants with HIV-1 sequence data, 204 were included (54%) in this analysis on the basis of having a CD4>350 at baseline, as has been reported elsewhere [16]. No other exclusion criteria were used. Apart from shorter duration of follow-up and lower CD4 count (by definition), there were no differences between excluded individuals compared to those included in the analysis (not shown). Clade A1 was approximately three times more common than clade D. Baseline and follow-up data for study participants are shown in Table 1. Although there were no statistically significant differences, clade A1-infected participants tended to be older (median 35 vs. 32, p = 0.32), more likely to be Kenyan (25.5 vs. 14.8%, p = 0.13), and have at least one protective class I HLA allele (29.1 vs. 18.9%, p = 0.20). Other participants were from Tanzania (65.5%) and Uganda (11.8%). Clade A1 participants tended to have longer follow-up (median 2,438 vs. 2,057 days, p = 0.159) and more CD4 measurements (median 11 vs. 8.5, p = 0.077). Many of these are consistent with the hypothesized longer survival time for clade A1 compared to D-infected individuals. Since VL is not standard of care, these were only available for a random selection of clade A1 (48.7%) and D (35.2%) participants.
Impact of Clade and Co-variates on Slope of CD4 Decline
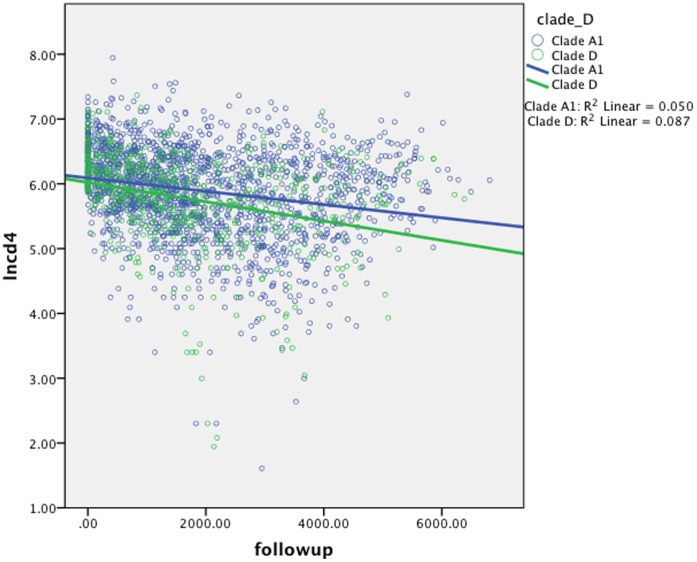
Because time of infection is left-censored for the majority of the cohort, survival analyses to determine the impact of clade on time to CD4 decline below a fixed threshold (e.g. 350) were not feasible. Therefore we used linear mixed models with random effects (slope and intercept) to determine the impact of clade on CD4 decline during prospective follow-up. The natural log of CD4 (lnCD4) was the dependent variable in all models; this measure declines linearly and is assumed to be independent of time of infection. In the most basic model (Table 2, Model 1), including time and the interaction between clade D and time, clade D was associated with a more rapid CD4 decline (p = 0.015, annual estimate = 0.061, 95% CI 0.012, 0.110). While clade D-infected individuals experienced an annual CD4 decline of 20.6%, this decline was 13.4% for clade A1, translating to a 1.53-fold more rapid CD4 decline for clade D than A1 (Figure 1). While CD4 cells declined by an average of 39.7 cells/year for clade A1, clade D infected participant CD4 counts declined by 50.6 cells/year. In an exploratory analysis including only participants with a CD4>500 at baseline, the impact of clade D on CD4 decline was also observed (not shown).
Table 2. Results from linear mixed models examining factors associated with CD4 decline.
| Variable | Model 1 | Model 2 | ||
| Estimate (95% CI) | P value | Estimate (95% CI) | P value | |
| Follow Up | −0.187 (−0.230, −0.144) | <0.001 | −0.084 (−0.156, −0.012) | 0.022 |
| Clade A1*follow-up | 0.061 (0.012, 0.110) | 0.015 | 0.048 (−0.001, 0.097) | 0.053 |
| No Protective HLA* follow-up | − | − | −0.054 (−0.102, −0.007) | 0.026 |
| Kenyan*follow-up | − | − | 0.070 (−0.119, −0.021) | 0.005 |
Figure 1. Scatter plot showing slopes of decline of natural log CD4 (lnCD4, y-axis) over time (x-axis).
Dots indicate individual CD4 measures, while lines indicate mean slope of decline for participants infected by Clades A1 (blue) and clade D (green). These were analyzed using linear mixed models (Table 2).
Next we controlled for various potential confounders (Table 2, Model 2). Age or duration of sex work had no impact on the association between clade and CD4 decline. Baseline CD4 was associated with time but also did not affect the clade association (not shown). No associations between baseline CD4 and clade, protective HLA, or country of origin were observed, and these were omitted from further models (not shown). In a multivariate model (Model 2), both protective HLA alleles and country of origin were independently associated with rate of CD4 decline (No protective HLA, p = 0.026, Estimate −0.054, 95% CI −0.102, −0.007; Non-Kenyan, p = 0.005, Estimate −0.070, 95% CI −0.119, −0.121), and these decreased the effect observed for clade (p = 0.053, Estimate 0.048, 95% CI −0.001, 0.097). Lastly, we added average VL as a co-variate to determine its impact on rates of CD4 decline. This was a sub-analysis that included VL data where available (92/204 participants with baseline CD4>350). While follow-up and country of origin remain significant in this model (p = 0.019 and p = 0.021, respectively), clade and protective HLA were no longer associated with rates of CD4 decline (not shown).
Discussion
These data support earlier findings that the rate of CD4 decline among individuals infected with clade D is more rapid, by approximately 50%, than those infected with clade A1. Despite the consistency of this observation, the reason(s) that underlie it remain unclear. One hypothesis is that clade D is associated with a higher VL, perhaps as a result of increased replicative fitness. Other hypotheses include that clade D viruses have a differential effect on other factors that impact HIV-1 disease progression, such as cellular tropism or immune activation. Resolution of these differences could provide important data for better understanding the heterogeneity of HIV-1 disease progression.
Our data suggest there is a slight (0.2 log10 copies/ml) increase in VL associated with clade D that was not statistically significant. While clade was a reasonable predictor of the slope of CD4 decline in models that include several relevant co-variates, the addition of VL nullified this association (while follow-up remained significant). It is important to note a major limitation of our study is that VL could only be included as a sub-analysis, and the time point of infection for VL remains unknown. Therefore it does not appear VL is a major determinant of the difference in rates of disease progression between clades. Another possible future direction would be to compare reservoir size between clades A1 and D by measuring cell-associated VL.
Our study is the first to compare differences in HIV-1 disease progression between clades while controlling for protective HLA alleles. These data suggest that both clade and HLA can contribute to rates of disease progression. HLA differences between populations could be an important confounder in studies of HIV pathogenesis. In addition, country of origin was a strong, independent predictor of the rate of CD4 decline in all models. The reasons for this are still not clear and suggest an impact of other, unmeasured, confounders.
Previous studies of HIV-1 clade and disease have employed several different approaches. While the methods to determine clade are likely all valid, the HIV-1 region under study could be important. In our study, clade was determined by sequencing and phylogenetics of the p24 protein of HIV-1. Therefore, one potential limitation is that recombinants may be missed due to misclassification bias. On the contrary, our data suggest that clade of p24 might be an important predictor of both VL and rates of CD4 decline. Studies of CTL escape suggest p24 has many conserved residues that are likely structurally important for virion function [17], [18]. Study of the p24 differences between clades therefore might be an important avenue for future study.
In summary, these data add to mounting evidence that clade D is more pathogenic than clade A1. Some studies suggest clade A1 is associated with increased transmission [19], [20], possibly because clade D viruses tend to be X4-tropic, and therefore do not readily establish infection [21]. The above data suggest that any difference in pathogenecity between clades will not necessarily increase the frequency of that clade over time. We compare the differences in clades including important co-variates such as protective HLA alleles and country of origin. Potential mediators of clade-associated differences in pathogenecity can only be resolved by further studies, particularly those that focus on the earliest events following HIV-1 infection.
Acknowledgments
We would like to thank the staff at Majengo clinic, the University of Nairobi, and the Kenyan AIDS Control Project. We would also like to thank the research participants for their patience and dedication to these studies.
Funding Statement
This research was supported by grants from the National Institutes of Health (R01 AI56980 A1), the Canadian Institutes of Health Research (HOP-43135), and the Bill and Melinda Gates Foundation and the CIHR through the Grand Challenges in Global Health Initiative to F.P. F.P. and R.K. hold Canada Research Chairs. L.M. is supported by a CIHR Biomedical/Clinical HIV/AIDS Research Fellowship and the International Infectious Diseases and Global Health Training Program (IID&GHTP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fauci AS (2007) Pathogenesis of HIV disease: opportunities for new prevention interventions. Clin Infect Dis 45 Suppl 4S206–212. [DOI] [PubMed] [Google Scholar]
- 2. Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM (2008) The challenge of HIV-1 subtype diversity. N Engl J Med 358: 1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Worobey M, Gemmel M, Teuwen DE, Haselkorn T, Kunstman K, et al. (2008) Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature 455: 661–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neilson JR, John GC, Carr JK, Lewis P, Kreiss JK, et al. (1999) Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J Virol 73: 4393–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kanki PJ, Hamel DJ, Sankale JL, Hsieh C, Thior I, et al. (1999) Human immunodeficiency virus type 1 subtypes differ in disease progression. J Infect Dis 179: 68–73. [DOI] [PubMed] [Google Scholar]
- 6. Baeten JM, Chohan B, Lavreys L, Chohan V, McClelland RS, et al. (2007) HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J Infect Dis 195: 1177–1180. [DOI] [PubMed] [Google Scholar]
- 7. Kiwanuka N, Laeyendecker O, Robb M, Kigozi G, Arroyo M, et al. (2008) Effect of human immunodeficiency virus Type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis 197: 707–713. [DOI] [PubMed] [Google Scholar]
- 8. Vasan A, Renjifo B, Hertzmark E, Chaplin B, Msamanga G, et al. (2006) Different rates of disease progression of HIV type 1 infection in Tanzania based on infecting subtype. Clin Infect Dis 42: 843–852. [DOI] [PubMed] [Google Scholar]
- 9. Kaleebu P, French N, Mahe C, Yirrell D, Watera C, et al. (2002) Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-positive persons in Uganda. J Infect Dis 185: 1244–1250. [DOI] [PubMed] [Google Scholar]
- 10. Kuritzkes DR (2008) HIV-1 subtype as a determinant of disease progression. J Infect Dis 197: 638–639. [DOI] [PubMed] [Google Scholar]
- 11. Kaleebu P, Nankya IL, Yirrell DL, Shafer LA, Kyosiimire-Lugemwa J, et al. (2007) Relation between chemokine receptor use, disease stage, and HIV-1 subtypes A and D: results from a rural Ugandan cohort. J Acquir Immune Defic Syndr 45: 28–33. [DOI] [PubMed] [Google Scholar]
- 12. Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR (1997) Change in coreceptor use coreceptor use correlates with disease progression in HIV-1–infected individuals. J Exp Med 185: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peters HO, Mendoza MG, Capina RE, Luo M, Mao X, et al. (2008) An integrative bioinformatic approach for studying escape mutations in human immunodeficiency virus type 1 gag in the Pumwani Sex Worker Cohort. J Virol 82: 1980–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goulder PJ, Watkins DI (2008) Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol 8: 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lipsitz SR, Ibrahim J, Molenberghs G (2000) Using a Box-Cox transformation in the analysis of longitudinal data with incomplete responses. Journal of the Royal Statistical Society Series C (Applied Statistics) 49: 287–296. [Google Scholar]
- 16.Yang H, Wu H, Hancock G, Clutton G, Sande N, et al. The antiviral inhibitory capacity of CD8+ T cells predicts the rate of CD4+ cell decline in HIV-1 infection. J Infect Dis. [DOI] [PMC free article] [PubMed]
- 17. Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, et al. (2004) HIV evolution: CTL escape mutation and reversion after transmission. Nat Med 10: 282–289. [DOI] [PubMed] [Google Scholar]
- 18. Martinez-Picado J, Prado JG, Fry EE, Pfafferott K, Leslie A, et al. (2006) Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J Virol 80: 3617–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kiwanuka N, Laeyendecker O, Quinn TC, Wawer MJ, Shepherd J, et al. (2009) HIV-1 subtypes and differences in heterosexual HIV transmission among HIV-discordant couples in Rakai, Uganda. Aids 23: 2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muller-Trutwin MC, Chaix ML, Letourneur F, Begaud E, Beaumont D, et al. (1999) Increase of HIV-1 subtype A in Central African Republic. J Acquir Immune Defic Syndr 21: 164–171. [PubMed] [Google Scholar]
- 21. Margolis L, Shattock R (2006) Selective transmission of CCR5-utilizing HIV-1: the ‘gatekeeper’ problem resolved? Nat Rev Microbiol 4: 312–317. [DOI] [PubMed] [Google Scholar]