Abstract
GNE myopathy, previously termed hereditary inclusion body myopathy (HIBM), is an adult-onset neuromuscular disorder characterized by progressive muscle weakness. The disorder results from biallelic mutations in GNE, encoding UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase, the key enzyme of sialic acid synthesis. GNE myopathy, associated with impaired glycan sialylation, has no approved therapy. Here we test potential sialylation-increasing monosaccharides for their effectiveness in prophylaxis (at the embryonic and neonatal stages) and therapy (after the onset of symptoms) by evaluating renal and muscle hyposialylation in a knock-in mouse model (Gne p.M712T) of GNE myopathy. We demonstrate that oral mannosamine (ManN), but not sialic acid (Neu5Ac), mannose (Man), galactose (Gal), or glucosamine (GlcN), administered to pregnant female mice has a similar prophylactic effect on renal hyposialylation, pathology and neonatal survival of mutant offspring, as previously shown for N-acetylmannosamine (ManNAc) therapy. ManN may be converted to ManNAc by a direct, yet unknown, pathway, or may act through another mode of action. The other sugars (Man, Gal, GlcN) may either not cross the placental barrier (Neu5Ac) and/or may be able to directly increase sialylation. Because GNE myopathy patients will likely require treatment in adulthood after onset of symptoms, we also administered ManNAc (1 or 2 g/kg/day for 12 weeks), Neu5Ac (2g/kg/day for 12 weeks), or ManN (2g/kg/day for 6 weeks) in drinking water to 6 month old mutant Gne p.M712T mice. All three therapies markedly improved the muscle and renal hyposialylation, as evidenced by lectin histochemistry for overall sialylation status and immunoblotting of specific sialoproteins. These preclinical data strongly support further evaluation of oral ManNAc, Neu5Ac and ManN as therapy for GNE myopathy and conceivably for certain glomerular diseases with hyposialylation.
Keywords: glomerulopathy, hyposialylation, mannosamine, myopathy, sialic acid, UDP-GlcNAc-2-epimerase/ManNAc kinase
1. Introduction
GNE myopathy, previously termed hereditary inclusion body myopathy (HIBM, IBM2; OMIM#600737), is a rare, autosomal recessive, neuromuscular disease, allelic to the Japanese disorder distal myopathy with rimmed vacuoles (DMRV), or Nonaka myopathy (OMIM#605820) [1–3]. GNE myopathy usually manifests after 20 years of age with foot drop and slowly progressive proximal and distal muscle weakness and atrophy. Histologically, it is associated with muscle fiber degeneration and formation of tubulofilamentous vacuoles [3, 4]. The disease is relentlessly progressive; patients become incapacitated and wheelchair-confined within 1 to 2 decades after onset of symptoms. There is no approved therapy [1, 2].
GNE myopathy results from predominantly missense mutations in the GNE gene, encoding the initial, bifunctional enzyme of sialic acid synthesis, UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase (GNE/MNK) [5–8]. The function and feedback regulation of GNE/MNK is depicted in Fig. 1. Sialic acids are negatively charged terminal sugar moieties on glycoproteins and glycolipids [9–11]. They act as molecular determinants of specific biological processes such as cellular adhesion, cell-cell interactions, and signal transduction [10, 12]. The exact pathophysiology of GNE myopathy remains unknown, but the dysfunction in GNE/MNK suggests involvement of impaired sialylation of (muscle) glycoproteins and glycolipids [1, 2, 13–18]. A complete Gne knock-out mouse is embryonic lethal, indicating that sialic acid is critical for viability [19].
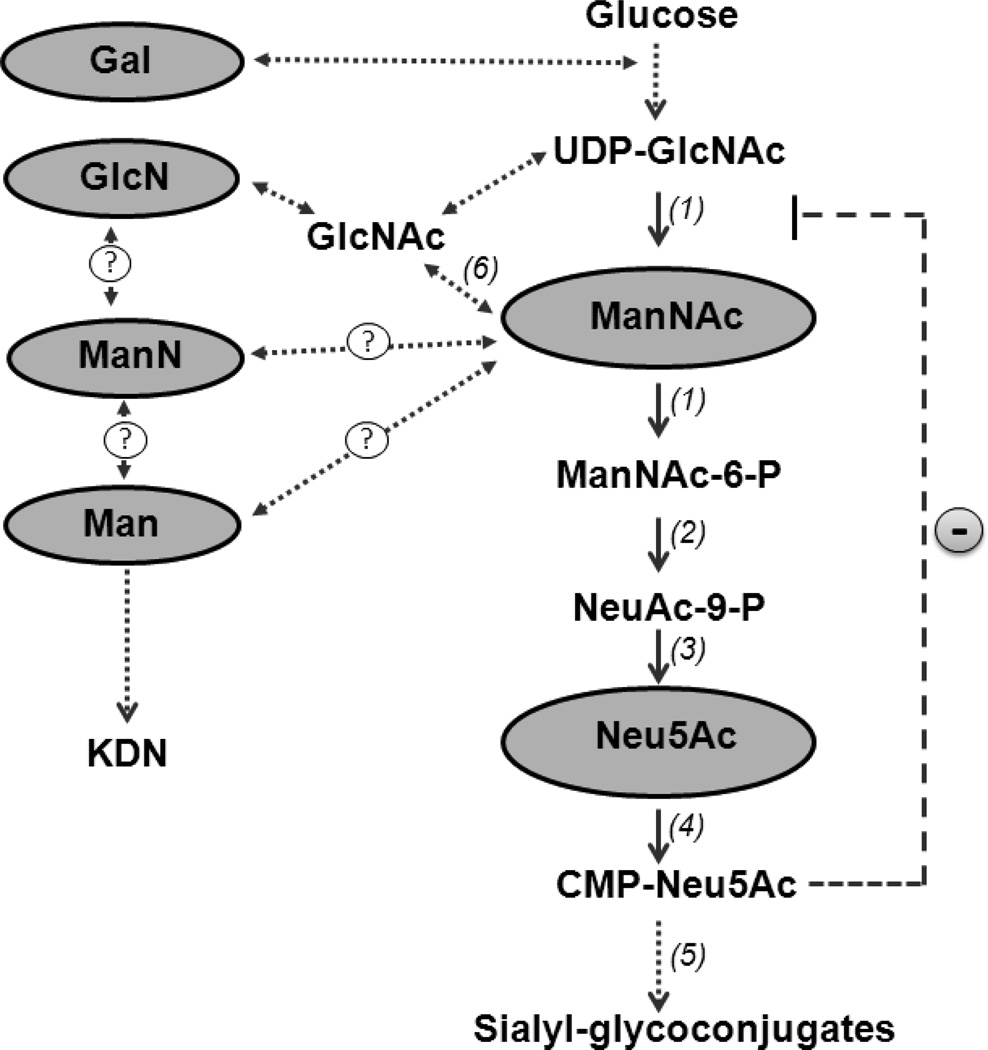
Fig. 1.
Sialic acid biosynthesis pathway. The synthesis of sialic acid is initiated in the cytosol, where glucose undergoes several modifications to eventually become sialic acid (Neu5Ac). The reactions catalyzed by the bifunctional enzyme UDP-GlcNAc 2-epimerase/ManNAc kinase (GNE/MNK) (1) are the initial and rate-limiting steps in this pathway. Subsequent steps are mediated by cytosolic NeuAc-9P synthetase (2) and NeuAc-9P phosphatase (3) to produce Neu5Ac, after which nuclear by CMP-Neu5Ac synthethase (4) produces CMP-Neu5Ac. Cytosolic CMP-Neu5Ac strongly feedback inhibits GNE-epimerase activity (−) and is utilized in the Golgi-complex by sialyltransferases (5) to produce sialyl-glycoconjugates. Monosaccharides that were orally administered to Gne p.M712T mice to test their sialylation-increasing potential are highlighted in gray. Neu5Ac and its precursor ManNAc are likely to increase sialylation by increasing the flux through the sialic acid synthesis pathway, since conversion of both these intermediates are unaffected by the feedback inhibition of the GNE-epimerase by CMP-Neu5Ac. GlcN may be converted into GlcNAc and then into ManNAc by GlcNAc 2-epimerase (6) or could increase UDP-GlcNAc pools and possibly increase flux into the sialic acid pathway. Gal, Man, or ManN may have potential, yet unknown (?), ways to enter the Neu5Ac biosynthesis pathway at one or more relevant steps, and may as such contribute to increased sialylation. Alternatively, Man or ManN may increase sialylation through production of an alternative sialic acid, KDN(2-keto-3-deoxy-D-glycero-D-galacto-nononic acid),which can be formed independent of the GNE/MNK-mediated Neu5Ac synthesis pathway.
To further study the pathology, pathogenesis, and proposed therapies of GNE myopathy, we previously created a knock-in mouse model mimicking the GNE p.M712T Persian Jewish founder mutation [20]. Unexpectedly, >90% of the homozygous mutant (GneM712T/M712T) pups died within 3 days of birth (postnatal day 3, P3) of severe glomerular disease with proteinuria, hematuria, effacement of the podocyte foot processes, and segmental splitting of the glomerular basement membrane. Biochemical analysis of mutant mouse kidneys revealed decreased UDP-GlcNAc 2-epimerase activity, deficient overall glomerular sialylation and poor sialylation of the major podocyte sialoproteins podocalyxin and nephrin, suggesting that decreased renal sialic acid production led to lethality in these mice [20, 21]. In contrast, no evidence of renal abnormalities has been reported in humans with GNE myopathy. The relative importance of sialic acid to the kidney may differ between humans and mice, and protein glycosylation patterns also vary. For example, the contingent of O-and N-linked glycosylation sites of podocalyxin differs among species [22]. The type of sialic acid present also differs among species; most mammals utilize the sialic acid N-glycolylneuraminic acid (Neu5Gc), but humans have lost the ability to synthesize Neu5Gc [23] and mainly utilize N-acetylneuraminic acid (Neu5Ac).
As a treatment for GneM712T/M712T mice, we explored dietary supplementation of the uncharged sialic acid precursor N-acetylmannosamine (ManNAc), the intermediate product of the GNE/MNK enzyme (Fig. 1). In the absence of MNK activity, ManNAc can be converted to ManNAc-6P by N-acetylglucosamine (GlcNAc) kinase, thus bypassing the kinase domain mutated in our knock-in mice [24]. Oral supplementation of ManNAc (1g/kg/day) to pregnant and nursing mice resulted in survival of 47% of their mutant offspring beyond P3. Mutant survivors displayed improved kidney histology, increased overall sialylation as well as podocalyxin and nephrin sialylation, and increased Gne/Mnk protein expression and Gne enzymatic activity [20, 21]. Mutant pups did not live long enough to develop a muscle phenotype. However, in mutant mice rescued from neonatal lethality by ManNAc administration, and receiving no further ManNAc after weaning, we detected muscle hyposialylation at age ~4–6 months, similar in appearance to the muscle tissue in human GNE myopathy [25, 26].
In the current study, we assessed whether oral administration of other monosaccharides besides ManNAc could also rescue mutant GneM712T/M712T pups from neonatal death. We tested sialic acid itself (N-acetylneuraminic acid, Neu5Ac), as well mannose (Man), mannosamine (ManN), galactose (Gal) and glucosamine (GlcN), monosaccharides with known or potential links to the sialic synthesis pathway (Fig. 1). Since GNE myopathy patients will likely require treatment in adulthood after onset of symptoms, we also evaluated whether muscle and kidney hyposialylation of mutant mice was alleviated by ManNAc or other monosaccharides administered after onset of the disease. These studies provide further preclinical data for therapeutic use of sialylation-increasing monosaccharides to rescue adult onset muscle hyposialylation in GNE myopathy patients, and possibly glomerular hyposialylation in yet to be identified human renal disorders with hyposialylation.
2. Methods
2.1. Mouse breeding, housing and genotyping
GneM712T/M712T knock-in mice were generated in the C57BL/6J background as described [20]. The mice were housed in a pathogen-free facility, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23). All mouse procedures were performed in accordance with protocol G04-3 and were approved by the Institutional Animal Care and Use Committee of the National Human Genome Research Institute. All mice were fed irradiated chow (Prolab 5P75 Isopro 3000; PMI Nutrition International) and sterile water ad libitum. Mouse genotyping was performed on tail-isolated genomic DNA by PCR amplification (using standard conditions) across the Gne p.M712T mutation, using the primer set 5'-CCACACTATGAATCCTTCCC-3' and 5'-TACATCTGAGTGCAGCTGCC-3'. PCR fragments were bi-directionally sequenced using the di-deoxy termination method (ABI BigDye Terminator v3.1) and analyzed on an ABI3130xl Genetic Analyzer (Applied Biosystems, Carlsbad, CA).
2.2. Oral monosaccharide administration
For neonatal survival and kidney tissue analysis, breeding pairs of approximately 8-week-old heterozygous Gne+/M712T mice received monosaccharides dissolved in sterile drinking water at 5 mg/ml (~1.0 g/kg/day; adult C57BL/6J mice given water ad libitum typically consume ~ 4–6 ml water each day [27]). We randomly verified and confirmed whether mice drank the approximate estimated amounts of water to which monosaccharides were added. Tested monosaccharides included N-acetyl-D-mannosamine (ManNAc; New Zealand Pharmaceuticals, Palmerston North, New Zealand), N-acetylneuraminic acid (Neu5Ac; Toronto Research Chemicals, North York, Canada), D-mannosamine hydrochloride (ManN; Toronto Research Chemicals), D-mannose (Man; Spectrum, New Brunswick, NJ), D-galactose (Gal; Sigma-Aldrich, St Louis, MO), and D-glucosamine hydrochloride (GlcN; Sigma-Aldrich). Water was changed twice per week. After giving birth, nursing females continued to receive monosaccharide-supplemented water until the pups were euthanized at postnatal day P5-10 (for genotyping and/or tissue analysis) or until weaning at P21, after which the pups were genotyped and continued to live without further monosaccharide supplementation. Untreated mice were euthanized at P1–P2 for genotyping and/or tissue analysis, or at P5 for genotyping. Heterozygous mice developed no clinical phenotype, normal glomerular podocyte foot processes were observed under electron microscopy, and no hyposialylation of podocalyxin or nephrin was observed by immunoblotting [20, 21]. Therefore, when indicated, heterozygous specimens were used as control tissues for some biochemical studies.
For monosaccharide treatment studies on adult mice, ~ 6 months old control (wild type or heterozygous, 1 or 2 mice per treatment group) and mutant mice (2 mutant mice per treatment group) were administered monosaccharides as described above in the following doses and time periods: ManNAc (1 g/kg/day and 2 g/kg/day) for 12 weeks; Neu5Ac (2 g/kg/day) for 12 weeks; or ManN (2 g/kg/day) for 6 weeks. Monosaccharide-containing water was changed two times per week. All mice were euthanized after the feeding period; in addition, age-matched unfed mutant and heterozygous mice (often littermates) were euthanized to serve as untreated controls.
2.3. Immunoblotting
Frozen mouse kidney specimens were homogenized in CelLytic buffer consisting of a mild detergent, bicine buffer, and 150 mM NaCl (Sigma-Aldrich) supplemented with protease inhibitors (Complete Mini; Roche Applied Science, Indianapolis, IN). The lysates were sonicated and cleared by centrifugation (13,200 rpm for 10 minutes), and the resulting supernatants were assayed for protein (BCA Protein Assay; Pierce Biotechnology, Rockford, IL). For the neuraminidase enzymatic treatments, protein homogenates (30µg) were incubated for 2 hours at 37°C with 50 Units of neuraminidase (P0720; New England Biolabs Ipswich, MA). This neuraminidase enzyme was cloned from Clostridium perfringens (and overexpressed in E. coli) and shows a preference for α2-3 and α2-6 sialic acid linkages over α2-8 linkages. Equal amounts of protein (30 µg for neonatal kidneys; 20 µg for adult kidneys) were electrophoresed on 4%–12% Tris-Glycine gels (Life Technologies, Grand Island, NY), and electroblotted onto 0.20 µm (neonatal kidneys) or 0.45 µm (adult kidneys) pore size Hybond ECL nitrocellulose membranes (Life Technologies). The membranes were blocked with either 10% fat-free milk (for neonatal kidney blots) or Odyssey Infrared Imaging System Blocking Buffer (for adult kidney blots; Li-Cor Biosciences, Lincoln, NE). The membranes were then incubated overnight with the primary antibodies goat-anti-mouse podocalyxin (AF1556; R&D Systems, Minneapolis, MN or guinea pig-anti-mouse nephrin (GP-N2; PROGEN Biotechnik, Heidelberg, Germany). Primary antibody incubation of neonatal kidneys was followed by horseradish peroxidase-conjugated secondary antibodies (GE Healthcare and Santa Cruz Biotechnology (Santa Cruz, CA), and detected with enhanced chemiluminescence (ECL Western blotting detection reagents; GE Healthcare) and exposure to CL-XPosure film (Pierce; Thermo Fisher Scientific, Rockford, IL). Primary antibody incubation of adult mouse kidneys was followed by IRDye-800 conjugated secondary antibodies (Li-Cor Biosciences) and visualized using the Odyssey Infrared Imaging System (Li-Cor Biosciences).
2.4. Lectin and antibody histochemistry
Mouse kidneys and gastrocnemius and/or gluteus muscles were harvested and fixed in 10% formalin for 48 hours at room temperature, followed by dehydration in 70% ethanol at 4°C for 4–10 days before paraffin embedding for sectioning (HistoServ, Germantown, MD). Tissue sections (5 µm) were deparaffinized in Hemo-De (Scientific Safety Solvents, Keller, TX) and rehydrated in a series of ethanol solutions. Antigen retrieval was performed by boiling slides in a citric acid-based solution. Slides were incubated in Tris-Buffered Saline (TBS) with 3% hydrogen peroxide and 10% methanol for 30 minutes, then blocked in Carbo-Free Blocking solution (Vector Laboratories, Burlingame, CA). Slides were then incubated overnight at 4°C with different FITC-conjugated lectins at 5 µg/mL in Carbo-Free Blocking solution. Washes were performed with 0.1% Triton-X-100 in TBS. The lectin-stained slides were incubated in 0.3% Sudan Black in 70% ethanol solution to reduce autofluorescence. Slides were mounted with Vectashield containing DAPI (Vector Laboratories). Representative muscle sections and junxtamedullary glomeruli were digitally imaged with a Zeiss LSM 510 META confocal laser-scanning microscope (Carl Zeiss, Microimaging Inc., Thornwood, NY). Images were acquired using a Plan-Apochromat 63X oil DIC objective. All images are 1D projections of confocal Z-stacks. The lectins used for this study included HPA (edible snail agglutinin from Helix pomatia) and VVA (hairy vetch agglutinin from Vicia villosa), purchased from EY Laboratories (San Mateo, CA), and SNA (elderberry bark agglutinin from Sambucus nigra) purchased from Vector Laboratories (Burlingame, CA). HPA and VVA predominantly bind GalNAc O-linked to serine or threonine residues of proteins [28–30], while SNA predominantly recognizes terminal sialic acid (Neu5Ac) in an α(2,6)-linkage with either galactose (prevalent in N-linked glycans) or with N-acetylgalactosamine (GalNAc) (found in O-linked glycans) [28, 31].
2.5. Electron microscopy
For transmission electron microscopy, mouse kidney samples were fixed overnight at 4°C in 2% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4) and washed with cacodylate buffer. The tissues were then fixed with 2% OsO4 for 2 h, washed again with 0.1 mol/L cacodylate buffer, washed with water, and placed in 1% uranyl acetate. The tissues were then serially dehydrated in ethanol and propylene oxide and embedded in EMbed 812 resin (Electron Microscopy Sciences, Hatfield, PA). Thin sections (~80 nm) were obtained using a Leica Ultracut-UCT ultramicrotome (Leica Microsystems, Deerfield, IL), placed onto 300-mesh copper grids, and stained with saturated uranyl acetate in 50% methanol followed by lead citrate. The grids were viewed under a JEM-1200EXII electron microscope (JEOL, Tokyo, Japan) at 80 kV; images were recorded on a XR611M, mid-mounted, 10.5 megapixel CCD camera (Advanced Microscopy Techniques, Danvers, MA).
2.7. Urine gel electrophoresis
Mouse urine samples were collected twice a week (early mornings), and were electrophoresed in 5 µL aliquots in Laemmli sample buffer supplemented with β-mercaptoethanol (Bio-Rad Laboratories, Hercules, CA) on 4–12% Tris-glycine polyacrylamide gradient gels (Novex; Invitrogen, Carlsbad, CA). Pure bovine serum albumin (Amersham; GE Healthcare, Piscataway, NJ) was loaded for quantification purposes. Gels were stained with Coomassie Brilliant Blue R-250 (Bio-Rad Laboratories) for 20 minutes and washed with R-250 destaining solution (Bio-Rad Laboratories) according to the manufacturer's protocols. Semi-quantitative densitometry was performed on the Coomassie-stained signals to quantify albumin concentrations using ImageJ software (version 1.44; NIH, Bethesda, MD).
3. Results
3.1. Rescue from neonatal death
Our previous investigations showed that the majority of untreated homozygous mutant pups died between birth and P3. When ManNAc (1 g/kg/day) was added to the drinking water during matings of heterozygous mice, subsequent pregnancy and nursing, ~47% of their mutant offspring survived beyond weaning (Fig. 2A) [20]. In the current study we explored whether oral feeding of other potential sialylation-increasing monosaccharides (highlighted in gray in Fig. 1) had similar effects on the survival rate of mutant pups. Neu5Ac, ManN, Man, Gal, and GlcN were added to the drinking water (1 g/kg/day) of heterozygous matings and whole litters were euthanized at P5 to assess mutant survival rate beyond P3 and to collect tissues for assessment of sialylation status. Administration of Man, Gal, or GlcN did not yield any mutant survivors at P5. Administration of Neu5Ac resulted in a similar sporadic survival rate of mutant pups (~10%) as found for untreated mice. Wild type and heterozygous pups had a normal survival rate at P5, indicating no neonatal toxicity of the tested sugars. Surprisingly, however, administration of ManN allowed ~55% of mutant pups to survive past P3, a significant increase compared to the untreated group. Kidneys were harvested at P1–P2 from whole litters of the untreated, Neu5Ac, Man, Gal and GlcN treatment groups, since the majority of mutant mice in these groups died before P3. Kidneys were harvested at P5 from the ManNAc and ManN treatment groups to investigate features of mutant survivors.
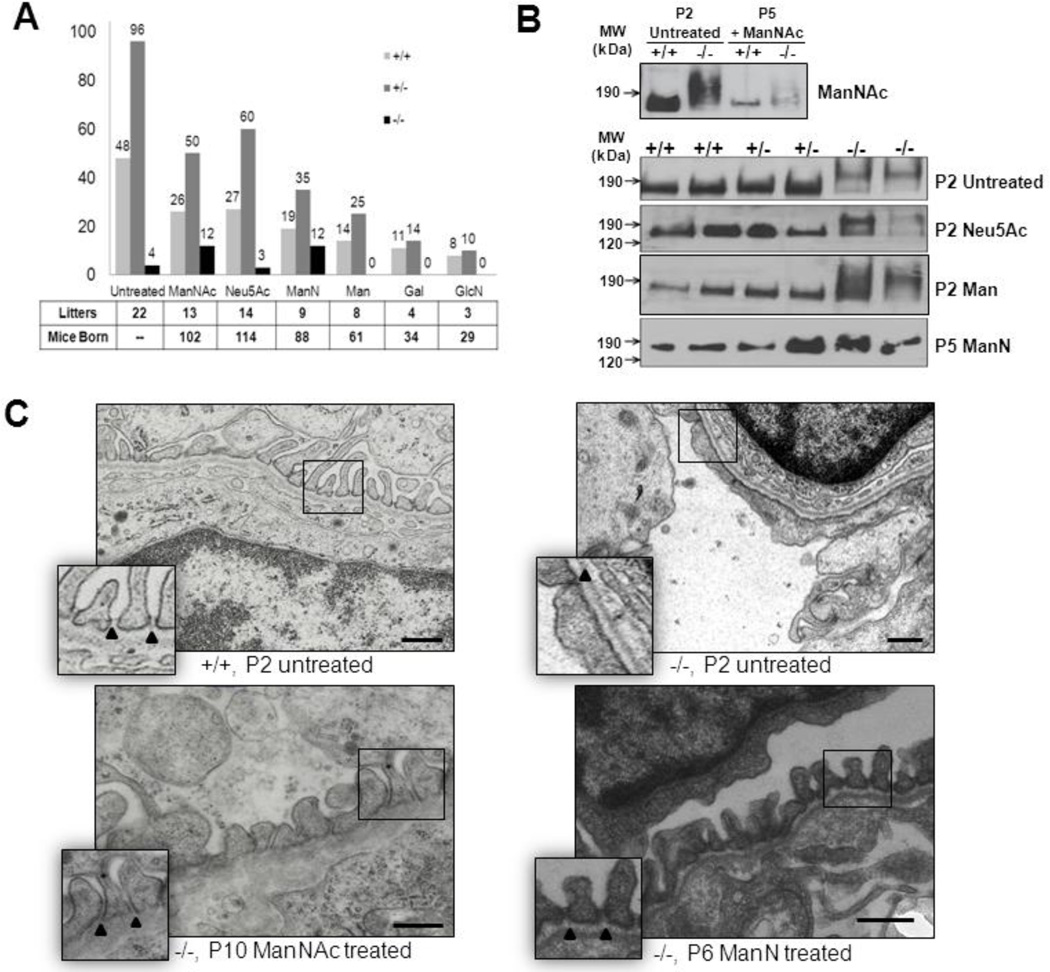
Fig. 2.
Assessment of the effects of oral monosaccharide administration on neonatal survival rate, kidney podocalyxin sialylation and renal ultrastructure in the Gne p.M712T mouse model. +/+ wild type Gne+/+; +/−, heterozygous Gne+/M712T; −/−, mutant GneM712T/M712T. Scale bars: 0.5 µm.
(A) Survival rates at P5 (past the critical age of P3) of litters born to heterozygous breeding pairs that were either untreated or treated with one of several monosaccharides (1 g/kg/day) in their drinking water. Total numbers of counted litters and mice born per treatment group are indicated. Note that the survival rate of mutant (−/−) pups significantly increased upon ManNAc (~47% survival rate) and ManN (~55% survival rate) therapy compared to the untreated group (~ 8%). Neu5Ac, Man, Gal, and GlcN therapy to pregnant and nursing mice did not improve survival rate of their mutant pups beyond P3.
(B) Immunoblots of kidney extracts (ages P2 or P5) labeled with antibodies against podocalyxin (~150 kDa). Wild-type (+/+) and heterozygote (+/−) specimens show normal migration, while slower podocalyxin migration (~190 kDa) occurs in untreated, Neu5Ac and Man treated mutant pups (P2), indicating hyposialylation. Podocalyxin migration in ManNAc and ManN treated mutant pups (P5) is within the range of wild type and heterozygous kidney extracts, indicating a rescued sialylation status.
(C) Representative electron microscopy images of juxtamedullary glomeruli in kidney sections of wild type (+/+, P2), mutant (−/−, P2), ManNAc (−/−, P10) or ManN treated (−/−, P6) pups. Wild type glomeruli contain interdigitating foot processes with open slit junctions (arrowheads), an intact glomerular basement membrane and fenestrated endothelial cells. Glomeruli of untreated mutant pups show effaced foot processes with occluding junctions (arrowhead), segmental splitting of the basement membrane and seemingly normally fenestrated endothelial cells. In ManNAc- or ManN-supplemented mutant pups, the podocyte effacement is reduced and basement membrane integrity is partially restored with mostly intact podocyte slit junctions.
Immunoblotting showed an upshift/slower migration of the band for the glomerular sialoprotein podocalyxin (resulting from the reduction in negative charge caused by hyposialylation) in untreated mutant mouse kidney extracts (Fig. 2B), as previously reported [20, 21]. We found similarly hyposialylated (upshifted) podocalyxin in mutant mouse kidney extracts of the Neu5Ac and Man (Fig. 2B), as well as the Gal and GlcN (not shown) treatment groups. We previously described that ManNAc therapy resulted in downshifting of this band (representing re-sialylation) back to the normal range in mutant kidney extracts [20, 21], as shown in Fig. 2B. A similar downshift to the normal range was seen in ManN treated mutant mouse kidney extracts (Fig. 2B), suggesting re-sialylation of podocalyxin after ManN therapy.
Ultrastructural kidney analysis showed severe defects in the glomerular filtration unit in mutant kidneys at P2 [20], including podocyte foot process effacement with tight slit junctions and segmental splitting of the glomerular basement membrane (Fig. 2C). These ultrastructural abnormalities are likely due to hyposialylation of membrane glycans, accounting for the early lethal phenotype of mutant pups. Similar to ManNAc treated pups [20], ManN treated surviving mutant pups showed significant improvement of glomerular ultrastructure, with increased podocyte foot process formation, mostly open filtration slits and improved glomerular membrane integrity (Fig. 2C). These changes were likely responsible for the survival of mutant mice beyond P3.
3.2. Rescue of adult onset kidney and muscle hyposialylation
Prophylactic administration of ManNAc before birth and during nursing allowed a large portion of mutant pups to survive beyond P3. Mutant survivors continue to live beyond weaning (P21) without further ManNAc administration, but they develop hyposialylation of muscle and kidney as well as proteinuria into adulthood. We explored the therapeutic rather than prophylactic potential of ManNAc and other monosaccharides, i.e., whether these sugars could rescue muscle and kidney hyposialylation after its onset.
Initially, ManNAc administration was tested at 1 g/k/day in the drinking water of age-matched mutant and control mice (6 months old or older). Improvement of proteinuria was followed as a noninvasive outcome parameter, since this could reflect improved renal sialylation status. However, the proteinuria did not markedly improve at this dose (Fig. 3A), therefore, the mice were euthanized and tissues collected after 12 weeks of treatment. In a following experiment, mice treated with a higher dose of ManNAc (2 g/kg/day) showed a significant decrease of proteinuria after only one week on the treatment (Fig. 3A). In contrast, administration of Neu5Ac (2 g/kg/day) or ManN (2 g/kg/day) did not decrease proteinuria during the treatment period (Fig. 3A).
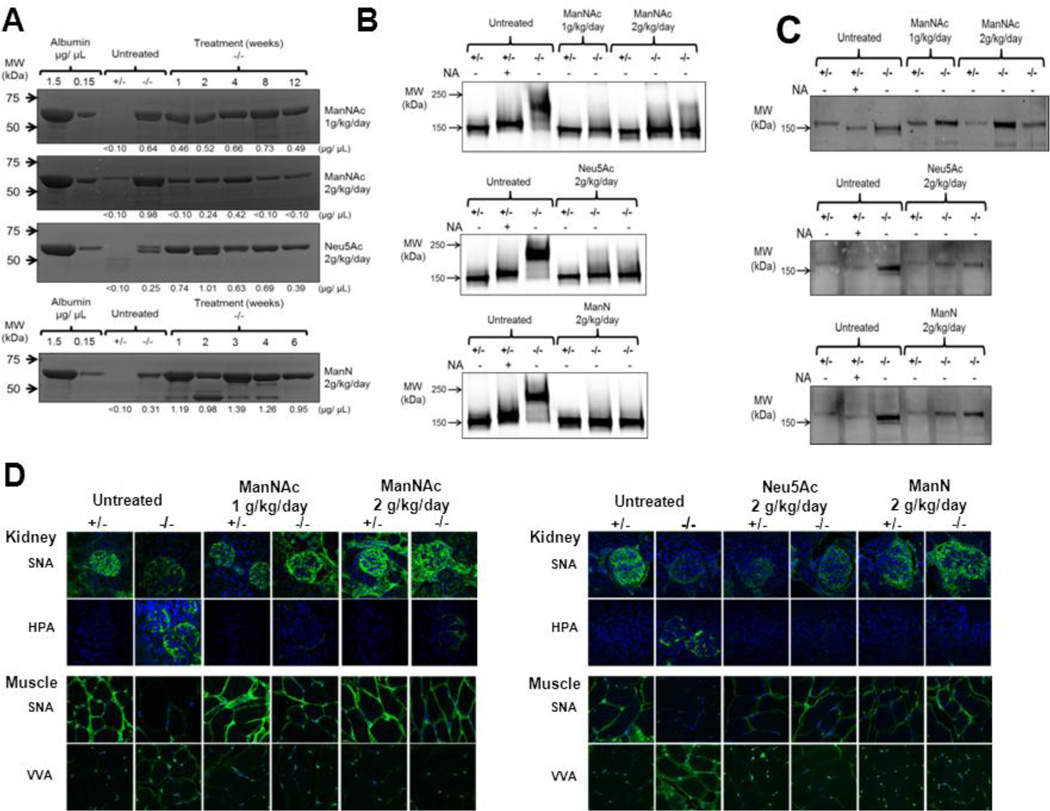
Fig. 3.
Effects of oral monosaccharide administration on adult onset proteinuria and kidney and muscle hyposialylation in the Gne p.M712T mouse model.
(A)Urinary proteinuria of mutant mice and the effects of monosaccharide therapy at different time points during treatment. Densitometry quantifications of albumin (66.7 kDa, the major protein band present) concentrations in each sample are indicated below the gel images.
(B) Immunoblots of kidney extracts with antibodies against podocalyxin (~150 kDa). The +/− specimens (treated and untreated) show normal migration. Slower migration (due to reduced negative charge) occurs in +/− specimen treated with neuraminidase (NA), which cleaves sialic acid residues, as well as in untreated −/− specimen, indicating hyposialylation. Podocalyxin migration in ManNAc, Neu5Ac and ManN treated −/− adult mice kidney extracts is in the normal range, indicating re-sialylation upon treatment.
(C) Immunoblots of kidney extracts labeled with antibodies against nephrin (~180 kDa). All (treated and untreated) +/− specimens show normal migration. Faster migration of nephrin (due to a reduced size) occurs in +/− kidney extracts treated with NA, as well as in untreated −/− extracts, indicating hyposialylation. Nephrin migration in ManNAc, Neu5Ac and ManN treated −/−adult mice kidney extracts is in the normal range, indicating re-sialylation upon treatment.
(D)Lectin histochemistry for kidney and muscle specimens. Paraffin-embedded sections were stained (green) with the lectins SNA (predominantly binding terminal α(2,6)-linked Neu5Ac on all glycans) and HPA or VVA (both predominantly binding terminal GalNAc, without Neu5Ac attached, O-linked to serine or threonine residues of glycoproteins), as well as the nuclear dye DAPI (blue). Untreated ~6 month old −/− specimen show hyposialylation, demonstrated by decreased SNA and increased HPA or VVA staining, compared to age-matched control (+/−) specimens. Oral supplementation of ManNAc (both 1 or 2 g/kg/day) or Neu5Ac (2 g/kg/day) for 12 weeks, or ManN (2 g/kg/day) for 6 weeks restored the sialylation status back to the normal range, demonstrated by increased SNA, HPA and VVA signal intensities similar to control (+/−) specimens. All confocal imaging was performed at the same microscope intensity settings per tissue and per lectin (with a 63X objective). Untreated +/− specimens were used for standard SNA settings and untreated −/− tissues for standard HPA and VVA settings. All images are 1D projections of confocal Z-stacks.
Even though proteinuria did not improve during the treatment period for some therapies, glomerular hyposialylation appeared improved to the normal range for all tested monosaccharides. In untreated mutant mouse kidney extracts, immunoblotting with antibodies against podocalyxin showed upshifted bands in neuraminidase treated wild type (which only show a slight upshift in Fig. 3B, likely due to incomplete digestion) and in untreated mutant kidney extracts (a large upshift) due to reduced charge caused by hyposialylation (Fig. 3B), and antibodies against nephrin showed downshifted bands in neuraminidase treated wild type and untreated mutant mouse kidney extracts due to reduced size caused by hyposialylation (Fig. 3C) [21]. After treatment with ManNAc, Neu5Ac, or ManN, the podocalyxin and nephrin bands recovered to the normal migration pattern. In addition, overall glomerular sialylation, visualized by lectin histochemistry, significantly improved with all three therapies. Specifically, improved kidney sialylation was demonstrated by increased SNA staining and decreased HPA staining in kidneys of treated mutant mice compared to those of untreated mutants (Fig. 3D).
Adult mutant mice (rescued from neonatal death by ManNAc supplementation to their mothers) showed adult onset hyposialylation of their muscle tissue at ~ 4–6 month of age (Fig. 3D), evidenced by decreased SNA and increased VVA lectin staining compared to that of control mice. After oral ManNAc administration (1 or 2 g/kg/day) for 12 weeks, the muscle sialylation status recovered significantly, to almost the normal range, demonstrated by increased SNA and decreased VVA staining compared to untreated specimens. Similarly, oral administration of Neu5Ac (2 g/kg/day) for 12 weeks or ManN (2 g/kg/day) for 6 weeks rescued the muscles from severe hyposialylation (Fig. 3D).
4. Discussion
GNE myopathy is an autosomal recessive inherited, adult onset neuromuscular disorder without an approved therapy. Patients have mild mutations (at least one allele contains a missense mutation) in GNE/MNK, the key enzyme of sialic acid synthesis. The exact pathology of the disorder remains puzzling, even a decade after identification of the causative gene [1, 2, 6]. The dysfunction in GNE/MNK suggests a systemic, congenital condition of impaired sialylation. Nevertheless, patients suffer from an adult onset muscular dystrophy, without inflammation, and no other tissues appear affected throughout life. Overall sialylation of GNE myopathy patients’ cells and tissues appears normal [32, 33]. So far, only specific glycoproteins and glycolipids in skeletal muscle tissues of patients were found to be affected, including alpha-dystroglycan [14], polysialylated neuronal cell adhesion molecule (PSA-NCAM) [18], neprilysin [34], O-glycans in general [16], and GM3 ganglioside [17]. The pathophysiologic consequences of these findings remain under investigation, but these findings suggest that the effects of sialic acid deficiency appear gradually due to residual GNE/MNK activity. Some glycoconjugates (such as N-linked glycans), might be more readily sialylated than others (such as O-linked or polysiaylated glycans). When a shortage of sialic acid occurs (apparently later in life), specific (muscle) glycans may be inadequately glycosylated, contributing to the pathology of GNE myopathy.
To further study the pathology of GNE myopathy and to test potential therapies, mouse models were created. A complete Gne knockout mouse was embryonic lethal [19]. A Gne knock-in mouse model with the p.M712T mutation, common among Persian-Jews, was studied by our group [20]. Mutant mice unexpectedly died within 72 hours of birth of severe glomerular disease due to hyposialylation. Oral administration of the uncharged, naturally occurring sialic acid precursor ManNAc to the pregnant and nursing mothers resulted in significant increase of survival of mutant GneM712T/M712T pups beyond 72 hours. Mutant survivors develop adult onset (4–6 months of age) muscle hyposialylation similar to GNE myopathy patients. These findings established the Gne p.M712T knock-in mice as the first genetic model not only of GNE myopathy, but also of podocyte injury due to hyposialylation [20, 21, 35]. A second mouse model of GNE myopathy, created by Malicdan et al. [36], was a transgenic mouse that expressed the human GNE cDNA with the p.D176V mutation, common among Japanese patients, on a mouse background with a disrupted mouse Gne gene, i.e., Gne(−/−)hGNED176V-Tg. The mutant offspring appeared normal at birth but had decreased levels of sialic acid in different organs. These mice developed adult onset GNE myopathy-like muscle pathology, starting with subtle changes at ~4 months of age and severe changes at ~10 months. Oral prophylactic treatment (starting at ~3 months) with ManNAc, Neu5Ac or the sialic acid conjugate sialyllactose (containing ~45% of sialic acid) prevented mutant mice from developing a severe muscle phenotype [37]. Further research is required to elucidate phenotypic differences between the transgenic Gne(−/−) hGNED176V-Tg and the knock-in p.M712T Gne models. In any event, these mouse findings strongly supported consideration of oral ManNAc (and possibly other sialylation-increasing compounds) as a therapy not only for GNE myopathy, but also for renal disorders with hyposialylation. The preclinical phase for the development of ManNAc for treatment of GNE myopathy patients was recently completed and a Phase I trial is currently ongoing (www.clinicaltrials.govNCT01634750).
The Gne p.M712T mouse emerged as an ideal model to assess efficacy of sialylation-increasing therapies by evaluating survival rate of mutant pups beyond 72 hours of life. Therefore, we used this model to assess whether supplementation of other monosaccharides with a possible sialylation-increasing capacity (Fig. 1) to pregnant and nursing mice could rescue their mutant offspring from neonatal death. Since the preclinical and clinical phases for development of ManNAc for therapy of patients with GNE myopathy is time consuming, possible other sialylation-increasing monosaccharides could be further explored/repurposed in a shorter timeframe for use in patients with GNE myopathy. Therefore, we used our mouse model to test monosaccharides that are already being administered to humans in other studies, including Man (indicated as therapy for carbohydrate-deficient glycoprotein syndrome (CDGS) type Ib [38, 39]), GlcN (a dietary supplement, indicated in relieving symptoms of osteoarthritis [40, 41]), and Gal (explored as a therapy for focal segmental glomerulosclerosis (www.clinicaltrials.gov:NCT01113385)[42]), and oral Neu5Ac (explored as therapy for GNE myopathy; www.clinicaltrials.gov:NCT01517880). In addition, we tested ManN (not pproved for human use), which may be converted to ManNAc by a direct, yet sparsely described pathway [43, 44]. Surprisingly, oral supplementation of ManN (which is not approved or currently tested for human use) to our mice resulted in a significant survival rate of mutant pups (Fig 2A) as well as re-sialylation of podocalyxin (Fig. 2B) and improved glomerular ultrastructure (Fig. 2C) in surviving pups, similar to ManNAc therapy. Even though ManN therapy appeared to reverse glomerular hyposialylation in adult mutant mice in a similar way as ManNAc therapy (Fig. 4B–D the proteinuria did not revert as dramatically upon administration of ManN compared to ManNAc (Fig. 4A). This discrepancy in the efficacy and extent of correction of the phenotypic abnormalities in the kidney of treated mice should be investigated more accurately in future studies.
Further studies are needed to determine the exact mechanism of ManN-induced sialylation. It is likely, but unproven, that ManN is directly converted into ManNAc, resulting in increased sialylation [43, 44]. It is also possible that ManN is converted through an alternative pathway (independent of the GNE/MNK-mediated Neu5Ac synthesis pathway) into Man to form the poorly studied sialic acid KDN (2-keto-3-deoxy-D-glycero-D-galacto-nononic acid) for subsequent incorporation into glycans (Fig. 1) [45]. However, the fact that Man administration did not increase neonatal survival rate of mutant GneM712T/M712T pups may argue against the formation and incorporation of KDN into glycoconjugates to rescue hyposialylation. Apart from ManN, none of the other tested monosaccharides significantly increased neonatal survival rate of mutant GneM712T/M712T pups and did not increase sialylation of podocalyxin in their kidney extracts (Fig. 2 A,B). We postulate that the negative charge of Neu5Ac may prevent it from efficiently entering cells and/or crossing the placental barrier. In addition, the neutral Man, GlcN and Gal molecules may not be able to efficiently cross membranes (the placental barrier or cell membranes) or may not have a direct link to increasing sialylation. Our mouse results are similar to those of a previous study with Lec3 Chinese hamster ovary cells harboring a G136E Gne mutation. In that study, ManNAc or ManN supplementation increased sialylation of polysialic acid (PSA) on neural crest adhesion molecules (NCAM), and supplementation of GlcN or Man did not increase sialylation [44].
To date, all preclinically tested oral therapies for GNE myopathy have been prophylactic, with treatment starting before the onset of symptoms [20, 37]. However, patients with GNE myopathy will likely be diagnosed and start treatment after onset of symptoms and/or tissue hyposialylation. Therefore, we explored whether oral ManNAc, Neu5Ac or ManN therapy in adult mutant mice could reverse the sialylation status of muscle and kidney tissue after onset of hyposialylation. ManNAc (1 and 2 g/kg/day) and Neu5Ac (2 g/kg/day) were administered in drinking water to mutant and control mice for 12 weeks, similar to the projected treatment period for an anticipated clinical trial of oral ManNAc in GNE myopathy patients. ManN was administered (2 g/kg/day) for 6 weeks. Even though urinary proteinuria/albuminuria decreased significantly only in mutant mice treated with 2 g/kg/day ManNAc (Fig. 3A), the sialylation status of kidney and muscle tissues recovered to the normal range in all treatment groups, as evidenced by podocalyxin and nephrin immunoblotting and lectin histochemistry (Fig. 3B–D). The persistent proteinuria in the Neu5Ac and ManN treated groups needs further research. It is possible that even though the renal sialylation status is recovered upon Neu5Ac or ManN therapy, the podocyte effacement causing proteinuria remains.
In sum, these preclinical findings combined with those of previous reports [20, 21, 37] strongly support the evaluation of oral ManNAc, Neu5Ac and ManN as therapy for GNE myopathy and conceivably for unexplained glomerular diseases with hyposialylation. Even though significant preclinical progress toward treatment of GNE myopathy through dietary supplementation of monosaccharides was made during the last 5 years, none of these compounds is approved for human use. The development of Neu5Ac therapy is progressing, with two phase I trials completed (http://clinicaltrials.gov:NCT01236898andNCT01359319) and a Phase II trial initiated (NCT01517880). A Phase I study for ManNAc in patients with GNE myopathy (NCT01634750) is awaiting approval. Apart from manipulating products and/or substrates in the sialic acid pathway, other therapies for GNE myopathy could lie in gene therapy [46] or (stem) cell transplantation, in particular directed to the muscle [47, 48]. A single patient with severe GNE myopathy has been treated by gene therapy, by intramuscular injections and by intravenous delivery of a normal GNE cDNA embedded in liposomes (GNE-Lipoplex) [25, 26]. The results were encouraging but inconclusive and more patients with different severities of muscle symptoms need to be assessed. With GNE myopathy research and treatment trials quickly evolving, elucidating the exact pathophysiology and effective therapeutic interventions of this devastating disorder will be required.
Highlights.
GNE myopathy is associated with impaired glycan sialylation
Sialylation-increasing monosaccharides were tested in a GNE myopathy mouse model
Oral ManNAc, Neu5Ac and ManN improved muscle and renal hyposialylation
Mouse tissue hyposialylation could be reversed after its onset
Oral monosaccharide therapy is promising for GNE myopathy patients
Acknowledgments
We thank Shelley Hoogstraten-Miller and Theresa Calhoun for their skilled assistance with mouse maintenance. We greatly appreciate the expert laboratory work of David Kurland, Maggie Lin, Mark Ziats, Katherine Patzel, Lisa Vincent, and Heidi Dorward. We thank the HIBM Research Group (Encino, CA, USA) for providing the Gne p.M712T knock-in mouse model. This work was performed in partial fulfillment of the requirements for a Ph.D. degree of T.Y. in the Sackler Faculty of Medicine of Tel Aviv University. This study was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
W.A.G. and M.H. are co-inventors on patent PCT/US2008/006895 “N-acetyl mannosamine as a therapeutc agent.”
References
- 1.Huizing M, Krasnewich DM. Hereditary inclusion body myopathy: a decade of progress. Biochim. Biophys. Acta. 2009;1792:881–887. doi: 10.1016/j.bbadis.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argov Z, Mitrani-Rosenbaum S. The hereditary inclusion body myopathy enigma and its future therapy. Neurotherapeutics. 2008;5:633–637. doi: 10.1016/j.nurt.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malicdan MC, Noguchi S, Hayashi YK, Nishino I. Muscle weakness correlates with muscle atrophy and precedes the development of inclusion body or rimmed vacuoles in the mouse model of DMRV/hIBM. Physiol Genomics. 2008;35:106–115. doi: 10.1152/physiolgenomics.90219.2008. [DOI] [PubMed] [Google Scholar]
- 4.Askanas V, Engel WK. Sporadic inclusion-body myositis and hereditary inclusion-body myopathies: current concepts of diagnosis and pathogenesis. Curr Opin Rheumatol. 1998;10:530–542. doi: 10.1097/00002281-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Hinderlich S, Stasche R, Zeitler R, Reutter W. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. Purification and characterization of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. J Biol Chem. 1997;272:24313–24318. doi: 10.1074/jbc.272.39.24313. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, Barash M, Shemesh M, Sadeh M, Grabov-Nardini G, Shmilevich I, Friedmann A, Karpati G, Bradley WG, Baumbach L, Lancet D, Asher EB, Beckmann JS, Argov Z, Mitrani-Rosenbaum S. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29:83–87. doi: 10.1038/ng718. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg I, Grabov-Nardini G, Hochner H, Korner M, Sadeh M, Bertorini T, Bushby K, Castellan C, Felice K, Mendell J, Merlini L, Shilling C, Wirguin I, Argov Z, Mitrani-Rosenbaum S. Mutations spectrum of GNE in hereditary inclusion body myopathy sparing the quadriceps. Hum Mutat. 2003;21:99. doi: 10.1002/humu.9100. [DOI] [PubMed] [Google Scholar]
- 8.Nishino I, Noguchi S, Murayama K, Driss A, Sugie K, Oya Y, Nagata T, Chida K, Takahashi T, Takusa Y, Ohi T, Nishimiya J, Sunohara N, Ciafaloni E, Kawai M, Aoki M, Nonaka I. Distal myopathy with rimmed vacuoles is allelic to hereditary inclusion body myopathy. Neurology. 2002;59:1689–1693. doi: 10.1212/01.wnl.0000041631.28557.c6. [DOI] [PubMed] [Google Scholar]
- 9.Varki A. Sialic acids as ligands in recognition phenomena. Faseb J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 10.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009 doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keppler OT, Hinderlich S, Langner J, Schwartz-Albiez R, Reutter W, Pawlita M. UDP-GlcNAc 2-epimerase: a regulator of cell surface sialylation. Science. 1999;284:1372–1376. doi: 10.1126/science.284.5418.1372. [DOI] [PubMed] [Google Scholar]
- 12.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14:351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparks SE, Ciccone C, Lalor M, Orvisky E, Klootwijk R, Savelkoul PJ, Dalakas MC, Krasnewich DM, Gahl WA, Huizing M. Use of a cell-free system to determine UDP-N-acetylglucosamine 2-epimerase and N-acetylmannosamine kinase activities in human hereditary inclusion body myopathy. Glycobiology. 2005;15:1102–1110. doi: 10.1093/glycob/cwi100. [DOI] [PubMed] [Google Scholar]
- 14.Huizing M, Rakocevic G, Sparks SE, Mamali I, Shatunov A, Goldfarb L, Krasnewich D, Gahl WA, Dalakas MC. Hypoglycosylation of alpha-dystroglycan in patients with hereditary IBM due to GNE mutations. Mol Genet Metab. 2004;81:196–202. doi: 10.1016/j.ymgme.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi S, Keira Y, Murayama K, Ogawa M, Fujita M, Kawahara G, Oya Y, Imazawa M, Goto Y, Hayashi YK, Nonaka I, Nishino I. Reduction of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase activity and sialylation in distal myopathy with rimmed vacuoles. J Biol Chem. 2004;279:11402–11407. doi: 10.1074/jbc.M313171200. [DOI] [PubMed] [Google Scholar]
- 16.Tajima Y, Uyama E, Go S, Sato C, Tao N, Kotani M, Hino H, Suzuki A, Sanai Y, Kitajima K, Sakuraba H. Distal myopathy with rimmed vacuoles: impaired O-glycan formation in muscular glycoproteins. Am J Pathol. 2005;166:1121–1130. doi: 10.1016/S0002-9440(10)62332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paccalet T, Coulombe Z, Tremblay JP. Ganglioside GM3 levels are altered in a mouse model of HIBM: GM3 as a cellular marker of the disease. PLoS One. 2010;5:e10055. doi: 10.1371/journal.pone.0010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricci E, Broccolini A, Gidaro T, Morosetti R, Gliubizzi C, Frusciante R, Di Lella GM, Tonali PA, Mirabella M. NCAM is hyposialylated in hereditary inclusion body myopathy due to GNE mutations. Neurology. 2006;66:755–758. doi: 10.1212/01.wnl.0000200956.76449.3f. [DOI] [PubMed] [Google Scholar]
- 19.Schwarzkopf M, Knobeloch KP, Rohde E, Hinderlich S, Wiechens N, Lucka L, Horak I, Reutter W, Horstkorte R. Sialylation is essential for early development in mice. Proc Natl Acad Sci U S A. 2002;99:5267–5270. doi: 10.1073/pnas.072066199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galeano B, Klootwijk R, Manoli I, Sun M, Ciccone C, Darvish D, Starost MF, Zerfas PM, Hoffmann VJ, Hoogstraten-Miller S, Krasnewich DM, Gahl WA, Huizing M. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest. 2007;117:1585–1594. doi: 10.1172/JCI30954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakani S, Yardeni T, Poling J, Ciccone C, Niethamer T, Klootwijk ED, Manoli I, Darvish D, Hoogstraten-Miller S, Zerfas P, Tian E, Ten Hagen KG, Kopp JB, Gahl WA, Huizing M. The Gne M712T mouse as a model for human glomerulopathy. Am J Pathol. 2012;180:1431–1440. doi: 10.1016/j.ajpath.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kershaw DB, Beck SG, Wharram BL, Wiggins JE, Goyal M, Thomas PE, Wiggins RC. Molecular cloning and characterization of human podocalyxin-like protein. Orthologous relationship to rabbit PCLP1 and rat podocalyxin. J Biol Chem. 1997;272:15708–15714. doi: 10.1074/jbc.272.25.15708. [DOI] [PubMed] [Google Scholar]
- 23.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinderlich S, Berger M, Keppler OT, Pawlita M, Reutter W. Biosynthesis of N-acetylneuraminic acid in cells lacking UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. Biol Chem. 2001;382:291–297. doi: 10.1515/BC.2001.036. [DOI] [PubMed] [Google Scholar]
- 25.Nemunaitis G, Maples PB, Jay C, Gahl WA, Huizing M, Poling J, Tong AW, Phadke AP, Pappen BO, Bedell C, Templeton NS, Kuhn J, Senzer N, Nemunaitis J. Hereditary inclusion body myopathy: single patient response to GNE gene Lipoplex therapy. J Gene Med. 2010;12:403–412. doi: 10.1002/jgm.1450. [DOI] [PubMed] [Google Scholar]
- 26.Nemunaitis G, Jay CM, Maples PB, Gahl WA, Huizing M, Yardeni T, Tong AW, Phadke AP, Pappen BO, Bedell C, Allen H, Hernandez C, Templeton NS, Kuhn J, Senzer N, Nemunaitis J. Hereditary inclusion body myopathy: single patient response to intravenous dosing of GNE gene lipoplex. Hum Gene Ther. 2011;22:1331–1341. doi: 10.1089/hum.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iskratsch T, Braun A, Paschinger K, Wilson IB. Specificity analysis of lectins and antibodies using remodeled glycoproteins. Anal Biochem. 2009;386:133–146. doi: 10.1016/j.ab.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez JF, Lescar J, Chazalet V, Audfray A, Gagnon J, Alvarez R, Breton C, Imberty A, Mitchell EP. Biochemical and structural analysis of Helix pomatia agglutinin. A hexameric lectin with a novel fold. J Biol Chem. 2006;281:20171–20180. doi: 10.1074/jbc.M603452200. [DOI] [PubMed] [Google Scholar]
- 30.Puri KD, Gopalakrishnan B, Surolia A. Carbohydrate binding specificity of the Tn-antigen binding lectin from Vicia villosa seeds (VVLB4) FEBS Lett. 1992;312:208–212. doi: 10.1016/0014-5793(92)80937-c. [DOI] [PubMed] [Google Scholar]
- 31.Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2–6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 32.Salama I, Hinderlich S, Shlomai Z, Eisenberg I, Krause S, Yarema K, Argov Z, Lochmuller H, Reutter W, Dabby R, Sadeh M, Ben-Bassat H, Mitrani-Rosenbaum S. No overall hyposialylation in hereditary inclusion body myopathy myoblasts carrying the homozygous M712T GNE mutation. Biochem Biophys Res Commun. 2005;328:221–226. doi: 10.1016/j.bbrc.2004.12.157. [DOI] [PubMed] [Google Scholar]
- 33.Savelkoul PJ, Manoli I, Sparks SE, Ciccone C, Gahl WA, Krasnewich DM, Huizing M. Normal sialylation of serum N-linked and O-GalNAc-linked glycans in hereditary inclusion-body myopathy. Mol Genet Metab. 2006;88:389–390. doi: 10.1016/j.ymgme.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Broccolini A, Gidaro T, De Cristofaro R, Morosetti R, Gliubizzi C, Ricci E, Tonali PA, Mirabella M. Hyposialylation of neprilysin possibly affects its expression and enzymatic activity in hereditary inclusion-body myopathy muscle. J Neurochem. 2008;105:971–981. doi: 10.1111/j.1471-4159.2007.05208.x. [DOI] [PubMed] [Google Scholar]
- 35.Quaggin SE. Sizing up sialic acid in glomerular disease. J Clin Invest. 2007;117:1480–1483. doi: 10.1172/JCI32482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malicdan MC, Noguchi S, Nonaka I, Hayashi YK, Nishino I. A Gne knockout mouse expressing human GNE D176V mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Human molecular genetics. 2007;16:2669–2682. doi: 10.1093/hmg/ddm220. [DOI] [PubMed] [Google Scholar]
- 37.Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, Nishino I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med. 2009;15:690–695. doi: 10.1038/nm.1956. [DOI] [PubMed] [Google Scholar]
- 38.Niehues R, Hasilik M, Alton G, Korner C, Schiebe-Sukumar M, Koch HG, Zimmer KP, Wu R, Harms E, Reiter K, von Figura K, Freeze HH, Harms HK, Marquardt T. Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. The Journal of clinical investigation. 1998;101:1414–1420. doi: 10.1172/JCI2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harms HK, Zimmer KP, Kurnik K, Bertele-Harms RM, Weidinger S, Reiter K. Oral mannose therapy persistently corrects the severe clinical symptoms and biochemical abnormalities of phosphomannose isomerase deficiency. Acta Paediatr. 2002;91:1065–1072. doi: 10.1080/080352502760311566. [DOI] [PubMed] [Google Scholar]
- 40.Miller KL, Clegg DO. Glucosamine and chondroitin sulfate. Rheum Dis Clin North Am. 2011;37:103–118. doi: 10.1016/j.rdc.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Altman RD. Glucosamine therapy for knee osteoarthritis: pharmacokinetic considerations. Expert Rev Clin Pharmacol. 2009;2:359–371. doi: 10.1586/ecp.09.17. [DOI] [PubMed] [Google Scholar]
- 42.De Smet E, Rioux JP, Ammann H, Deziel C, Querin S. FSGS permeability factor-associated nephrotic syndrome: remission after oral galactose therapy, Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24:2938–2940. doi: 10.1093/ndt/gfp278. [DOI] [PubMed] [Google Scholar]
- 43.Raisys VA, Winzler RJ. Metabolism of exogenous D-mannosamine. The Journal of biological chemistry. 1970;245:3203–3208. [PubMed] [Google Scholar]
- 44.Hong Y, Stanley P. Lec3 Chinese hamster ovary mutants lack UDP-N-acetylglucosamine 2-epimerase activity because of mutations in the epimerase domain of the Gne gene. The Journal of biological chemistry. 2003;278:53045–53054. doi: 10.1074/jbc.M309967200. [DOI] [PubMed] [Google Scholar]
- 45.Inoue S, Kitajima K. KDN (deaminated neuraminic acid): dreamful past and exciting future of the newest member of the sialic acid family. Glycoconj J. 2006;23:277–290. doi: 10.1007/s10719-006-6484-y. [DOI] [PubMed] [Google Scholar]
- 46.Miyagoe-Suzuki Y, Takeda S. Gene therapy for muscle disease. Exp Cell Res. 2010;316:3087–3092. doi: 10.1016/j.yexcr.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Sohn RL, Gussoni E. Stem cell therapy for muscular dystrophy. Expert Opin Biol Ther. 2004;4:1–9. doi: 10.1517/14712598.4.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Skuk D, Tremblay JP. Intramuscular cell transplantation as a potential treatment of myopathies: clinical and preclinical relevant data. Expert Opin Biol Ther. 2011;11:359–374. doi: 10.1517/14712598.2011.548800. [DOI] [PubMed] [Google Scholar]