Abstract
Maternal phenylketonuria (MPKU) is a syndrome including cardiovascular malformations (CVMs), microcephaly, intellectual impairment, and small for gestational age, caused by in-utero exposure to elevated serum phenylalanine (Phe) due to PKU in the mother. It is becoming a public health concern as more women with PKU reach child bearing age. Although a mouse model of PKU, BTBR Pahenu2, has been available for 20 years, it has not been well utilized for studying MPKU. We used this model to delineate critical parameters in Phe cardiovascular teratogenicity and study the effect of genetic background. Dosing and timing experiments were performed with the BTBR Pahenu2 mouse. A dose response curve was noted, with CVM rates at maternal serum Phe levels <360 μM (control), 360 – 600 μM (low), 600 – 900 μM (mid), and >900μM (high) of 11.86%, 16.67%, 30.86%, and 46.67% respectively. A variety of CVMs were noted on the BTBR background, including double outlet right ventricle (DORV), aortic arch artery (AAA)abnormalities, and ventricular septal defects (VSDs). Timed exposure experiments identified a teratogenic window from embryonic day 8.5-13.5, with higher rates of conotruncal and valve defects occurring in early exposure time and persistent truncus arteriosus (PTA) and aortic arch branching abnormalities occurring with late exposure. Compared to the BTBR strain, N10+ Pahenu2 congenics on the C3H/HeJ background had higher rates of CVMs in general and propensity to left ventricular outflow tract (LVOT) malformations, while the C57B/L6 background had similar CVM rates but predominately AAA abnormalities. We have delineated key parameters of Phe cardiovascular teratogenicity, demonstrated the utility of this MPKU model on different mouse strains, and shown how genetic background profoundly affects the phenotype.
1. Introduction
Congenital cardiovascular malformations (CVMs) are common birth defects occurring at a rate of 5-8/1000 live births and accounting for ¼ of all birth defects [1, 2]. Despite improvements in medical therapy and surgical palliation, CVMs cause substantial morbidity and mortality, particularly in infancy. Both genetic and environmental factors are known to cause CVMs [3, 4], with genetic background playing an important role in the frequency and manifestation of CVMs. Multiple epidemiology studies of heart defects have noted ethnic disparities in the rates of different CVMs, [1, 5, 6] with similar findings identified within specific genetic syndromes. Studies of individuals with Down syndrome (trisomy 21) demonstrated that atrioventricular septal defects (ASDs) among Blacks were twice the rate of Whites, while Hispanics had half the rate of Whites [7]. In addition to trisomy 21, specific known causes of CVMs include other chromosomal defects (Turner syndrome, del 22q11), single gene disorders (GATA4, NKX2.5, NOTCH1), prenatal ethanol exposure, maternal diabetes, and maternal phenylketonuria (MPKU) [3, 4].
Phenylketonuria (PKU – MIM 261600) is an inborn error of metabolism that occurs in 1 in 10,000 – 15,000 live births, due to mutations in the Phenylalanine hydroxylase (PAH) gene that encodes the liver enzyme (PAH; EC 1.14.16.1) required to catabolize the amino acid Phenylalanine (Phe) to Tyrosine. Individuals with PKU exposed to dietary Phe lack the ability to control serum Phe levels. Early detection and the development of strict dietary restriction have made it possible for PKU patients to achieve nearly normal cognitive development and live a relatively normal life. PKU patients were previously treated only until 12 years of age, but with increasing evidence of harm from discontinuing treatment [8-11], recent consensus recommends Phe restriction for life [12]. Despite the recent addition of new therapies (sapropterin, large neutral amino acids (LNAA)) and promise of enzyme replacement therapy, the backbone of treatment remains dietary restriction, which is difficult to maintain with waning compliance over time [11].
Pregnant women with PKU (Maternal PKU) who do not remain on a Phe restricted diet during pregnancy place the developing fetus at high risk. The high maternal serum Phe levels are extremely teratogenic to the fetus, with up to 95% of exposed offspring exhibiting growth retardation, microcephaly, dysmorphic features, intellectual disability, and CVMs [13]. MPKU-associated CVMs are documented to occur in 7-15% of cases with the most common defects being coarctation of the aorta (COA), tetralogy of Fallot (TOF), ventricular septal defects (VSD), and hypoplastic left heart syndrome (HLHS) [14, 15]. The risk of defects is positively correlated with the maternal serum Phe level. Eighty percent of CVMs reported in the International Maternal PKU Collaborative Study resulted when maternal serum Phe levels were >900 μmol/L during the first 8 weeks of gestation. Strict dietary Phe control maintaining serum levels between 120-360 μmol/L during this time can reduce the incidence of CVMs to that of the general population (1%). As a result, any woman with PKU in child-bearing age is now recommended to restrict dietary Phe to maintain a serum Phe level between 120-360 μm/L [15]. However, the success rate of pregnant women achieving this goal by 8 weeks is estimated to be only 50%. MPKU is now becoming a significant public health problem threatening to undermine the success of newborn screening for PKU, as the number of babies born to mothers with PKU is now estimated to equal the number of babies born affected with PKU [16].
A mouse model mimicking classic PKU, BTBR Pahenu2, was developed in the 1990's. The mutation is a g.835G>T, causing a missense change (p.Phe263Ser) in the active site of the enzyme, resulting in essentially no enzyme activity [17]. Studies of MPKU in this strain revealed fetuses of homozygous mutant BTBR Pahenu2 dams exposed to high maternal serum phenylalanine are affected with a wide array of cardiac and vascular defects in up to 46% of cases [18]. CVMs in exposed pups include abnormal aortic arch patterning, interrupted aortic arch (IAA), ventricular septal defects (VSD), pulmonic valve stenosis (PVS), double outlet right ventricle (DORV), transposition of the great arteries (TGA), and persistent truncus arteriosus (PTA) [18]. Compared to human, there appeared to be more defects involving the great arteries. Detailed morphologic and molecular studies have not been previously performed in this strain. This model of MPKU has been underutilized, with only two publications to date. Additionally, the cardiovascular malformations have not been well characterized, and the specifics of teratogenicity and pathogenesis of CVMs have not been investigated [18, 19].
There are numerous strains of the laboratory mouse (Mus musculus) which have been classified into different phylogenetic groups of inbred mice [20]. The C57B/L6 strain, belonging to the C57/58 group, has been extensively studied and phenotypically characterized, and is a common strain employed in constructing genetic knock-outs. The C3H/HeJ mouse of the Bagg albino group has a higher propensity for spontaneous CVMs [21-23]. The exact origin of the BTBR T+ tf mouse strain is uncertain, but recent SNP genotyping studies suggest it is derived from the 129 group of Castle's mice [24], a group separate from the above two strains.
Similar to human, genetic background plays an important role in the expression and penetrance of congenital CVMs in numerous genetic mouse models. The jumonji gene knock-out shows no cardiac phenotype on the C57BL/6J background, a low frequency of CVMs on the BALB/cA and 129/Ola strain, and nearly 100% frequency when on the C3H/He background [22]. Similarly, a hypomorph epithelial growth factor receptor [Egfr] mutant leads to aortic valve defects on the C57BL/6 background, but not on the 129S1/SvImJ strain [25]. Background strain differences on the presence of pharyngeal arch artery anomalies have also been observed in the TS16 mouse, which is a model of human trisomy 21 [23]. Finally, in the largest genetic background experiment in mouse to date, modifier genes affected the frequency and type of heart defects seen due to Nkx2-5 gene knock-out [26].
Given the increasing importance of MPKU and the difficulty with current therapies, it is vital that we better understand the precise pathogenesis of Phe teratogenicity, so that novel therapeutics may be developed. Based upon studies conducted in humans we hypothesized genetic background would affect CVM phenotype, CVMs would occur in a dose dependent manner and exposure of high Phe during isolated times in heart development would cause specific types of CVMs, allowing us to determine the probable developmental mechanism perturbed.
2. Materials and Methods
2.1 Mouse Colony Maintenance
All breeding procedures, animal care and experimental protocols were approved by the Institutional Animal Care and Use Committee at The Research Institute at Nationwide Children's Hospital. Carbon dioxide euthanasia was used to sacrifice animals.
BTBR Pahenu2 mice were obtained from Jackson Laboratories, Bar Harbor, Maine (stock #002232) and have been previously described [27]. The breeding colony was maintained by crossing heterozygous females to homozygous mutant males. The resulting homozygous mutant females were used for experiments detailed below. Mice homozygous for the Pahenu2 were fed a Phe deficient mouse chow from Harlan Teklad (TD97152) and supplementation of 0.7g/L Phe in the water to maintain serum Phe levels between 120-360 μM. Every 10th generation, animals were backcrossed to the BTBR T+ tf/J (stock# 002282) to reduce genetic variation specific to our colony.
2.2 Establishment of Congenic Lines
Wild-type breeder pairs of C57B/L6J (stock#000664) and C3H/HeJ (stock#000659) were obtained from Jackson Laboratories. Heterozygous BTBR Pahenu2 animals were backcrossed to animals from each of the recipient strains. The offspring of each generation were genotyped and those heterozygous for the Pah mutation were crossed back to inbred mice of each recipient strain. Alternate sexes of heterozygotes were used each generation. Animals were backcrossed until the N10+ generation was obtained. The N10+ homozygous females were used for the experiments detailed below. Every 10 generations of animals were backcrossed to the Jackson Laboratory stock strains to reduce genetic variation specific to our colony and to enhance breeder quality.
2.3 Phenylalanine Analysis
The equivalency of serum Phe levels measured by an amino acid analyzer and blood spot Phe levels by tandem mass spectrometry was established, by comparing submandibular blood samples for blood spots obtained immediately premortem with blood from cardiac puncture postmortem. The submandibular bleeding technique was used to collect a few drops of whole blood on filter paper from live animals. Samples were processed in-house at the Clinical Biochemical Laboratory at Nationwide Children's Hospital. Phe levels were measured by tandem mass spectrometry (MS/MS) Model Quattro Micro with a Waters 2795 Separation Module.
2.4 Phenylalanine Dosage Experiments
Homozygous mutant BTBR Pahenu2 females were bred to homozygous wild-type males to produce heterozygous fetuses, to avoid any effect of PKU in the fetal animals. Based upon studies conducted in humans, we established four treatment groups to investigate the critical maternal serum level as follows: control (<360μM), low (360-600μM), mid (600-900μM) and high (>900μM). The control and treatment group females were placed on a Phe deficient mouse chow and 0.7g/L Phe in the water to maintain maternal serum Phe levels <360μM. On day E5.5, the water supplement of Phe was changed to various increased concentrations to achieve target serum Phe levels for each of the treatment groups while the control group remained unchanged. Pregnant females were sacrificed on day E18.5, and fetuses were dissected as detailed in the Fetal Dissection section below. Phe levels were obtained at baseline, E5.5, E10.5, E15.5 and at sacrifice. Similar experiments were performed using congenic N10+ homozygous mutant females from C57B/L6J Pahenu2 and C3H/HeJ Pahenu2 congenic strains.
2.5 Timed Phenylalanine Exposure Experiment
Homozygous mutant BTBR Pah enu2 females were again bred to homozygous wild-type males and placed initially on a Phe deficient mouse chow and 0.7g/L Phe in the water to maintain maternal serum Phe levels <360μM. Groups of three homozygous mutant BTBR Pahenu2 female mice were exposed to a high Phe diet for time periods staggered over days E7.5-E15.5. Each exposure group was administered a high Phe diet to elevate maternal serum Phe levels at the specific 48hr time periods during gestation. One day of no Phe intake (Phe deficient chow and no Phe in water) was administered after Phe exposure to rapidly decrease serum Phe levels to that of the control. Females were again sacrificed at E18.5 and fetuses dissected (below) Maternal Phe levels were collected by submandibular bleeding technique prior to increasing the dietary Phe, during the 48 hour exposure, and 24+ hours after exposure was discontinued.
2.6 Fetal Dissection
All fetuses were harvested by C-section at E18.5. The fetuses were removed from the uterus starting at the right rostral to the right caudal and continuing the same order on the left. The fetuses were weighed, then exsanguinated and blood was spotted on filter paper for fetal serum Phe analysis. Each fetus was dissected and ribcage removed to expose the heart and vasculature. The right ventricle was injected with 10% India ink to provide a contrast agent with surrounding tissues. Gross cardiac morphology and positioning as well as variations in vascular organization were documented. Then, the tissue was fixed in 10% buffered formalin, paraffin embedded, and serial sections cut at 16μm in the transverse plane. The sections were stained with hematoxylin and eosin and examined for internal morphology.
CVMs were catalogued and classified. If more than one defect was present, the most severe defect was used to define the CVM for that fetus; e.g. simultaneous presence of a VSD and DORV was classified as DORV. CVMs were grouped by collapsing individual defects using the classification scheme of Botto et al [28].
2.7 Power Calculations and Statistical Analysis
Sample size calculations indicate we needed 52 total pups for each exposure group to detect a 25% difference in proportion of CVMs of at a power of 0.80. Summary statistics (mean, standard deviation, or proportions) were calculated for all groups. Comparisons of groups were made by Chi square or Fisher's exact test; in the case of ordered groups (dosing experiments), a non-parametric test for trends was used. Continuous variable comparisons were made with t-test or ANOVA with Dunnett post-hoc test. Maternal serum Phe levels in relation to dietary intake were studied using pharmacokinetic analysis. Multivariate modeling was performed using logistic regression analysis. All statistical analysis was performed using STATA version 11.0 (Stata Corp, College Station, TX).
3 RESULTS
3.1 Phe Intake and Serum Maternal Phe levels in BTBR Pahenu2
We first established equivalency in the two methods of measurement of serum Phe. Excellent correlation was observed between the tandem mass spectrometry and amino acid analyzer [Supplemental Figure 1]. Phe levels were roughly 15% lower by the tandem method, similar to observations in human [29].
Next, we ascertained the concentration of Phe supplementation in the drinking water that was required to achieve the appropriate maternal serum Phe level for each treatment group [Supplemental Figure 2]. We then determined the response times of serum Phe levels to dietary manipulation [Supplemental Figure 3]. The half-life of Phe in mouse is approximately 13.5 hours as compared to approximately 48 hours in humans [30]. Our results support that the administration of one day of a Phe null diet will reduce high serum Phe levels to a normal range to restrict fetal Phe exposure to our desired time in the developmental time of insult experiments.
We noted an increase in pregnancy loss in females exposed to high dietary Phe intake, particularly if exposed from E0-E4.5 (data not shown). This observation allowed us to optimize our breeding program by utilizing the mid Phe exposure in the timed exposure experiment, and not starting high Phe diets until E4.5. We also observed the maternal Phe at E13.5 negatively correlated with the weight of the fetus at E18.5 (maternal Phe at E13.5 <360μM, fetal weight at E18.5 = 0.97 +/- 0.14 g; 360-600μM, 0.94 +/- 0.12; 600-900μM, 0.81 +/- 0.18; >900μM, 0.71 +/- 0.13 [F (3, 166) = 22.78; p<0.0001]), similar to low birth weight seen in human MPKU.
3.2 BTBR Pahenu2 Dosage Dependent Response
We have screened 207 BTBR Pahenu2 fetuses for CVMs. Fetal Phe exposure category (control, low, mid, high) positively correlated with its affected status [p=0.04]. We observed a variety of defects affecting the OFT and AAA organization [Figure 1] including tissue migration abnormalities, abnormal intracardiac blood flow, cell death abnormalities, and extracellular matrix abnormalities. CVMs occurred in a maternal serum Phe dose-dependent manner [nonparametric test for trends, p<0.0001]. Specifically in control, low, mid and high Phe diet groups the observed incidence of affected fetuses was 11.86%, 16.67%, 30.86%, and 46.67%, respectively [Figure 2, Table 1]. Abnormal aortic arch arteries were the most common defect observed in all treatment groups [Table 1]. We detected many defects of AAA remodeling including abnormal origin of the right subclavian artery (RSA) and the right common carotid artery (RCCA). More severe forms of AAA remodeling included retroesophageal aorta (REA) [Supplemental Figure 4]. We also observed defects in valve development. Bicuspid aortic valve (BAV) was found in three fetuses and aortic valve stenosis or atresia (AVS) was observed in three additional fetuses. All three BAVs were type-A or left coronary-right coronary cusp fusion [Figure 3]. VSDs of all types were observed including, muscular, membranous, and supracristal [Supplemental Figure 5]. Although there were more TGAs in the low exposure group (3/6 total defects) and more AAA remodeling defects in the high exposure group (8/14 total defects), the numbers were too small to realistically infer any true differences in CVM type based on Phe dosage effect. Also, we observed a pattern of defects that resemble human HLHS including IAA (type B), plus aortic stenosis, and left ventricular hypertrophy (with hypoplastic LV cavity) [Supplemental figure 6].
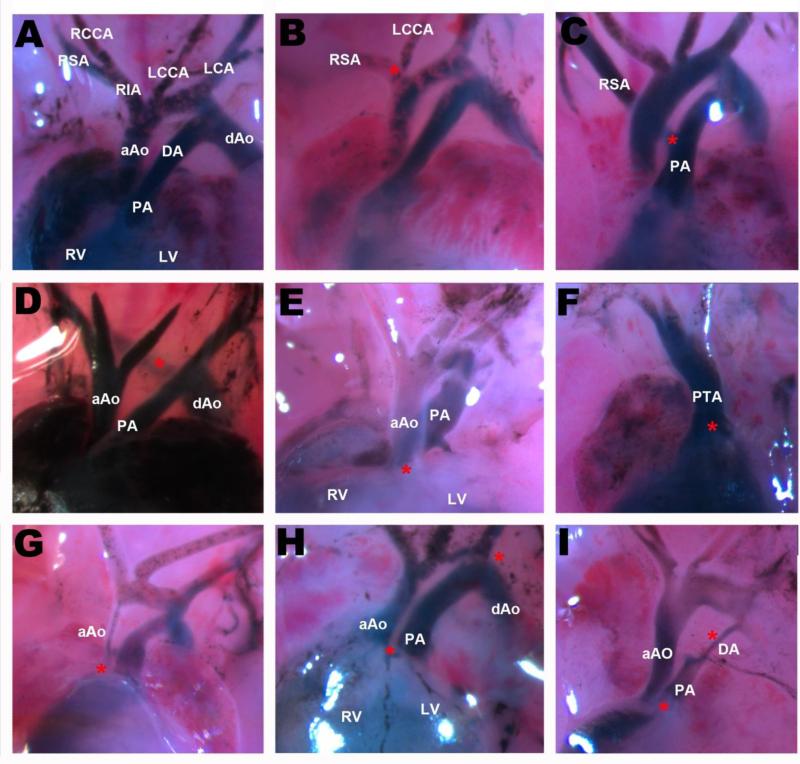
FIGURE 1.
Outflow tract (OFT) defects observed in the BTBR Pahenu2 strain. (A-E) Frontal view of the heart and OFT region of E18.5 embryos. The RVs were injected with 10% India ink. (A) Normal morphology the OFT and AA arteries. (B) Abnormal origin of the LCCA; branches from the RIA. (C) Abnormal origin of the RSA; branches from the PA. (D) Type B Interrupted Aortic Arch. (E,H) Transposition of the Great Arteries (TGA). (F) Persistent Truncus Arteriosis (PTA). (G) Aortic Stenosis. (I) Aortic Stenosis, Pulmonary Stenosis, and premature closure of the Ductus Arteriosis. aAo: ascending Aorta; dAo: descending Aorta; PA: Pulmonary Artery; DA: Ductus Arteriosis; RIA: Right Inominate Artery; RSA: Right Subclavian Artery; LSA: Left Subclavian Artery; RCCA: Right Common Carotid Artery; LCCA: Left Common Carotid Artery; RV: Right Ventricle; LV: Left Ventricle
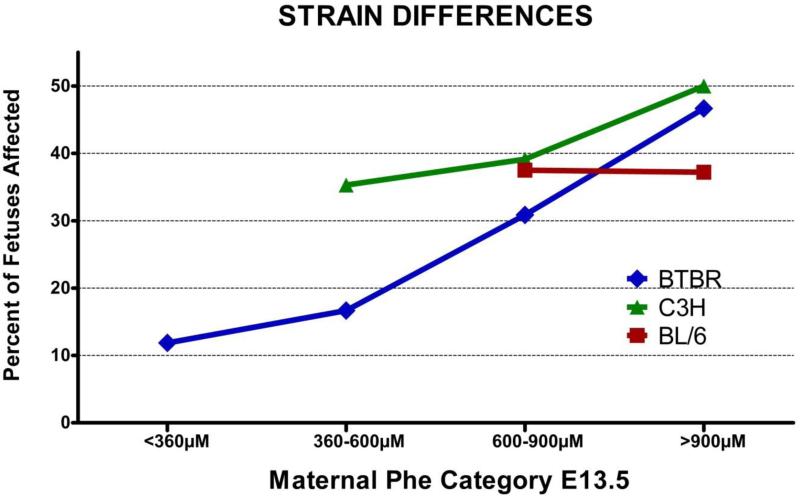
FIGURE 2.
Graphical representation of the dosage response in the incidence of CVMs among all three Pahenu2 strains. Blue) BTBR embryos. Green) C3H embryos. Red) C57BL6
TABLE 1.
Defect types observed in the BTBR Pahenu2 strain by maternal serum phenylalanine level at embryonic day 13.5.
| Maternal Phe Level E13.5 | |||||
|---|---|---|---|---|---|
| CHD No. (%) | <360μM | 360-600μM | 600-900μM | >900μM | Total |
| PVS | 0 (0) | 0 (0) | 1 (1.23) | 0 (0) | 1 |
| PTA | 2 (3.39) | 1 (2.78) | 2 (2.47) | 0 (0) | 5 |
| TGA | 0 (0) | 3 (8.33) | 4 (4.94) | 1 (3.33) | 8 |
| DORV | 2 (3.39) | 1 (2.78) | 6 (7.41) | 2 (6.67) | 11 |
| VSD | 1 (1.69) | 1 (2.78) | 3 (3.7) | 2 (6.67) | 7 |
| BRANCH | 2 (3.39) | 0 (0) | 6 (7.41) | 8 (26.67) | 16 |
| BAV | 0 (0) | 0 (0) | 2 (2.47) | 1 (3.33) | 3 |
| COA | 0 (0) | 0 (0) | 1 (1.23) | 0 (0) | 1 |
| NONE | 52 (88.14) | 30 (83.33) | 56 (69.14) | 16 (53.33) | 154 |
| Total | 59 | 36 | 81 | 30 | 206 |
BAV – bicuspid aortic valve; BRANCH – branching abnormalities of the aortic arch; COA – coarctation of the aorta; DORV – double outlet right ventricle; PTA – persistent truncus arteriosus; PVS – pulmonic valve stenosis; TGA – transposition of the great arteries
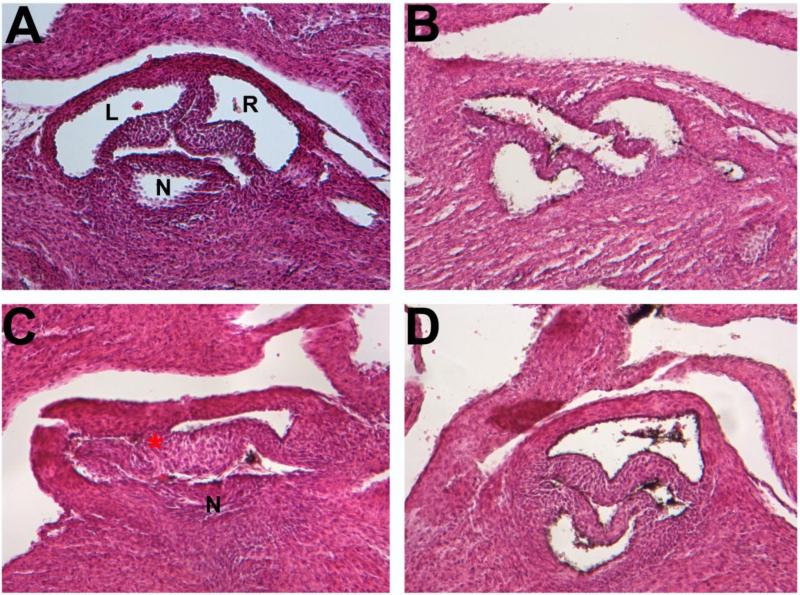
FIGURE 3.
BICUSPID AORTIC VALVE Histological sections of aortic valves in E18.5 BTBR embryos. (A) Normal tricuspid valve morphology (B,C,D) Type A bicuspid aortic valves, fusion of the LC-RC leaflets.
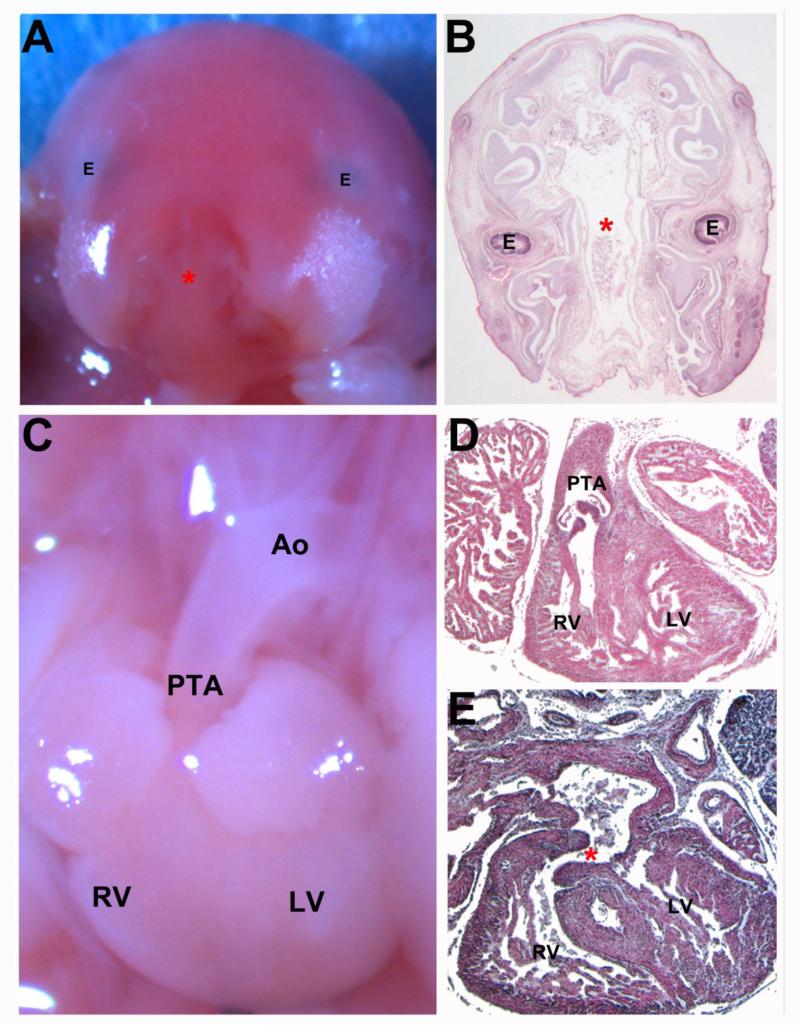
Extra-cardiac defects associated with MPKU were also found in our model including craniofacial abnormalities. A fetus with a cleft palate also had PTA and VSD [Figure 4]. A case of severe orofacial clefting has been reported in human MPKU [31].
FIGURE 4.
E18.5 BTBR Embryo displaying severe facial clefting and persistent truncus arteriosis (PTA) (A,C) Gross morphological images (B,D,E) Transverse histological sections (A) Gross image showing the large facial cleft. (B) Transverse section showing the large cleft (astrick). (C) Gross image of the heart displaying PTA. (D) Section showing the common valve of the PTA. (E)Section showing the VSD. E: Eye; RV: Right Ventricle; LV: Left Ventricle; Ao: Aorta; PTA: Persistent Truncus Arteriosis
3.3 Genetic Background
We generated congenic mice by breeding the Pahenu2 locus onto the C57BL6 and C3H/HeJ strains to investigate the effect of genetic background upon incidence and phenotypic variation of CVMs. There are differences in the CVM incidence between the three strains at the low and mid exposure levels, but this did not reach statistical significance [Figure 1,5]. The C3H.BTBR.Pahenu2 strain responds in a dose dependent manner but has higher incidence of CVMs increasing from 35.29%, 39.13%, and 50.00% across the low, mid and high treatment groups, respectively. Although, B6.BTBR.Pahenu2 fetuses have similar incidence of CVMs in the mid Phe group (37.5%), this effect plateaus in the high Phe exposure group (37.2%) [Figure 1]. Additionally, the BL6.BTBRPahenu2 strain required less Phe intake to achieve higher serum levels [Supplemental Figure 7]. Combined data of all three strains revealed that maternal Phe concentration at E13.5 significantly affects the likelihood of fetal CVM occurrence [p=0.01].
FIGURE 5.
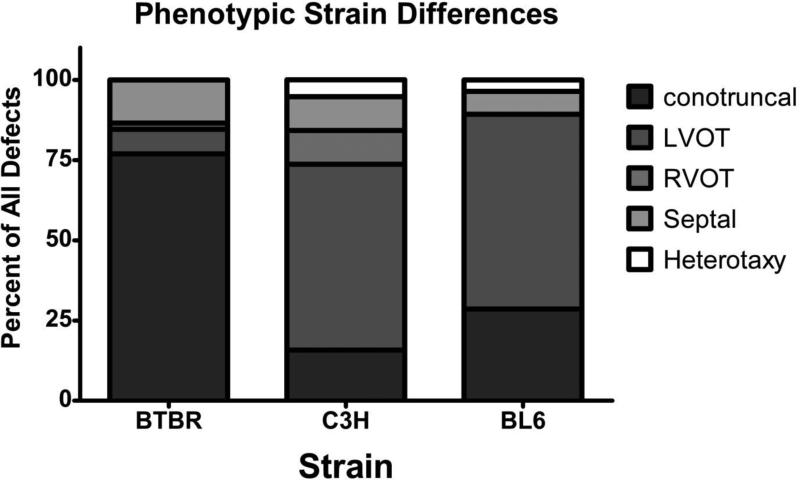
Graphical display of phenotypic differences between strains based on Botto's categorization scheme. BTBR display a majority of conotruncal defects while C3H and BL6 strains display LVOT defects.
We observed statistically significant phenotypic differences on each of the three Pahenu2 strains [Table 2, Figure 2,6; Fishers exact test p<0.001]. The majority of affected fetuses on the BTBR background have VSDs, branching abnormalities, or DORV. Affected B6.BTBRPahenu2 fetuses have right aortic arch (RAA) with interrupted aortic arch (IAA) type B, while C3H.BTBRPahenu2 have sub-aortic valve stenosis and ventricular hypertrophy. Utilizing the categorization method based on developmental mechanism developed by Botto, et al., [28] a clear difference in the cardiac phenotype emerged [Figure 5; Fishers exact p<0.0001]. Greater than 75% of the affected BTBR fetuses exhibit conotruncal defects. C3H and BL6 fetuses are most affected by left ventricular outflow tract (LVOT) defects.
TABLE 2.
Cardiovascular malformations of E18.5 fetuses by anatomic defect, sorted by background strain
| Cardiovascular Malformation Type | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | AVS | PVS | PTA | TGA | DORV | VSD | IAA | AAA | VH | DEX | BAV | COA | RAA | Total |
| BTBR, no. | 0 | 1 | 5 | 8 | 11 | 7 | 0 | 16 | 0 | 0 | 3 | 1 | 0 | 52 |
| % | 0 | 1.9 | 9.6 | 15.4 | 21.2 | 13.5 | 0 | 30.8 | 0 | 0 | 5.8 | 1.9 | 0 | 100 |
| C3H, no. | 10 | 0 | 0 | 0 | 1 | 3 | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 19 |
| % | 52.6 | 0 | 0 | 0 | 5.3 | 15.8 | 10.5 | 0 | 10.5 | 5.3 | 0 | 0 | 0 | 100 |
| BL6, no. | 4 | 0 | 0 | 0 | 1 | 2 | 6 | 1 | 12 | 0 | 1 | 0 | 1 | 28 |
| % | 14.3 | 0 | 0 | 0 | 3.6 | 7.1 | 21.4 | 3.6 | 42.9 | 0 | 3.6 | 0 | 3.6 | 100 |
| Total, no. | 14 | 1 | 5 | 8 | 13 | 12 | 8 | 17 | 14 | 1 | 4 | 1 | 1 | 99 |
| % | 14.1 | 1 | 5.1 | 8.1 | 13.1 | 12.1 | 8.1 | 17.2 | 14.1 | 1 | 4 | 1 | 1 | 100 |
AVS – aortic valve stenosis; PVS – pulmonic valve stenosis; PTA – persistent truncus arteriosus; TGA – transposition of the great arteries; DORV – double outlet right ventricle; VSD – ventricular septal defect; IAA – interrupted aortic arch; AAA – aortic arch anomalies (branching defects); VH – ventricular hypertrophy; DEX – dextrocardia; BAV – bicuspid aortic valve; COA – coarctation of the aorta; RAA – right aortic arch.
FIGURE 6.
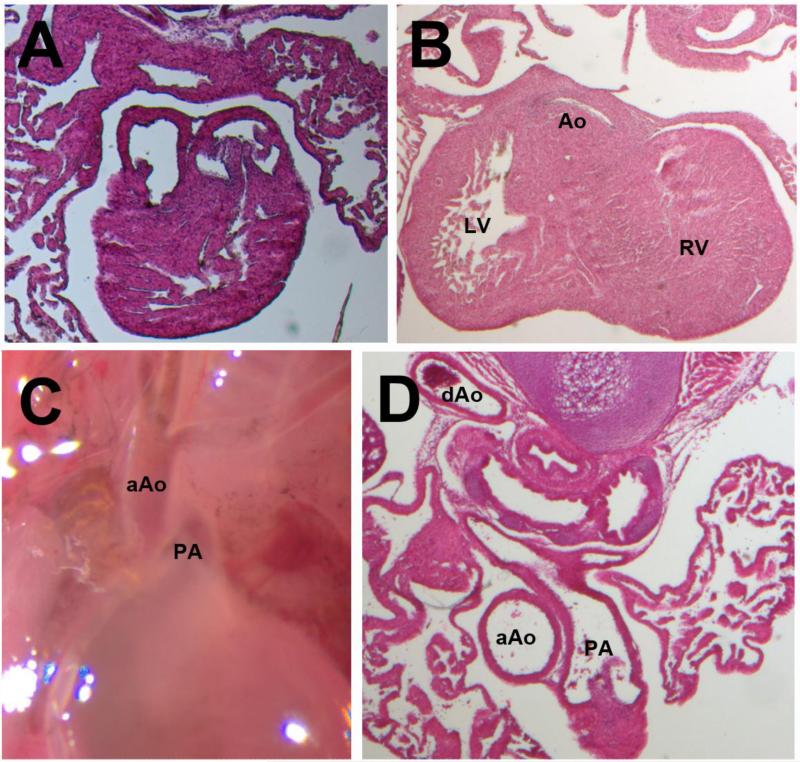
Gross and Histological Sections of most common defects in each strain. (A) Double Outlet Right Ventricle (DORV) in BTBR (B) Subaortic valve stenosis and left ventricular hypertrophy in C3H (C, D) BL6 fetus affected with right aortic arch. aAO: ascending Aorta; PA: Pulmonary Artery; dAo: descending Aorta.
3.4 Time of Insult by Phe
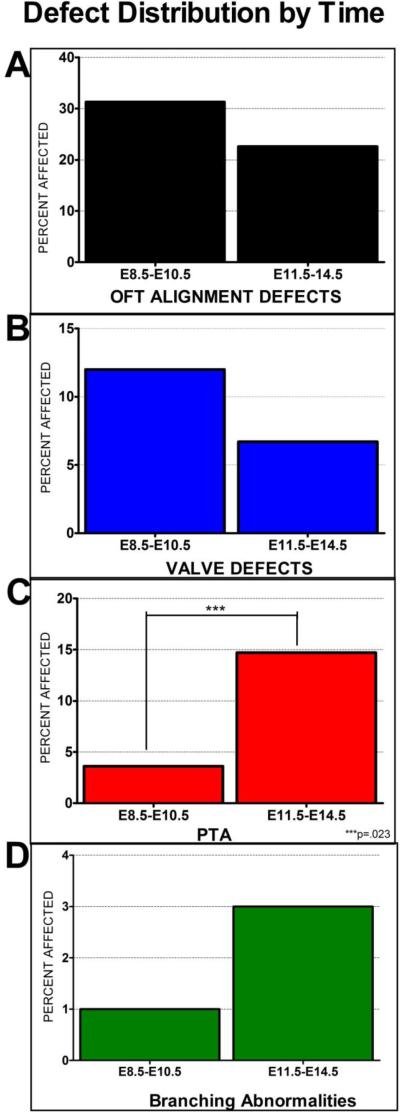
We exposed BTBR Pahenu2 embryos to high maternal Phe during an isolated period of development to investigate the period of exposure required to induce CVMs. Our data supports that exposure at any time point between embryonic days 8.5-13.5 is sufficient to cause defects affecting the OFT and AAA organization. No defects were observed in fetuses exposed to high Phe after E14.5 (n=10). This correlates well with what is observed in human MPKU as E14.5 in mouse corresponds to 7.25 weeks [14, 32, 33]. Analysis of the effect of exposure before E7.5 was impeded due to high embryonic loss and complete pregnancy termination (data not shown). Our data revealed trends in different defects including OFT alignment, valve defects, PTA and AAA branching abnormalities. A decreasing trend was observed in the percent of affected embryos with OFT and valve defects in early (E8.5-E10.5) vs late (E11.5-E14.5) exposure [Figure 7], with an increasing trend in PTA and branching abnormalities from early to late exposure. Only the proportion of PTA difference over time was statistically significant (Fisher's exact p=0.023).
FIGURE 7.
Incidence of defects based on early (E8.5-E10.5) (n=83) vs late (E11.5-E14.5) (n=75) Phe exposure. There is a clear trend in OFT alignment and valve defects and branching abnormalities. There is a statistically significant difference in the rate of PTA in early vs late Phe exposure.
4 DISCUSSION
This study provides the following major findings: 1) MPKU associated cardiovascular defects occur in a dose-dependent manner according to maternal Phe levels, 2) Timing of insult by Phe exposure corresponds to the human pattern, and 3) Background strain influences the incidence and phenotype of MPKU associated cardiovascular defects.
The effect of maternal Phe levels on the incidence of CVMs has been well described in human MPKU. In mouse, it was demonstrated that a very high, uncontrolled level (>900μM) of Phe caused CVMs in up to 47% of offspring [18]. Here we demonstrate the effect of lower dosages on the incidence of CVMs in the BTBR Pahenu2 model. We have demonstrated that the BTBR Pahenu2 model responds in a dose-dependent manner to the amount of maternal Phe. In addition, we also note, similar to human, pregnancy loss when maternal serum Phe levels are high, and a dose dependent response of near term (E18.5) fetal weight. We have also defined the serum Phe level in the fetus at E18.5, providing an indication of the Phe level required in the developing fetus to cause cardiac defects, confirming earlier data [34]. This information will be crucial for design of cellular assays of Phe teratogenicity. The dosage dependent response further confirms the accuracy of the Pahenu2 as a model of MPKU.
Previous studies to characterize the MPKU associated cardiovascular phenotype on the BTBR Pahenu2 mouse model have been limited. This study confirmed CVMs noted in previous work [18], and also noted additional defects of BAV and COA. Timing of Phe exposure showed abnormalities in aortic arch remodeling and great vessel septation occurred with higher frequency in later exposure (E11.5-14.5), while earlier exposure tended to produce OFT alignment defects. These results will be utilized to better understand the most likely mechanism involved, so we may refine future mechanistic studies to the most important time points. Isolating the time of exposure that leads to CVMs in the BTBR Pahenu2-/- model will aid in determining the cell population, genes, and genetic pathways affected by Phe teratogenicity.
Many studies have been conducted to determine the effect of genetic background on the incidence of CVMs in single gene mutants as well as models environmental exposures [22, 35]. To date, all of the MPKU experiments have been conducted on the BTBR background; therefore it was important to determine the phenotypic differences in MKPU in additional inbred strains. We anticipated each congenic strain would demonstrate a phenotype of CVM incidence and CVM type unique to each strain. Based upon previous studies, we predicted the C3H/HeJ genetic background would demonstrate an increase in the incidence of CVMs [22] but we could not predict the outcome for the C57BL/6. We note clear CVM type differences among the three strains, with higher rates of conotruncal type defects in the BTBR, higher rates of aortic arch patterning defects in the C57B/L6 and higher rates of left ventricular outflow tract defects in the C3H/HeJ strain. We identified CVMs not observed in the BTBR strain, including IAA (type B), plus aortic stenosis, and left ventricular hypertrophy (with hypoplastic LV cavity) that resemble human HLHS. The C57B/L6 was more sensitive to dietary intake of Phe, developing high serum levels at lower dietary Phe intake. The reason for this is unclear, and may represent a true difference in Phe metabolism, greater GI uptake of Phe or a difference in water intake. These data present different strain attributes in CVMs that will be well suited for future studies mapping modifier genes [36] and performing crosses with cardiac phenotype knock-outs and transgenics.
The stages and timing of heart development in the mouse are well defined [37], allowing reasonable assumptions on the developmental process disturbed by Phe in MPKU. Both human and mouse MPKU CVMs appear to primarily involve the outflow tract and aortic arch, with the type of defects suggesting abnormalities of the cardiac neural crest cells (as suggested previously [18, 38]) or secondary heart field. Normal development of the outflow tract (traditionally called the conotruncus) depends on migration of additional extra-cardiac cells into the arterial pole of the heart [39]. The secondary heart field contributes cells to the developing outflow tract during looping (E10.5). Migration of cardiac neural crest cells from pharyngeal arches 3, 4, and 6 into the outflow cushions and aortic arch arteries occurs from E8.5-9.5. During heart remodeling (E12.5 – 15.5) cNCCs are responsible for outflow tract septation (conal ventricular septum, great artery division) and repatterning of the aortic arch, respectively [40].
Although these observations strongly support a hypothesis of cNCC abnormalities as a cause of CVMs due to Phe teratogenicity, little research has been conducted to determine the influence of Phe on neural crest cells. A single study in chick had unclear findings, as timing and dosage of Phe did not correspond with the development of the cardiac neural crest, all treatment groups were pooled, and similar results were noted between treatment and control groups, with CVMs detected in 39% of sham injected embryos and 43% of Phe treated embryos [38]. Additional studies are merited and necessary to understand the role of cardiac neural crest cells in the development of CVMs in MPKU.
A more general disturbance of other neural crest cell populations may also account for other parts of the MPKU phenotype. It has recently been shown that cephalic neural crest cells contribute more to brain development than previously appreciated [41], thus providing a link with the microcephaly and intellectual disabilities. However, other typical neural crest cell phenotypes, such as pigmentary changes or colonic aganglionosis, have not been observed in MPKU. This suggests either Phe teratogenicity affects only a sub-population of neural crest cells, or possibly also affects mediators of neural crest cells in brain and heart development such as FGF8.
The teratogenic effect of Phe on heart development may involve many causes as with other common teratogens (ie ethanol and maternal diabetes) [42]. Deficiencies in other dietary components as well as deficiencies in LNAAs have been explored [43, 44]. Deficiency in protein intake has been correlated with CVMs, but it has not been determined to be a direct cause. Also, LNAAs compete for the Lat-1 transporter thus implicating a deficiency in non- Phe LNAAs as a cause for neurological dysfunction and CVM development; however this also has not been determined as a direct cause.
The presence of LVOT abnormalities in MPKU, both in human and now observed in mouse on the C3H and C57B/L6 strains, is intriguing. LVOT defects are not thought of as defects of cNCCs. This may suggest that the cNCCs may play a role in the pathogenesis of LVOT malformations in general, or Phe teratogenicity may operate on different developmental mechanisms in causing the spectrum of CVMs in MPKU. Additionally, the differences in CVM phenotype between mouse and human are an important observation to consider. The developmental anatomy of the heart in mouse and human is surprisingly similar so much of the research conducted in mouse can be extrapolated the humans [45]. However, genetic studies conducted in mouse and human have demonstrated that mutations in different genes can result in similar CVMs while mutations in the same gene can lead to varying defects. For example, the mouse knockout of Notch1 and genes in that pathway have left ventricle hypoplasia and aortic valve defects but not COA or HLHS [46], while mutations in NOTCH1 have been found among human subjects with AVS, COA and HLHS [47]. This observation in genetic models combined with the complexity of teratogenic effect makes it very difficult to explain why the difference in CVMs exists between mouse and human.
There are several limitations to our study. We are not powered to detect small differences in proportion of CVMs, thus we were unable to identify statistically significant differences for individual defects. As we relied on dietary intake of Phe as opposed to gavage, precise timing of Phe exposure could not be guaranteed, and may have contributed to our inability to identify specific time points for individual defects. The complex nature of teratogens includes a time and dosage sensitivity as well as genetic/environmental influences. All of these qualities make it difficult to pinpoint the direct cause of CVMs induced by Phe exposure in MPKU offspring.
In summary, we have confirmed the utility of the BTBR Pahenu2 mouse as a model for MPKU, defining several critical parameters for future studies to delineate the precise mechanism of Phe teratogenicity. We have also demonstrated the effect of genetic background on the MPKU phenotype, in the process generating two useful congenics for studying modifiers, and for the C57B/L6 strain, for use in crosses of known cardiac phenotype knock-outs and transgenics. Understanding a model of environmentally induced CVMs will be an invaluable tool in the determination and understanding of the mechanisms of CVMs in general. Ultimately, determining the mechanism of cardiac malformations caused by Phe teratogenicity will provide the possibility to develop therapeutic interventions.
Supplementary Material
Highlights.
Cardiovascular defects occur in a dose-dependent manner in a mouse model of MPKU
Phe exposure during E8.5-13.5 is the critical time period that causes heart defects
Genetic background strain influences the MPKU phenotype and rate of heart defects
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ferencz C, et al. Congenital cardiovascular malformations: questions on inheritance. Baltimore-Washington Infant Study Group. J Am Coll Cardiol. 1989;14(3):756–63. doi: 10.1016/0735-1097(89)90122-8. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins KJ, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115(23):2995–3014. doi: 10.1161/CIRCULATIONAHA.106.183216. [DOI] [PubMed] [Google Scholar]
- 4.Pierpont ME, et al. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115(23):3015–38. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- 5.Pradat P, et al. The epidemiology of cardiovascular defects, part I: a study based on data from three large registries of congenital malformations. Pediatr Cardiol. 2003;24(3):195–221. doi: 10.1007/s00246-002-9401-6. [DOI] [PubMed] [Google Scholar]
- 6.McBride KL, et al. Epidemiology of noncomplex left ventricular outflow tract obstruction malformations (aortic valve stenosis, coarctation of the aorta, hypoplastic left heart syndrome) in Texas, 1999-2001. Birth Defects Res A Clin Mol Teratol. 2005;73(8):555–61. doi: 10.1002/bdra.20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman SB, et al. Ethnicity, sex, and the incidence of congenital heart defects: a report from the National Down Syndrome Project. Genet Med. 2008;10(3):173–80. doi: 10.1097/GIM.0b013e3181634867. [DOI] [PubMed] [Google Scholar]
- 8.Channon S, et al. Effects of dietary management of phenylketonuria on long-term cognitive outcome. Arch Dis Child. 2007;92(3):213–218. doi: 10.1136/adc.2006.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmann R, et al. Frontal lobe-dependent functions in treated phenylketonuria: blood phenylalanine concentrations and long-term deficits in adolescents and young adults. J Inherit Metab Dis. 2005;28(4):445–55. doi: 10.1007/s10545-005-0445-7. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan JE. Emotional outcome of adolescents and young adults with early and continuously treated phenylketonuria. J Pediatr Psychol. 2001;26(8):477–84. doi: 10.1093/jpepsy/26.8.477. [DOI] [PubMed] [Google Scholar]
- 11.Walter JH, et al. How practical are recommendations for dietary control in phenylketonuria? Lancet. 2002;360(9326):55–7. doi: 10.1016/s0140-6736(02)09334-0. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health Consensus Development Conference Statement: phenylketonuria: screening and management, October 16-18, 2000. Pediatrics. 2001;108(4):972–82. doi: 10.1542/peds.108.4.972. [DOI] [PubMed] [Google Scholar]
- 13.Lenke RR, Levy HL. Maternal phenylketonuria and hyperphenylalaninemia. An international survey of the outcome of untreated and treated pregnancies. N Engl J Med. 1980;303(21):1202–8. doi: 10.1056/NEJM198011203032104. [DOI] [PubMed] [Google Scholar]
- 14.Levy HL, et al. Congenital heart disease in maternal phenylketonuria: report from the Maternal PKU Collaborative Study. Pediatr Res. 2001;49(5):636–42. doi: 10.1203/00006450-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Platt LD, et al. The international study of pregnancy outcome in women with maternal phenylketonuria: report of a 12-year study. Am J Obstet Gynecol. 2000;182(2):326–33. doi: 10.1016/s0002-9378(00)70219-5. [DOI] [PubMed] [Google Scholar]
- 16.Resta R. Generation n + 1: Projected numbers of babies born to women with PKU compared to babies with PKU in the United States in 2009. Am J Med Genet A. 2012;158A(5):1118–23. doi: 10.1002/ajmg.a.35312. [DOI] [PubMed] [Google Scholar]
- 17.McDonald JD, Charlton CK. Characterization of mutations at the mouse phenylalanine hydroxylase locus. Genomics. 1997;39(3):402–5. doi: 10.1006/geno.1996.4508. [DOI] [PubMed] [Google Scholar]
- 18.McDonald JD, et al. Cardiovascular defects among the progeny of mouse phenylketonuria females. Pediatr Res. 1997;42(1):103–7. doi: 10.1203/00006450-199707000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Matalon R, et al. Abnormal expression of genes associated with development and inflammation in the heart of mouse maternal phenylketonuria offspring. Int J Immunopathol Pharmacol. 2005;18(3):557–65. doi: 10.1177/039463200501800316. [DOI] [PubMed] [Google Scholar]
- 20.Beck JA, et al. Genealogies of mouse inbred strains. Nat Genet. 2000;24(1):23–5. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 21.Festing MF, Blackmore DK. Life span of specified-pathogen-free (MRC category 4) mice and rats. Lab Anim. 1971;5(2):179–92. doi: 10.1258/002367771781006564. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi T, et al. jumonji gene is essential for the neurulation and cardiac development of mouse embryos with a C3H/He background. Mech Dev. 1999;86(1-2):29–38. doi: 10.1016/s0925-4773(99)00100-8. [DOI] [PubMed] [Google Scholar]
- 23.Villar AJ, et al. Effects of genetic background on cardiovascular anomalies in the Ts16 mouse. Dev Dyn. 2005;232(1):131–9. doi: 10.1002/dvdy.20216. [DOI] [PubMed] [Google Scholar]
- 24.Petkov PM, et al. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14(9):1806–11. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrick CJ, et al. Reduced EGFR causes abnormal valvular differentiation leading to calcific aortic stenosis and left ventricular hypertrophy in C57BL/6J but not 129S1/SvImJ mice. Am J Physiol Heart Circ Physiol. 2009;297(1):H65–75. doi: 10.1152/ajpheart.00866.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winston JB, et al. Heterogeneity of genetic modifiers ensures normal cardiac development. Circulation. 2010;121(11):1313–21. doi: 10.1161/CIRCULATIONAHA.109.887687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shedlovsky A, et al. Mouse models of human phenylketonuria. Genetics. 1993;134(4):1205–10. doi: 10.1093/genetics/134.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botto LD, et al. Seeking causes: Classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79(10):714–27. doi: 10.1002/bdra.20403. [DOI] [PubMed] [Google Scholar]
- 29.Gregory CO, Yu C, Singh RH. Blood phenylalanine monitoring for dietary compliance among patients with phenylketonuria: comparison of methods. Genet Med. 2007;9(11):761–5. doi: 10.1097/GIM.0b013e318159a355. [DOI] [PubMed] [Google Scholar]
- 30.Langenbeck U, et al. Metabolic phenotypes of phenylketonuria. Kinetic and molecular evaluation of the Blaskovics protein loading test. J Inherit Metab Dis. 2009;32(4):506–13. doi: 10.1007/s10545-009-1152-6. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney E, Fryer A. Nasomaxillary hypoplasia and severe orofacial clefting in a child of a mother with phenylketonuria. J Inherit Metab Dis. 2002;25(1):77–9. doi: 10.1023/a:1015102320233. [DOI] [PubMed] [Google Scholar]
- 32.O'Rahilly R. Early human development and the chief sources of information on staged human embryos. Eur J Obstet Gynecol Reprod Biol. 1979;9(4):273–80. doi: 10.1016/0028-2243(79)90068-6. [DOI] [PubMed] [Google Scholar]
- 33.Theiler K. The house mouse; development and normal stages from fertilization to 4 weeks of age. Springer-Verlag; Berlin, New York: 1972. p. 168. [Google Scholar]
- 34.Cho S, McDonald JD. Effect of maternal blood phenylalanine level on mouse maternal phenylketonuria offspring. Mol Genet Metab. 2001;74(4):420–5. doi: 10.1006/mgme.2001.3255. [DOI] [PubMed] [Google Scholar]
- 35.Downing C, et al. Ethanol teratogenesis in five inbred strains of mice. Alcohol Clin Exp Res. 2009;33(7):1238–45. doi: 10.1111/j.1530-0277.2009.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts A, et al. The polymorphism architecture of mouse genetic resources elucidated using genome-wide resequencing data: implications for QTL discovery and systems genetics. Mamm Genome. 2007;18(6-7):473–81. doi: 10.1007/s00335-007-9045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey RP. Patterning the vertebrate heart. Nat Rev Genet. 2002;3(7):544–56. doi: 10.1038/nrg843. [DOI] [PubMed] [Google Scholar]
- 38.Kirby ML, Miyagawa ST. The effects of high phenylalanine concentration on chick embryonic development. J Inherit Metab Dis. 1990;13(4):634–40. doi: 10.1007/BF01799518. [DOI] [PubMed] [Google Scholar]
- 39.Waldo KL, et al. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128(16):3179–88. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- 40.Waldo K, et al. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Dev Biol. 1998;196(2):129–44. doi: 10.1006/dbio.1998.8860. [DOI] [PubMed] [Google Scholar]
- 41.Creuzet SE, Martinez S, Le Douarin NM. The cephalic neural crest exerts a critical effect on forebrain and midbrain development. Proc Natl Acad Sci U S A. 2006;103(38):14033–8. doi: 10.1073/pnas.0605899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa LG, et al. Developmental neurotoxicity: do similar phenotypes indicate a common mode of action? A comparison of fetal alcohol syndrome, toluene embryopathy and maternal phenylketonuria. Toxicol Lett. 2002;127(1-3):197–205. doi: 10.1016/s0378-4274(01)00501-x. [DOI] [PubMed] [Google Scholar]
- 43.Matalon KM. Congenital Heart Disease in Maternal Phenylketonuria: Effects of blood Phenylalanine and Nutrient Intake. Mental Retardation and Developmental Disabilities Research Reviews. 1999;5:122–124. [Google Scholar]
- 44.de Groot MJ, et al. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99(Suppl 1):S86–9. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Wessels A, Sedmera D. Developmental anatomy of the heart: a tale of mice and man. Physiol Genomics. 2003;15(3):165–76. doi: 10.1152/physiolgenomics.00033.2003. [DOI] [PubMed] [Google Scholar]
- 46.de la Pompa JL, Epstein JA. Coordinating tissue interactions: Notch signaling in cardiac development and disease. Dev Cell. 2012;22(2):244–54. doi: 10.1016/j.devcel.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McBride KL, et al. NOTCH1 mutations in individuals with left ventricular outflow tract malformations reduce ligand-induced signaling. Hum Mol Genet. 2008;17(18):2886–93. doi: 10.1093/hmg/ddn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.