Abstract
The neurodevelopmental model of schizophrenia, which posits that the illness is the end state of abnormal neurodevelopmental processes that started years before the illness onset, is widely accepted, and has long been dominant for childhood-onset neuropsychiatric disorders. This selective review updates our 2005 review of recent studies that have impacted, or have the greatest potential to modify or extend, the neurodevelopmental model of schizophrenia. Longitudinal whole-population studies support a dimensional, rather than categorical, concept of psychosis. New studies suggest that placental pathology could be a key measure in future prenatal high-risk studies. Both common and rare genetic variants have proved surprisingly diagnostically nonspecific, and copy number variants (CNVs) associated with schizophrenia are often also associated with autism, epilepsy and intellectual deficiency. Large post-mortem gene expression studies and prospective developmental multi-modal brain imaging studies are providing critical data for future clinical and high-risk developmental brain studies. Whether there can be greater molecular specificity for phenotypic characterization is a subject of current intense study and debate, as is the possibility of neuronal phenotyping using human pluripotent-inducible stem cells. Biological nonspecificity, such as in timing or nature of early brain development, carries the possibility of new targets for broad preventive treatments.
Keywords: CNV, genetics, imaging, neurodevelopment, schizophrenia, placenta
Introduction
Schizophrenia is a severe disabling disorder of unknown etiology, and its pathophysiology remains poorly understood. The neurodevelopmental model posits that the illness is the end stage of abnormal neurodevelopmental processes that began years before the onset of the illness. There has been considerable relevant work since the 2005 update on the Neurodevelopmental Model of Schizophrenia in this journal,1 which summarized several decades of research.
This highly selective review covers clinical, epidemiological, brain imaging and genetic studies since 2005 that elaborate on, or that we judge has the most potential to elaborate on, neurodevelopmental models of schizophrenia. We review research on antecedent risks, brain development and genetics of schizophrenia. In brief, we note that pre- and perinatal studies of placental pathology should be included in population/high-risk studies, and that environmental risk factors such as urbanicity, childhood trauma and social adversity have received strong replication. The importance of rare genetic variants as risk for schizophrenia is widely recognized, but with marked phenotypic nonspecificity pointing to common brain developmental pathways across disorders. The case for some molecular specificity is unclear but may be strengthened by ongoing sequencing studies, at least for rare copy number variants (CNVs).
Brain development is a continuous process, and early and later periods are relevant for study. Postmortem brain studies across the lifespan document the striking time- and region-specific nature of gene expression, including risk genes for schizophrenia (and other) disorders. Longitudinal cohort studies and emerging genetic and brain imaging technology provide new levels of complexity, including prenatal imaging and brain connectivity measures for understanding early brain precursors to illness.
New studies of typical brain development are looking at multiple cognitive and behavioral outcomes rather than a disease-based approach.2 The neurodevelopmental model of schizophrenia has long existed as a model for other childhood-onset conditions, including attention deficit hyperactivity disorder, intellectual deficiency, autism spectrum disorders (ASD) and epilepsy. Optimally, broader (less specific) risk could lead to translational research enabling broad preventive measures.
Longitudinal epidemiology
Cohort studies continue to provide valuable insights with the power to overcome the ‘noise’ of cross-sectional analyses. Individuals who later develop schizophrenia have lower cognitive and motor performance in childhood.3 Developmental delays may interact with other factors such as obstetrical complications, and even low Apgar scores, to provide as much as a fivefold risk of schizophrenia.4 However, early disorders of cognition, language and motor performance are both general risks for adult psychopathology and too common to be effective in predicting schizophrenia.5,6
Longitudinal studies support the dimensional nature of psychosis and risk. Psychotic symptoms were examined at age 12 for a twin cohort of over 2000 British children followed since age 5.7 There was a prevalence of 5% with psychotic symptoms, similar to that found in other birth cohorts, fivefold higher than the rate for schizophrenia; psychotic symptoms actually represent a trait diathesis, which in some cases convert to schizophrenia. There was broad comorbidity for psychosis across mood and antisocial behaviors. Psychotic symptoms were familial and heritable; associated with adverse upbringing and impaired cognitive functioning; and related to urban residence and lower birth weight.
Ongoing prospective studies in Europe, Asia and the United States are evaluating specific environmental, genetic and epistatic contributions to the development of schizophrenia. One outstanding example is the Generation R study based in Rotterdam, which is following a cohort of 10 000 children recruited during fetal life.8 Prospective imaging was carried out during pregnancy and will be continued until young adulthood. Maternal and fetal DNA is obtained for all subjects, and environmental measures include fetal exposure to medications and drugs, making this a promising study for gene–environment interaction analyses.
Environmental measures
Pre- and perinatal risk
Infection/famine
Clinical, epidemiological and translational studies continue to link prenatal infection and schizophrenia.9 Prenatal exposure to rubella, toxoplasma and herpes simplex virus type 2 are known causes of developmental disorders, including mental retardation, learning disabilities and sensorineural dysfunction.10 Studies most consistently find association between Toxoplasma gondii infection and schizophrenia; mothers with the highest immunoglobulin G level had a relative risk of 1.73.11 In a study of Danish infants using newborn filter paper blood spots taken at birth, T. gondii immunoglobulin G antibody was also associated with schizophrenia.12 The mechanism of such risk factors remains obscure, as does the phenotypic specificity of prenatal infection.
Follow-up of previous work on the Dutch Hunger Winter revealed that individuals who were in utero during this famine showed an increased risk of brain abnormalities, schizophrenia and depression. The increase in schizophrenia risk has been replicated in two separate studies in two different regions of China, which sustained famine during the cultural revolution and had a twofold increase.13,14 Exposure to famine is also associated with mood disorders and antisocial behavior, as well as with diabetes and body size.15
Placental pathology
As reviewed previously,1 a large number of studies find some relationship between obstetric complications and later onset of schizophrenia. Obstetrical events may be causal in themselves or reflect other causal processes. The past 5 years have seen particular interest in placental pathology, as recent reports indicate a high rate of placental pathology in infants with signs of perinatal brain injury16 or cerebral palsy.17 In addition to traditional gross and histological examination, the placenta produces substances important for infant brain development, such as serotonin,18 other monoamines,19 vasoactive intestinal peptide20 and mammalian target of rapamycin.21 As psychiatric outcomes may not be recognizable until years after, large prospective studies with banked tissue, more systematic description of placental appearance, and long-term follow-up are needed.16 This has already proved successful with respect to risk of cerebral palsy.22 Animal studies indicate an even wider role for the placenta in supporting fetal brain development during maternal food deprivation and stress that could be through a program of catabolic gene expression, resulting in placental catabolization to provide fuel to the developing brain.23
There are major obstacles to the study of placenta and psychiatric outcomes. Placentas are typically only examined when the babies are visibly ill at delivery. However, automated high-throughput image analyses are now feasible to standardize and speed identification of placental histopathology. As of this writing, at least three large cohort studies have begun incorporating this approach.
Low birth weight
Epidemiological studies have long identified preterm birth ( < 37 weeks, < 2055 g) as a significant, albeit nonspecific, risk factor for psychiatric disorders including schizophrenia. There is a broad increase in childhood disorders such as attention deficit hyperactivity disorder, intellectual deficiency, and most recently, ASD, and most are found with other comorbidities, particularly cognitive impairment. White matter (WM) abnormalities, ventricular enlargement and cerebellar hemorrhagic injury, all associated with ASD (see 2011 review by Johnson and Marlow24), indicate the probability of more subtle early insults for other low-birth-weight survivors.
Premorbid risk
Urban environment
Meta-analyses show consistent association in a dose–response manner with the urban environment in many settings,25 after controlling for a range of possible confounders, such as urban drift and minority status.26 It remains unclear as to how social factors mediate this relationship, as the effect of risk factors may vary depending on whether they are the norm or the exception within the social environment.27 There is probably a general stress processing factor,28 but here too, the effect appears diagnostically nonspecific, as mood and anxiety disorders are also elevated,29 and interaction effects with family liability have been shown repeatedly.25
Childhood trauma
Mounting evidence shows a significant relationship between early childhood trauma and risk of psychosis. Many studies are small, and the etiological importance is still controversial.30 Sexual abuse may be of particular importance,31 and interaction between developmental trauma (for example, physical, sexual and neglect) and decreased cortical thickness has been found.7,32-40 A prospective longitudinal twin study from the United Kingdom found trauma (characterized by intent to harm) to be associated with children’s later report of psychotic symptoms (relative risk 3.15).41 Given the long-lasting effects of stress for many mental health outcomes, this remains an area of active research.
Ethnic minority/immigrant status
Minority group position is associated with psychotic symptoms across different cultures.42 These effects appear mediated by chronic social adversity. This is one of the best documented and strongest environmental risk factors, particularly in interaction with family risk25 and specificity of age of exposure.43
Biological mechanisms
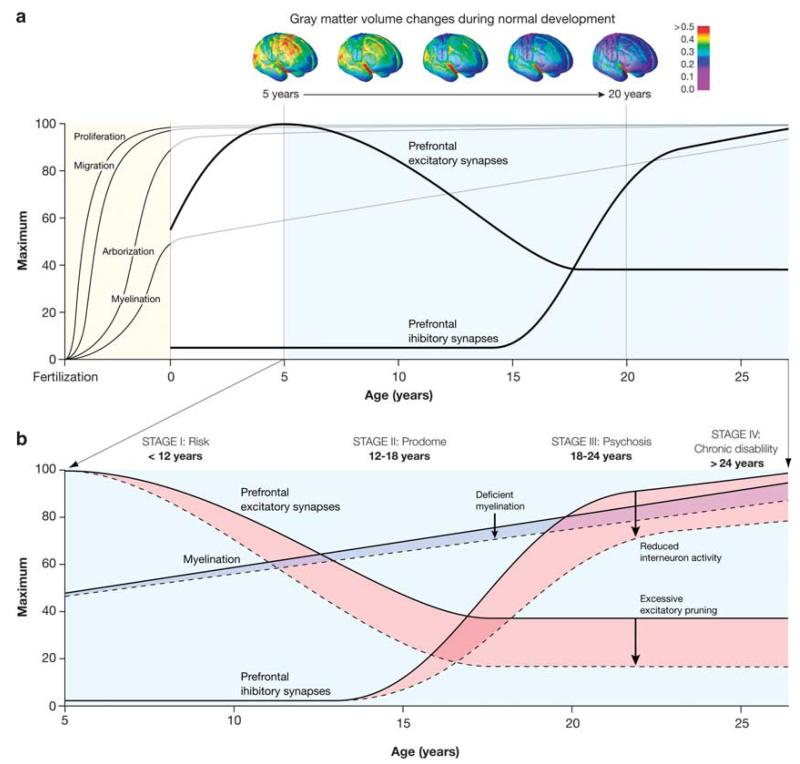
Biological mechanisms for environmental impact have been proposed. Urban environment has been associated with amygdala activation (current city living) and altered perigenual anterior cingulated cortex excitation (urban upbringing).28 In addition, the neuropeptides oxytocin and vasopressin, complex regulators of social interaction, have been proposed as mediators of social alienation.44 These models remain general, with presumed sensitive periods for brain developmental processes that share mechanisms across psychiatric disorders.25 In the central nervous system, neuronal proliferation, cell migration, morphological and biochemical differentiation, and circuit formation all depend on cell and cell–environment interactions that control developmental processes, and so can cause altered trajectories45,46 (see Figure 1).
Figure 1.
(a and b) Neuronal proliferation, cell migration, morphological and biochemical differentiation, and circuit formation all depend on cell and cell–environment interactions that control developmental processes, and so can cause altered trajectories.46
Neuroimaging
Technical and empirical advances in neuroimaging have been applied to further our understanding of neurodevelopmental mechanisms, including: (1) large prospective studies through childhood and adolescence producing normative data; (2) parallel longitudinal studies on non-psychotic siblings and ultra-high-risk groups; (3) acquisition of prenatal scans; (4) application of complementary methodologies such as tensor-based morphometry, diffusion tensor imaging and graph theoretical approaches to characterize circuitry; and (5) imaging genetic approaches.
Longitudinal studies on early onset/childhood-onset schizophrenia
Studying childhood-onset schizophrenia (COS) continues to provide unique neurodevelopmental data as probands and siblings are younger, and developmental brain changes are less influenced by illness or its treatment. The gray matter (GM) loss in COS seen in the adolescent years becomes localized to prefrontal and temporal cortices by age 20, as has been seen in most adult studies, supporting biological continuity between childhood-onset and adult forms of the illness.47
High-risk studies
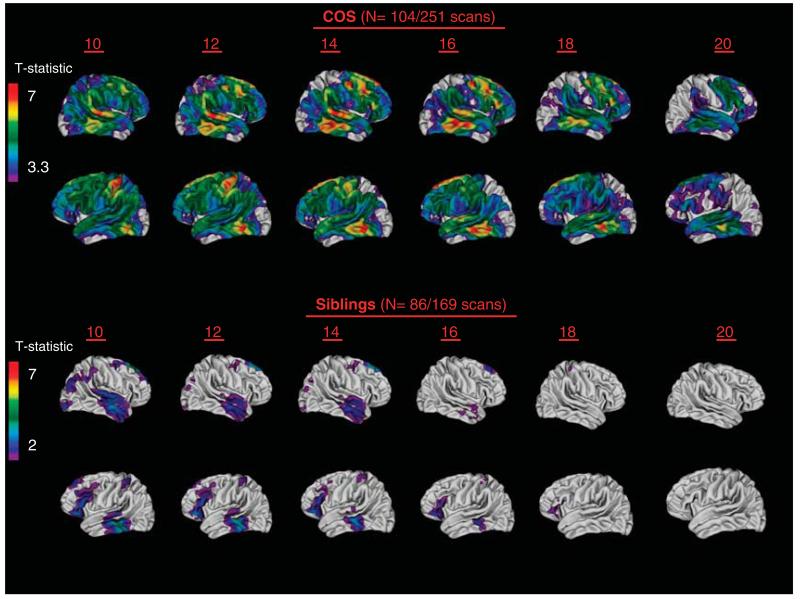
Non-psychotic siblings provide a valuable contrast group to address whether the observed brain trajectories represent familial/genetic traits in an unmedicated and asymptomatic sample. Healthy siblings of adult-onset schizophrenia generally have not evidenced cortical GM reduction, although some have shown enlarged ventricles and reduction in thalamic GM volume.48 Stronger evidence for the trait nature of GM abnormalities has come from discordant twin studies of adult patients.49-51 Prospective imaging studies of non-psychotic full siblings of COS patients have shown a pattern of prefrontal and temporal GM deficits in childhood that appear to ‘normalize’ by the time the subjects reach late adolescence.52 This initial observation was recently replicated in a ‘non-overlapping’ sample of healthy siblings and matched healthy controls.53 These combined data are shown in Figure 2.
Figure 2.
Cortical gray matter (GM) deficits across age 10–20 years in patients with childhood-onset schizophrenia (COS; upper panel) and their healthy siblings compared with matched healthy controls. Statistically significant gray matter cortical thickness deficits, using mixed-effect models across over 40 000 cortical points in each hemisphere, are represented by colors corresponding to t-values shown in the scale bars. Figure 2 adapted from the research of Greenstein et al.47 (upper panel), Gogtay et al.52 (lower panel) and Mattai et al.53 (lower panel).
The normalization of cortical GM loss may represent a ‘resilient phenotype’ where early GM loss in siblings is due to genetic vulnerabilities (first/genetic hit). A second/subsequent ‘hit’ around the typical age of onset in late adolescence may be absent or overcome by this plastic response of ‘normalization’ of the healthy siblings’ early GM abnormalities.
As with investigations of GM, longitudinal studies of WM show that the WM integrity changes throughout the course of illness, most prominently in the prefrontal cortex.54,55 Diffusion tensor imaging studies in adolescents and adults with schizophrenia consistently indicate widespread WM abnormalities,56-59 and prospective follow-up studies have found abnormal development in probands,58,60-62 expanding the case for progressive post-onset brain abnormalities. More recently, using a novel method called tensorbased morphometry, a significant lack of WM growth in COS patients was observed during adolescent years.63
Similarly, in a prospective study on ultra-high-risk individuals, Walterfang et al.54 reported that individuals who later developed psychosis showed a reduction in WM volume in the left fronto-occipital fasciculus. More recently, Bloemen et al.64 identified lower fractional anisotropy values in the medial frontal cortex in ultra-high-risk individuals developing psychosis.
Acquisition of prenatal and infant scans
Healthy siblings and other high-risk subjects provide one avenue of looking at very early neurodevelopment. Recent studies have assessed brain maturation in the first 2 years of postnatal life, during which cortical GM increases 185%.65 Gilmore et al.66,67 examined offspring of mothers with schizophrenia or schizo-affective disorder and controls combining prenatal ultrasound scans, neonatal structural magnetic resonance imaging and diffusion tensor imaging during this period. Unexpectedly, the results were strongly sexually dimorphic; high-risk males, but not females, showed larger intracranial volume, cerebrospinal fluid, total GM and lateral ventricle volumes.
The larger GM volume in the high-risk male children is counterintuitive, as COS children and adolescents (typically studied after age 7) are found to have smaller GM volume.1,68 At the same time, the larger GM findings are consistent with findings from subgroups of autistic children at this age.69 Twin studies from these same investigators67 indicate a GM volume heritability of 0.56 (lower than adults) and a lateral ventricle volume heritability of 0.71 (higher than adults) at this age, suggesting that gene×environment interaction may be relevant for GM development, but less so for ventricular volumes.
Schizophrenia: a disorder of connectivity
Schizophrenia is increasingly considered a disorder of cortical connectivity.70,71 Many neuroimaging modalities attempt to address this either directly, with methods such as diffusion tensor imaging, or indirectly, by correlating cortical voxels using multivariate analyses on either structural or functional data.
Multivariate analyses, including default mode networks of the brain obtained either through resting state functional magnetic resonance imaging or resting state MEG analyses,72 have gained prominence in developmental neuroimaging. Various analytical approaches are used to study these data. Graph theory provides a mathematical framework in which to describe the relationship between ‘nodes’ (for example, a brain structure or region) and ‘edges’ (for example, a physical or correlative connection between nodes). This involves the assumption that brain network organization is guided by a tendency to maximize communication efficiency while minimizing connection cost, which results in cost-efficient, ‘small-world’ networks having both low cost and high efficiency. Recent evidence from both anatomical and functional imaging suggests that these properties are under genetic control70 and are altered in schizophrenia generally,73-76 as well as in our COS cohort, consistent with models of regional synaptic deficit with intact long-distance connections.77,78 This finding fits with the synaptic pruning model of schizophrenia.
Imaging, genetics and neurodevelopment
Brain abnormalities in childhood may pre-date onset of illness. For example, brain cortical thinning of the left entorhinal regions was seen in relation to APOE allele status for children having no current cognitive impairment,79 and subjects carrying the risk gene for Huntington’s show striatal abnormalities and smaller intracranial volume80 many years before symptom onset. Twin studies in typically developing twins have indicated that heritability of cortical and subcortical regions varies by region and time of development, with some areas becoming more (frontal cortex) and others less (cerebellum) genetically determined with time.81
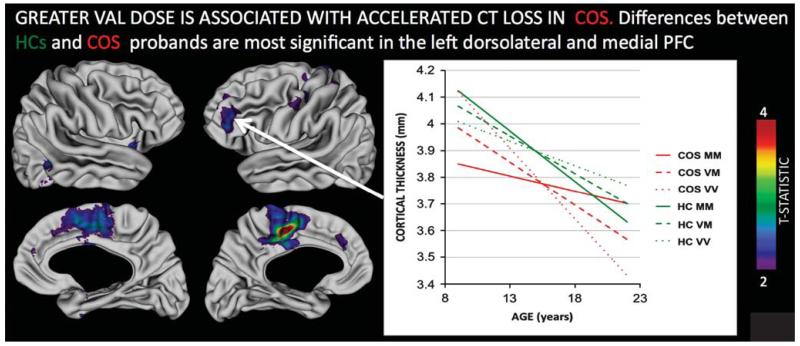
Neuroimaging methodologies that combine the genetic and imaging data sets are now starting to link results between risk alleles (small-to-moderate effect size) and the trait nature of morphological and functional brain abnormalities.82,83 This is based on the assumption that the brain phenotype, if indeed it is an intermediate phenotype,84 is likely to show greater association with the underlying risk alleles than with the overall disease diagnosis. This is supported by our recent observations in COS patients and their siblings, where increased Val dosing of COMT Val158Met genotype resulted in accelerated loss of cortical GM in the prefrontal cortex in both COS probands and their healthy siblings, while COMT genotype per se had no diagnostic association in this group (see Figures 3). Interestingly, in both COS probands and siblings, the Met–Met genotype appeared to ‘normalize’ the GM loss, but this occurred at an earlier age (late adolescence) in siblings and even later in COS probands, indicating age-dependent genetic influence on development.85
Figure 3.
Regions where the relationship between COMT Val158Met Val allele dose and cortical thickness change is significantly different in healthy controls (HCs) as compared to probands with childhood-onset schizophrenia (COS). The inset plot illustrates this interaction for the left dorsolateral prefrontal region, where increased Val dose attenuates cortical thinning on HCs, but accelerates it in probands with COS. Note that by adulthood, COS Val homozygotes have persistent cortical thickness deficits compared with HCs, whereas Met homozygotes do not. All colored regions shown survive false discovery rate correction for multiple comparisons at q < 0.05.85
Copy number variants
Recent research strongly supports the role for rare DNA CNVs as risk factors for schizophrenia. The best supported loci are deletions at 1q21, 2p53, 3q29, 15p11.2, 15q11.3, 17q12, 22q11.2 and Neurexin 1 (NRXN1),86-91 and duplications at 7q36.3, 25q11–13, 16p11.2 and 16p13.1.92-94 Both immunological and brain developmental pathways are implicated.95 The number of risk CNVs will certainly grow as larger patient and control samples increase sensitivity. CNVs vary considerably with respect to frequency and penetrance; a role for rare causal variants is emerging, and de novo mutations are seen particularly for CNVs that incur a large degree of selection against the phenotype.96
Extreme populations have been informative for the genetics of disease and in line with this concept; CNVs may be more frequent in very early-onset schizophrenia.97,98 MYT1 duplication and deletions at 16p11.2 and 22q11 were all recurrent within the small sample of childhood-onset patients.97,99
CNVs for 16p11.2 duplications were associated with smaller head circumference, and within the COS population, associated with smaller brain WM volume.92 As larger populations are screened for CNVs and larger 16p11 deletion/duplication samples are identified, it will be of interest to see if brain developmental patterns interact with deletion/duplication patterns within and across ASD and schizophrenia. It is possible that haplo-insufficiency or rare recessive effects of single base pair mutations in the non-deleted chromosome could account for some of the pleiotropy of CNVs.100
In addition, a ‘two-hit’ model has been proposed to explain severity/phenotypic choice.101 A ‘second hit’ could be another CNV, a disruptive single base pair mutation, or an environmental event influencing the phenotype.
Psychiatric disorder-based CNV studies need to be supplemented by CNV-based large population studies. Because CNVs such as 22q11 (the DiGeorge syndrome) may have pharyngeal, cardiac or immune deficits phenotypes, and 16p13.1 duplications have been associated with aortic dissection,102 disease-based studies omit other disorders related to the same CNV. Understanding this predisposition to disorders involving more than one organ system is critical to understanding the role of recurrent CNVs in human disease and gives true ‘overall’ prevalence and penetrance information that is crucial for understanding mechanisms, and for genetic counseling.
Human-induced pluripotent stem cells
The ability to generate neurons from fibroblasts can, in principle, lead to the discovery of cellular defects that may be fundamental to disease.103 Moreover, this technique lends itself to the study of individual differences given the unique genetic background propagated for each patient and has generated remarkable interest. Recently, skin cells taken from three patients with Alzheimer’s disease have been reported to produce higher levels of amyloid B, which is linked to Alzheimer’s disease.104 Using Rett syndrome as an ASD genetic model, human-induced pluripotent stem cell (hiPSC)-derived neurons from this group had fewer synapses, reduced spine density, smaller soma size and electrophysiological defects.105 Smaller neuronal soma size was also found in a second study with hiPSC-derived neurons from a single Rett syndrome patient.106
The hiPSC approach would seem ideal for the study of neurodevelopmental disorders, but there are still major limitations to this approach. These include variability across neurons, and hiPSC variability within and across populations. Direct reprogramming of fibroblasts to neurons has been reported,107 but a potential problem is that this transformation could miss the developmental window of neuronal differentiation and maturation.
In schizophrenia, there is a very wide choice of cellular phenotypes to examine, but further study of neuronal development would be suggested from the neurodevelopmental models of the disorder. Brennand et al.108 reprogrammed fibroblasts from four patients with schizophrenia into hiPSCs and subsequently differentiated them into neurons. The patient cells showed diminished neuronal connectivity in conjunction with decreased neurite number, PSD 95 protein levels and glutamate receptor expression.
An important next step is to carry out synaptic assays on a larger scale with large, well-characterized patient and control groups for which family, genetic and brain developmental (imaging) data are also available.
Post-mortem brain studies
Developmental neuroanatomy
Because schizophrenia typically starts in late adolescence, the remodeling of cortical connections during this period is thought to be critical.109,110 A recent study of normal microanatomic brain development replicates and extends Huttenlocher’s findings of frontal synaptic changes during adolescence.111 In the largest study of its kind, Petanjek et al.112 studied the synaptic spine density in the human prefrontal cortex during an extended period from newborn to age 91 years for 32 post-mortem subjects. They also found evidence that overproduction and developmental remodeling continues beyond adolescence and throughout the third decade of life before stabilizing. This extended developmental period has implications for genetic/environmental effects on late onset of neuropsychiatric disorders such as schizophrenia.
Post-mortem brain gene expression
Important new normative studies have set the stage for future clinical and high-risk work. A recent report on 269 post-mortem brains across the lifespan from individuals without psychiatric disorder have shown that, in spite of the great amount of individual genetic differences, there is a striking common pattern of how 650 000 messenger RNA or transcript variations influence gene expression in the frontal lobe across the age span. High gene expression during fetal life becomes much diminished after birth and gradually declines until middle age. At later ages, overall gene expression increases, mirroring the reversal in infancy.113,114 Newer (primate-specific) genes have expression bias toward early brain development compared with older genes, particularly in evolutionary newer (prefrontal) brain regions.115 A parallel study looked at gene expression across multiple brain regions across the same time period with 57 of these brains. In all, 86% of the genes analyzed were expressed, and 90% of these were differentially regulated across brain regions or across time. Schizophrenia risk genes are associated with transcripts that are enriched in, or unique to, the human brain. Some also show preferential expression in the fetal brain.113,116
Studies of post-mortem brains from patients who had schizophrenia will provide patterns of abnormality in development that will inform neurodevelopmental models. While the patient samples will all be adults, in principle, high-risk post-mortem brain samples, such as from first-degree relatives of patients with schizophrenia, should be obtained across a wider age range to permit comparable developmental study.
It is almost certain that both dopamine and glutamate transmission are abnormal in this disorder,117-119 and striatal dopamine over-activity may be critical to conversion to psychosis or psychotic symptoms generally.120,121 Several post-mortem brain studies have been designed around neurodevelopmental models relating to GABA, indicating vulnerable brain developmental periods122 and a risk genotype possibly reflecting immature GABA physiology.123 Defects in GABAergic neurotransmission have been linked to multiple neuropsychiatric disorders, including schizophrenia and autism. The most common abnormalities have been in the number of parvalbumin-containing interneurons (PV+) or their synaptic function. Alterations in timing of developmental disruption of GABAergic interneurons as the basis for several different neurodevelopmental disorders are gaining increasing support.124
This has been a particularly productive era for postmortem brain studies. Transcription studies have already proved important in autism where both developmental and immunological patterns of abnormalities have been identified.125
Summary
There is continued support for a broad neurodevelopmental model of schizophrenia. Age periods have been extended for human developmental brain imaging studies, but there is not yet satisfactory explanation for mechanisms mediating risk or of phenotype ‘choice’. Large prospective normative cognitive and brain imaging studies with contrasting clinical populations are providing valuable data. It may be that the phenotypic specificity will be found at the cellular level with hiPSC126 or that there will be distinct anatomic abnormalities for various cognitive/social abnormalities in local and long-range connectivity.127 Future studies that are chromosome-based, such as a large population study of risk CNVs for neurodevelopmental disorders, will better identify predictive genetic and brain developmental features.128-130
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- 2.Gur RC, Irani F, Seligman S, Calkins ME, Richard J, Gur RE. Challenges and opportunities for genomic developmental neuropsychology: examples from the Penn–Drexel collaborative battery. Clin Neuropsychol. 2011;25:1029–1041. doi: 10.1080/13854046.2011.585142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med. 2012;42:743–755. doi: 10.1017/S0033291711001693. [DOI] [PubMed] [Google Scholar]
- 4.Clarke MC, Tanskanen A, Huttunen M, Leon DA, Murray RM, Jones PB, et al. Increased risk of schizophrenia from additive interaction between infant motor developmental delay and obstetric complications: evidence from a population-based longitudinal study. Am J Psychiatry. 2011;168:1295–1302. doi: 10.1176/appi.ajp.2011.11010011. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone EC, Ebmeier KP, Miller P, Owens DG, Lawrie SM. Predicting schizophrenia: findings from the Edinburgh High-Risk Study. Br J Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- 6.Lawrie SM, Olabi B, Hall J, McIntosh AM. Do we have any solid evidence of clinical utility about the pathophysiology of schizophrenia? World Psychiatry. 2011;10:19–31. doi: 10.1002/j.2051-5545.2011.tb00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polanczyk G, Moffitt TE, Arseneault L, Cannon M, Ambler A, Keefe RS, et al. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67:328–338. doi: 10.1001/archgenpsychiatry.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofman A, Jaddoe VW, Mackenbach JP, Moll HA, Snijders RF, Steegers EA, et al. Growth, development and health from early fetal life until young adulthood: the Generation R Study. Paediatr Perinat Epidemiol. 2004;18:61–72. doi: 10.1111/j.1365-3016.2003.00521.x. [DOI] [PubMed] [Google Scholar]
- 9.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remington J, Klein J, Wilson C, Baker DJ. Infectious Diseases of the Fetus and Newborn Infant. 6th edn Elsevier Saunders; Philadelphia, PA: 2006. [Google Scholar]
- 11.Pedersen MG, Stevens H, Pedersen CB, Norgaard-Pedersen B, Mortensen PB. Toxoplasma infection and later development of schizophrenia in mothers. Am J Psychiatry. 2011;168:814–821. doi: 10.1176/appi.ajp.2011.10091351. [DOI] [PubMed] [Google Scholar]
- 12.Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Torrey EF, et al. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol Psychiatry. 2007;61:688–693. doi: 10.1016/j.biopsych.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 13.St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- 14.Xu MQ, Sun WS, Liu BX, Feng GY, Yu L, Yang L, et al. Prenatal malnutrition and adult schizophrenia: further evidence from the 1959–1961 Chinese famine. Schizophr Bull. 2009;35:568–576. doi: 10.1093/schbul/sbn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumey LH, Stein AD, Susser E. Prenatal famine and adult health. Annu Rev Public Health. 2011;32:237–262. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson KB, Blair E. The placenta and neurologic and psychiatric outcomes in the child: study design matters. Placenta. 2011;32:623–625. doi: 10.1016/j.placenta.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Blair E, de Groot J, Nelson KB. Placental infarction identified by macroscopic examination and risk of cerebral palsy in infants at 35 weeks of gestational age and over. Am J Obstet Gynecol. 2011;205:124.e1–7. doi: 10.1016/j.ajog.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKay R. Developmental biology: remarkable role for the placenta. Nature. 2011;472:298–299. doi: 10.1038/472298a. [DOI] [PubMed] [Google Scholar]
- 20.Passemard S, Sokolowska P, Schwendimann L, Gressens P. VIP-induced neuroprotection of the developing brain. Curr Pharm Des. 2011;17:1036–1039. doi: 10.2174/138161211795589409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen LF, Schendel D, Grove J, Hvidtjorn D, Jacobsson B, Josiassen T, et al. Asphyxia-related risk factors and their timing in spastic cerebral palsy. BJOG. 2008;115:1518–1528. doi: 10.1111/j.1471-0528.2008.01896.x. [DOI] [PubMed] [Google Scholar]
- 23.Zeltser LM, Leibel RL. Roles of the placenta in fetal brain development. Proc Natl Acad Sci USA. 2011;108:15667–15668. doi: 10.1073/pnas.1112239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. 2011;69(Part 2):11R–18R. doi: 10.1203/PDR.0b013e318212faa0. [DOI] [PubMed] [Google Scholar]
- 25.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 26.March D, Hatch SL, Morgan C, Kirkbride JB, Bresnahan M, Fearon P, et al. Psychosis and place. Epidemiol Rev. 2008;30:84–100. doi: 10.1093/epirev/mxn006. [DOI] [PubMed] [Google Scholar]
- 27.Zammit S, Lewis G, Rasbash J, Dalman C, Gustafsson JE, Allebeck P. Individuals, schools, and neighborhood: a multilevel long-itudinal study of variation in incidence of psychotic disorders. Arch Gen Psychiatry. 2010;67:914–922. doi: 10.1001/archgenpsychiatry.2010.101. [DOI] [PubMed] [Google Scholar]
- 28.Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- 29.Peen J, Schoevers RA, Beekman AT, Dekker J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr Scand. 2010;121:84–93. doi: 10.1111/j.1600-0447.2009.01438.x. [DOI] [PubMed] [Google Scholar]
- 30.Morgan C, Fisher H. Environment and schizophrenia: environmental factors in schizophrenia: childhood trauma-a critical review. Schizophr Bull. 2007;33:3–10. doi: 10.1093/schbul/sbl053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cutajar MC, Mullen PE, Ogloff JR, Thomas SD, Wells DL, Spataro J. Schizophrenia and other psychotic disorders in a cohort of sexually abused children. Arch Gen Psychiatry. 2010;67:1114–1119. doi: 10.1001/archgenpsychiatry.2010.147. [DOI] [PubMed] [Google Scholar]
- 32.Habets P, Marcelis M, Gronenschild E, Drukker M, van Os J. Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biol Psychiatry. 2011;69:487–494. doi: 10.1016/j.biopsych.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Arseneault L, Cannon M, Fisher HL, Polanczyk G, Moffitt TE, Caspi A. Childhood trauma and children’s emerging psychotic symptoms: a genetically sensitive longitudinal cohort study. Am J Psychiatry. 2011;168:65–72. doi: 10.1176/appi.ajp.2010.10040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salum GA, Polanczyk GV, Miguel EC, Rohde LA. Effects of childhood development on late-life mental disorders. Curr Opin Psychiatry. 2010;23:498–503. doi: 10.1097/YCO.0b013e32833ead33. [DOI] [PubMed] [Google Scholar]
- 35.Polanczyk G, Laranjeira R, Zaleski M, Pinsky I, Caetano R, Rohde LA. ADHD in a representative sample of the Brazilian population: estimated prevalence and comparative adequacy of criteria between adolescents and adults according to the item response theory. Int J Methods Psychiatr Res. 2010;19:177–184. doi: 10.1002/mpr.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polanczyk G, Caspi A, Houts R, Kollins SH, Rohde LA, Moffitt TE. Implications of extending the ADHD age-of-onset criterion to age 12: results from a prospectively studied birth cohort. J Am Acad Child Adolesc Psychiatry. 2010;49:210–216. [PubMed] [Google Scholar]
- 37.Polanczyk G, Bigarella MP, Hutz MH, Rohde LA. Pharmacogenetic approach for a better drug treatment in children. Curr Pharm Des. 2010;16:2462–2473. doi: 10.2174/138161210791959872. [DOI] [PubMed] [Google Scholar]
- 38.Moffitt TE, Caspi A, Taylor A, Kokaua J, Milne BJ, Polanczyk G, et al. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol Med. 2010;40:899–909. doi: 10.1017/S0033291709991036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canino G, Polanczyk G, Bauermeister JJ, Rohde LA, Frick PJ. Does the prevalence of CD and ODD vary across cultures? Soc Psychiatry Psychiatr Epidemiol. 2010;45:695–704. doi: 10.1007/s00127-010-0242-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauermeister JJ, Canino G, Polanczyk G, Rohde LA. ADHD across cultures: is there evidence for a bidimensional organization of symptoms? J Clin Child Adolesc Psychol. 2010;39:362–372. doi: 10.1080/15374411003691743. [DOI] [PubMed] [Google Scholar]
- 41.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bourque F, van der Ven E, Malla A. A meta-analysis of the risk for psychotic disorders among first- and second-generation immigrants. Psychol Med. 2010;41:897–910. doi: 10.1017/S0033291710001406. [DOI] [PubMed] [Google Scholar]
- 43.Veling W, Hoek H, Selten JP, Susser E. Age at migration and future risk of psychotic disorder among immigrants in the Netherlands: a 7-year incidence study. Am J Psychiatry. 2011;168:1278–1285. doi: 10.1176/appi.ajp.2011.11010110. [DOI] [PubMed] [Google Scholar]
- 44.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 45.Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev. 2002;26:471–483. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 46.Insel T. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 47.Greenstein D, Lerch J, Shaw P, Clasen L, Giedd J, Gochman P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry Allied Disciplines. 2006;47:1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 48.Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 49.Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci USA. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ettinger U, Picchioni M, Landau S, Matsumoto K, van Haren NE, Marshall N, et al. Magnetic resonance imaging of the thalamus and adhesio-interthalamica in twins with schizophrenia. Arch Gen Psychiatry. 2007;64:401–409. doi: 10.1001/archpsyc.64.4.401. [DOI] [PubMed] [Google Scholar]
- 51.Ettinger U, Schmechtig A, Toulopoulou T, Borg C, Orrells C, Owens S, et al. Prefrontal and striatal volumes in monozygotic twins concordant and discordant for schizophrenia. Schizophr Bull. 2010;38:192–203. doi: 10.1093/schbul/sbq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gogtay N, Greenstein D, Lenane M, Clasen L, Sharp W, Gochman P, et al. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch Gen Psychiatry. 2007;64:772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- 53.Mattai AA, Weisinger B, Greenstein D, Stidd R, Clasen L, Miller R, et al. Normalization of cortical gray matter deficits in non-psychotic siblings of patients with childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2011;50:697–704. doi: 10.1016/j.jaac.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walterfang M, McGuire PK, Yung AR, Phillips LJ, Velakoulis D, Wood SJ, et al. White matter volume changes in people who develop psychosis. Br J Psychiatry. 2008;193:210–215. doi: 10.1192/bjp.bp.107.043463. [DOI] [PubMed] [Google Scholar]
- 55.Walterfang M, Wood AG, Reutens DC, Wood SJ, Chen J, Velakoulis D, et al. Morphology of the corpus callosum at different stages of schizophrenia: cross-sectional study in first-episode and chronic illness. Br J Psychiatry. 2008;192:429–434. doi: 10.1192/bjp.bp.107.041251. [DOI] [PubMed] [Google Scholar]
- 56.Kyriakopoulos M, Frangou S. Recent diffusion tensor imaging findings in early stages of schizophrenia. Curr Opin Psychiatry. 2009;22:168–176. doi: 10.1097/YCO.0b013e328325aa23. [DOI] [PubMed] [Google Scholar]
- 57.Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, et al. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry. 2007;64:1270–1280. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- 58.Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol Psychiatry. 2008;63:519–523. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 59.Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130(Part 9):2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- 60.Douad G. Longitudinal structural and diffusion imaging in adolescent-onset schizophrenia: a delayed brain maturation story?; Proceedings of the 14th Annual Meeting of the Organization of Human Brain Mapping; Melbourne, Australia. 15–19 June 2008.2008. [Google Scholar]
- 61.Carpenter DM, Tang CY, Friedman JI, Hof PR, Stewart DG, Buchsbaum MS, et al. Temporal characteristics of tract-specific anisotropy abnormalities in schizophrenia. NeuroReport. 2008;19:1369–1372. doi: 10.1097/WNR.0b013e32830abc35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberger G, Kubicki M, Nestor PG, Connor E, Bushell GB, Markant D, et al. Age-related deficits in fronto-temporal connections in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2008;102:181–188. doi: 10.1016/j.schres.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gogtay N, Lu A, Leow AD, Klunder AD, Lee AD, Chavez A, et al. Three-dimensional brain growth abnormalities in childhoodonset schizophrenia visualized by using tensor-based morphometry. Proc Natl Acad Sci USA. 2008;105:15979–15984. doi: 10.1073/pnas.0806485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bloemen OJ, de Koning MB, Schmitz N, Nieman DH, Becker HE, de Haan L, et al. White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol Med. 2010;40:1297–1304. doi: 10.1017/S0033291709991711. [DOI] [PubMed] [Google Scholar]
- 65.Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilmore JH, Kang C, Evans DD, Wolfe HM, Smith MD, Lieberman JA, et al. Prenatal and neonatal brain structure and white matter maturation in children at high risk for schizophrenia. Am J Psychiatry. 2008;167:1083–1091. doi: 10.1176/appi.ajp.2010.09101492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilmore JH, Schmitt JE, Knickmeyer RC, Smith JK, Lin W, Styner M, et al. Genetic and environmental contributions to neonatal brain structure: a twin study. Hum Brain Mapp. 2010;31:1174–1182. doi: 10.1002/hbm.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frazier JA, Giedd JN, Hamburger SD, Albus KE, Kaysen D, Vaituzis AC, et al. Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry. 1996;53:617–624. doi: 10.1001/archpsyc.1996.01830070065010. [DOI] [PubMed] [Google Scholar]
- 69.Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 70.Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmitt A, Hasan A, Gruber O, Falkai P. Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci. 2011;261(Suppl 2):150–154. doi: 10.1007/s00406-011-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 73.Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131(Part 4):945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 75.van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci Off J Soc Neurosci. 2010;30:15915–15926. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, et al. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80–89. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alexander-Bloch AF, Gogtay N, Meunier D, Birn R, Clasen L, Lalonde F, et al. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front Syst Neurosci. 2010;4:147. doi: 10.3389/fnsys.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alexander-Bloch A, Lambiotte R, Roberts B, Giedd J, Gogtay N, Bullmore E. The discovery of population differences in network community structure: new methods and applications to brain functional networks in schizophrenia. NeuroImage. 2011;59:3889–3900. doi: 10.1016/j.neuroimage.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, et al. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- 80.Nopoulos PC, Aylward EH, Ross CA, Mills JA, Langbehn DR, Johnson HJ, et al. Smaller intracranial volume in prodromal Huntington’s disease: evidence for abnormal neurodevelopment. Brain. 2011;134(Part 1):137–142. doi: 10.1093/brain/awq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lenroot RK, Giedd JN. The changing impact of genes and environment on brain development during childhood and adolescence: initial findings from a neuroimaging study of pediatric twins. Dev Psychopathol. 2008;20:1161–1175. doi: 10.1017/S0954579408000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate–amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 83.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 84.Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, et al. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;63:465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raznahan A, Greenstein D, Lee Y, Long R, Clasen L, Gochman P, et al. Catechol-o-methyl transferase (COMT) val158-met polymorphism and adolescent cortical development in patients with childhood-onset schizophrenia, their non-psychotic siblings, and healthy controls. NeuroImage. 2011;57:1517–1523. doi: 10.1016/j.neuroimage.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moreno-De-Luca D, Mulle JG, Kaminsky EB, Sanders SJ, Myers SM, Adam MP, et al. Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am J Hum Genet. 2010;87:618–630. doi: 10.1016/j.ajhg.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stone JL, O’Donovan MC, Gurling H, Kirov GK, Blackwood DH, Corvin A, et al. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, et al. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 micro-deletions and VIPR2 duplications. Am J Psychiatry. 2011;168:302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mulle JG, Dodd AF, McGrath JA, Wolyniec PS, Mitchell AA, Shetty AC, et al. Microdeletions of 3q29 confer high risk for schizophrenia. Am J Hum Genet. 2010;87:229–236. doi: 10.1016/j.ajhg.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stefansson H, Rujescu D, Cichon S, Ingason A, Steinberg S, Fossdal R, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kirov G, Rujescu D, Ingason A, Collier DA, O’Donovan MC, Owen MJ. Neurexin 1 (NRXN1) deletions in schizophrenia. Schizophr Bull. 2009;35:851–854. doi: 10.1093/schbul/sbp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, et al. Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41:1223–1227. doi: 10.1038/ng.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ingason A, Rujescu D, Cichon S, Sigurdsson E, Sigmundsson T, Pietilainen OP, et al. Copy number variations of chromosome 16p13.1 region associated with schizophrenia. Mol Psychiatry. 2011;16:17–25. doi: 10.1038/mp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, et al. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B. 2005;134:60–66. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- 95.Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rees E, Moskvina V, Owen MJ, O’Donovan MC, Kirov G. De novo rates and selection of schizophrenia-associated copy number variants. Biol Psychiatry. 2011;70:1109–1114. doi: 10.1016/j.biopsych.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 97.Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 98.Lee Y, Mattai A, Long R, Rapoport J. Microduplications disrupting the MYT1L gene (2p25.3) are associated with schizophrenia. Psychiatric Genet. doi: 10.1097/YPG.0b013e328353ae3d. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O’Tuathaigh CM, Desbonnet L, Waddington JL. Neuregulin-1 signaling in schizophrenia: ‘Jack of all trades’ or master of some? Expert Rev Neurother. 2009;9:1–3. doi: 10.1586/14737175.9.1.1. [DOI] [PubMed] [Google Scholar]
- 100.Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet. 2010;11:415–425. doi: 10.1038/nrg2779. [DOI] [PubMed] [Google Scholar]
- 101.Girirajan S, Rosenfeld JA, Cooper GM, Antonacci F, Siswara P, Itsara A, et al. A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet. 2010;42:203–209. doi: 10.1038/ng.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuang SQ, Guo DC, Prakash SK, McDonald ML, Johnson RJ, Wang M, et al. Recurrent chromosome 16p13.1 duplications are a risk factor for aortic dissections. PLoS Genet. 2011;7:e1002118, 1–10. doi: 10.1371/journal.pgen.1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dolmetsch R, Geschwind DH. The human brain in a dish: the promise of iPSC-derived neurons. Cell. 2011;145:831–834. doi: 10.1016/j.cell.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, et al. Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell. 2011;146:359–371. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheung AY, Horvath LM, Grafodatskaya D, Pasceri P, Weksberg R, Hotta A, et al. Isolation of MECP2-null Rett syndrome patient hiPS cells and isogenic controls through X-chromosome inactivation. Hum Mol Genet. 2011;20:2103–2115. doi: 10.1093/hmg/ddr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, et al. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46:266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harris LW, Lockstone HE, Khaitovich P, Weickert CS, Webster MJ, Bahn S. Gene expression in the prefrontal cortex during adolescence: implications for the onset of schizophrenia. BMC Med Genomics. 2009;2:28. doi: 10.1186/1755-8794-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huttenlocher PR. Synaptogenesis in human cerebral cortex. In: Dawson G, Fischer K, editors. Human Behavior and the Developing Brain. Guilford Press; New York: 1994. pp. 137–152. [Google Scholar]
- 112.Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13–13. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, et al. Spatiotemporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Colantuoni C, Lipska BK, Ye T, Hyde TM, Tao R, Leek JT, et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature. 2011;478:519–523. doi: 10.1038/nature10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang YE, Landback P, Vibranovski MD, Long M. Accelerated recruitment of new brain development genes into the human genome. PLoS Biol. 2011;9:e1001179. doi: 10.1371/journal.pbio.1001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kleinman JE, Law AJ, Lipska BK, Hyde TM, Ellis JK, Harrison PJ, et al. Genetic neuropathology of schizophrenia: new approaches to an old question and new uses for postmortem human brains. Biol Psychiatry. 2011;69:140–145. doi: 10.1016/j.biopsych.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 118.Beneyto M, Lewis DA. Insights into the neurodevelopmental origin of schizophrenia from postmortem studies of prefrontal cortical circuitry. Int J Dev Neurosci. 2011;29:295–304. doi: 10.1016/j.ijdevneu.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Henn F. Dopamine: a marker of psychosis and final common driver of schizophrenia psychosis. Am J Psychiatry. 2011;168:1239–1240. doi: 10.1176/appi.ajp.2011.11091346. [DOI] [PubMed] [Google Scholar]
- 120.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Valmaggia L, et al. Dopamine synthesis capacity before onset of psychosis: a prospective 18F-DOPA PET imaging study. Am J Psychiatry. 2011;168:1311–1317. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 123.Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11–11. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 125.Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brennand KJ, Gage FH. Modeling psychiatric disorders through reprogramming. Dis Model Mech. 2011;5:26–32. doi: 10.1242/dmm.008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meyer-Lindenberg A. The future of fMRI and genetics research. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.10.063. doi:10.1016/j.neuroimage.2011.10.063. [DOI] [PubMed] [Google Scholar]
- 128.Mitchell KJ, Porteous DJ. Rethinking the genetic architecture of schizophrenia. Psychol Med. 2011;41:19–32. doi: 10.1017/S003329171000070X. [DOI] [PubMed] [Google Scholar]
- 129.Mitchell KJ. The genetics of neurodevelopmental disease. Curr Opin Neurobiol. 2011;21:197–203. doi: 10.1016/j.conb.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 130.Mitchell KJ. The miswired brain: making connections from neurodevelopment to psychopathology. BMC Biol. 2011;9:23. doi: 10.1186/1741-7007-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]