Abstract
Acute kidney injury (AKI) is frequently complicated by extra-renal multi-organ injury including intestinal and hepatic dysfunction. In this study, we hypothesized that a discrete intestinal source of pro-inflammatory mediators drives multi-organ injury in response to AKI. After induction of AKI in mice by renal ischemia-reperfusion or bilateral nephrectomy, small intestinal Paneth cells increased the synthesis and release of IL-17A in conjunction with severe intestinal apoptosis and inflammation. We also detected significantly increased IL-17A in portal and systemic circulation after AKI. Intestinal macrophages appear to transport released Paneth cell granule constituents induced by AKI, away from the base of the crypts into the liver. Genetic or pharmacologic depletion of Paneth cells decreased small intestinal IL-17A secretion and plasma IL-17A levels significantly and attenuated intestinal, hepatic, and renal injury after AKI. Similarly, portal delivery of IL-17A in macrophage depleted mice decreased markedly, and intestinal, hepatic, and renal injury following AKI was attenuated without affecting intestinal IL-17A generation. In conclusion, AKI induces IL-17A synthesis and secretion by Paneth cells to initiate intestinal and hepatic injury by hepatic and systemic delivery of IL-17A by macrophages. Modulation of Paneth cell dysregulation may have therapeutic implications by reducing systemic complications arising from AKI.
Keywords: acute renal failure, apoptosis, cytokine, inflammation, ischemia reperfusion, nephrectomy
Introduction
Acute kidney injury (AKI) is a major unresolved clinical problem costing more than 10 billion dollars per year in the Unites States (1,2). Morbidity and mortality from AKI is very high in part due to the high incidence of extra-renal complications (2), leading to intestinal barrier disruption, liver dysfunction, respiratory failure and a systemic inflammatory response syndrome that may progress to sepsis and multi-organ failure (3-6). Extra-renal systemic complications secondary to AKI are leading causes of mortality in the intensive care unit (3,7), but the mechanisms that lead to extra-renal organ dysfunction remain unclear.
Profound hepatic injury and systemic inflammation occur after ischemic and non-ischemic AKI with marked rises in plasma bilirubin, ALT, creatinine and BUN (8-10). In previous studies, hepatic injury induced prominent peri-portal hepatocyte necrosis, apoptosis and inflammation, and rapid (<5 hr) intestinal injury characterized by endothelial apoptosis, epithelial necrosis and inflammation was evident (10). Significantly higher IL-17A levels were detected in the small intestine and in portal venous blood relative to levels in liver and the systemic circulation, respectively, implicating the small intestine as a major source of IL-17A generation after AKI.
Small intestinal Paneth cells produce and release IL-17A to mediate TNF-α-induced shock (11), and hepatic ischemia and reperfusion (IR) caused Paneth cell degranulation and elevated secretion of Paneth cell-derived IL-17A (12). These findings suggest that Paneth cells may function as a source and reservoir of pro-inflammatory IL-17A to contribute to intestinal and hepatic injury as well as systemic inflammation after AKI. In contrast, intestinal IR injury causes rapid Paneth cell apoptosis with subsequent bacterial translocation to the liver and spleen, and Paneth cell granule depletion by zinc chelation exacerbated bacterial translocation and systemic inflammation after hemorrhagic shock, implicating the lineage as protective against systemic inflammation (13). .
Therefore, the role of Paneth cells in systemic inflammation and multi-organ dysfunction after AKI remains unresolved, and the mechanisms of extra-intestinal delivery of Paneth cell-derived IL-17A are not known. In this study, we report on the role of small intestinal Paneth cell derived IL-17A and subsequent mechanisms involved in the pathogenesis of remote organ injury and systemic inflammation after AKI.
Materials and Methods
Murine model of renal IR or bilateral nephrectomy
C57BL/6 mice (20-25 g) were obtained from Harlan, Indianapolis, IN. Paneth cell deficient mice (Sox9 flox/flox/ Villin Cre+/−) were generated as described (14). Sox9 flox/flox/ Villin Cre−/− mice were used as wild type controls. After Columbia University IACUC approval, male mice under pentobarbital anesthesia were subjected to sham surgery, 30 min. renal IR or bilateral nephrectomy as described previously (15,16). Sham-operated mice underwent identical abdominal manipulations as mice subjected to renal ischemia or bilateral nephrectomy (laparotomy, bilateral intestinal retraction and positioning). In a separate cohort of mice, recombinant murine IL-17A (0.3 or 1 μg per mouse, i.v.) or vehicle (saline) was injected instead subjecting to AKI to determine whether IL-17A can recapitulate hepatic, renal and intestinal injury in mice. We also injected recombinant murine IL-17A (1 μg per mouse, i.v.) after Paneth cell granule depletion with dithizone treatment (100 mg/kg, i.v. 6 hrs prior to IL-17A injection). In some mice, we measured systolic blood pressure after sham-surgery or AKI via indwelling carotid artery catheter. We ruled out blood pressure changes as a cause of systemic injury after AKI as neither renal IR nor nephrectomy resulted in post-surgical hypotension (Supplementary Figure 1).
Assessment of hepatic and renal injury
Plasma ALT activity, bilirubin, creatinine and BUN levels were determined as described previously (17,18). Markers of liver and kidney injury was sampled at 5 hrs after bilateral nephrectomy and 24 hrs after renal IR. Plasma ALT activity and bilirubin level were measured using the Infinity™ ALT assay kit (Thermo Fisher Scientific, Waltham, MA) and the QuantiChrom™ Bilirubin Assay kit (BioAssay Systems, Hayward, CA, USA). Plasma creatinine was measured by an enzymatic creatinine reagent kit (Thermo Fisher Scientific, Waltham, MA). Blood urea nitrogen (BUN) was measured by the Infinity™ BUN assay kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA).
Macrophage depletion
Macrophages were depleted by intraperitoneal injection of liposome-encapsulated dichloromethylene bisphosphonate (1 mg clodronate liposome per mouse, 200 μL) as described (19,20) 48 hrs prior to induction of AKI. Clodronate liposome was purchased from Encapsula NanoSciences (Nashville, TN). Control mice received the same volume of phosphate-buffered saline-containing liposome (PBS liposome). We determined the efficiency of macrophage depletion by performing spleen macrophage immunohistochemistry.
Enzyme Linked ImmunoSorbent Assay for plasma IL-17A
Five hrs after induction of AKI, plasma, jejunum and isolated crypt IL-17A levels were measured with mouse specific IL-17A ELISA kit according to the manufacturer’s instructions (eBiosciences, San Diego, CA) as described (10).
Portal vein plasma cryptdin-1 immunoblotting
Five hrs after sham-operation or induction of AKI, portal vein plasma were obtained and processed for cryptdin-1 immunoblotting with Anti-(6C/A)-Crp1 antibody as described previously (21,22).
Pharmacological Paneth cell depletion
To deplete Paneth cell granules, mice were treated with dithizone (100 mg/kg, i.v.) 6 hrs prior to renal ischemia or nephrectomy as described (23,24). Dithizone is dissolved as 10 mg/mL of final concentration in the saturated lithium carbonate (1 g/100 mL).
Small intestine immunohistochemistry
Immunohistochemistry for macrophages (F4/80, 1:200 dilution, T-2006, Bachem, Torrance, CA), lysozyme (1:100 dilution, PA1-29680, Thermo fisher scientific) or cryptdin-1 (25) was performed as described previously in paraffin-embedded tissue sections (10,18).
Immunofluorescence staining for small intestine macrophage/cryptdin-5 and macrophage/IL-17A
Small intestines were embedded in Tissue-Tek oxytetracycline compound (Fisher Scientific, Pittsburgh, PA, USA) and cut into 5 μm sections. After fixation with 3.7% paraformaldehyde, the sections were blocked in 10% BSA dissolved in PBS for 1 hr at room temperature. The sections were then incubated with CD68 primary macrophage antibody (1:100 dilution, MCA1957, Serotec, Raleigh, NC) in a humidified chamber for 16 hrs at 4°C. Secondary antibody incubation, using red fluorescent goat anti-rat immunoglobulin G (1:200 dilution), was performed at room temperature for 30 min. Subsequently, cryptdin-5 primary antibody (1:200 dilution, generated in house) or IL-17A primary antibody (1:100 dilution, sc-7927, Santa Cruz Biotechnology) was applied in a humidified chamber for 16 hrs at 4°C. The sections were incubated with green fluorescent secondary antibodies with donkey anti-goat immunoglobulin G for cryptdin-5 or donkey anti-rabbit immunoglobulin G for IL-17A. Finally, sections were then washed twice in PBS and mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA).
Reverse Transcriptase - PCR
Conventional RT-PCR was performed to analyze the expression of IL-17A and cryptdin-1 (as a quantitative marker for mature murine Paneth cells as descried (16,26,27) (Table 1). We also performed quantitative real-time RTPCR (Q-RTPCR) with the MyiQ Real Time Detection System (Bio-Rad, Hercules, CA) using SYBR Green I Brilliant Mastermix (Stratagene, La Jolla, CA). The Ct values were determined by using Mx3000P software. Values were normalized for GAPDH mRNA and relative expression of pro-inflammatory mRNA was calculated with the ΔΔCt method.
Table 1.
Primers used to amplify mRNAs encoding mouse GAPDH, IL-17A and cryptdin 1 based on published GenBank sequences for mice. Respective anticipated RT-PCR product size, PCR cycle number for linear amplification and annealing temperatures used for each primer are also provided.
| Primer | Accession Number | Sequence (Sense, Antisense) | Product Size (bp) |
Cycle Number |
Annealing Temp (°C) |
|---|---|---|---|---|---|
| Mouse Cryptdin-1 | NM_010031 | 5′- GCTGCCTGCTCATCCTAATC -3′ 5′- CAGCATCAGTGGCCTCAGTA -3′ |
376 | 27 | 64 |
| Mouse IL-17A | NM_010552 | 5′- TCCAGAAGGCCCTCAGACTA -3′ 5′- ACACCCACCAGCATCTTCTC -3′ |
248 | 32 | 66 |
| Mouse GAPDH | M32599 | 5′- ACCACAGTCCATGCCATCAC -3′ 5′- CACCACCCTGTTGCTGTAGCC -3′ |
450 | 15 | 65 |
Vascular permeability of liver and intestine tissues
Changes in liver, kidney and small intestine vascular permeability were assessed by quantitating extravasation of Evans blue dye (EBD) into the tissue as described by Awad et al. (28) with some modifications. Two percent EBD (Sigma Biosciences, St. Louis, MO) was administered intravenously at a dose of 20 mg/kg after ischemic AKI or bilateral nephrectomy. One hr later, mice were killed and perfused through the heart 5 mM EDTA in 10 mL cold saline with heparin (100 U/mL). Liver and small intestine tissues were then removed, allowed to dry overnight at 60°C, and the dry weights were determined. EBD was extracted in formamide (20 mL/g dry tissue; Sigma Biosciences), homogenized, and incubated at 60°C overnight. Homogenized samples were centrifuged at 12,000 g for 30 min. and the supernatants were measured at 620 and 740 nm in a spectrophotometer. The extravasated EBD concentration was calculated against a standard curve and the data expressed as micrograms of EBD per gram of dry tissue weight.
Detection of small intestine apoptosis
In situ TUNEL staining was used for detecting DNA fragmentation in apoptosis using a commercially available in situ cell death detection kit (Roche, Nutley, NJ) according to the manufacturer’s instructions. We further confirmed that the TUNEL positive cells are endothelial cells by staining serial small intestine (jejunum) sections with TUNEL and CD34 (an endothelial cell marker, Abcam Inc., Cambridge, MA). For DNA laddering, apoptotic DNA fragments were extracted according to the methods of Herrmann et al. (29) and was electrophoresed at 70 V in a 2.0% agarose gel in Tris-acetate-EDTA buffer. This method of DNA extraction selectively isolates apoptotic, fragmented DNA and leaves behind the intact chromatin. The gel was stained with ethidium bromide and photographed under UV illumination. DNA ladder markers (100 bp) were added to a lane of each gel as a reference for the analysis of internucleosomal DNA fragmentation.
Laser capture micro-dissection (LCM) of Paneth cells
LCM of individual Paneth cells was performed with the PixCell I LCM System (Arcturus Engineering, Mountain View, Calif., USA) as described (30). Small intestines were embedded in Optimum Cutting Temperature (OCT) compound (Sakura, Torrance, CA), sectioned at a thickness of 10 μM and mounted on 1.0 PEN Membrane Slides (Carl Zeiss, Thornwood, NY). The sections were then prepared for micro-dissection using an LCM staining kit (Ambion, Austin, Tx) through a graded alcohol series (95%, 75%, 50%) followed by cresyl violet staining. After de-staining via second graded alcohol series (50%, 75%, 95%), they were dehydrated in 100% ethanol followed by xylene. LCM was performed on a Zeiss Axiovert 200M microscope equipped with PALM RoboSoftware (Carl Zeiss, Thornwood, NY) and the total area of tissue collected per slide was tracked and recorded. RNA was isolated from the dissected tissue by following the protocol provided by the RNAqueous-Micro kit (Ambion, Austin, TX) via column purification.
Electron Microscopy
Small intestines were fixed in 4% paraformaldehyde/3% glutaraldehyde in 10 mM sodium phosphate buffer (pH 7.4) for 48 hrs. All samples were postfixed with 1% osmium tetroxide in 100 mM cacodylate buffer (pH 7.4) on ice for 1 hr. Samples were then treated with 0.5% aqueous uranyl acetate, dehydrated in graded alcohol, treated with propylene oxide, and embedded in Embed 812 (Electron Microscopy Sciences). The resin was polymerized in a 60°C oven for 2-3 days. Sections were cut with a Dupont diamond knife in Reichert-Jung UltraCut E ultramicrotome, collected on copper grids, and doubly stained with saturated aqueous uranyl acetate and lead citrate. Ultrathin sections were imaged for Paneth cells using a JEM-1200EX electron microscope manufactured by JEOL.
Isolation of intestinal crypts
Intact small intestinal crypts were isolated with the distended intestinal sac method as described by Traber et al. (31) with slight modifications. Small intestine from the duodenum to the ileum was removed and rinsed thoroughly with intestinal wash solution [0.15 M NaCl, 1 mM dithiothreitol (DTT), and 40 pg/mL phenylmethylsulfonyl fluoride (PMSF)] and then filled with buffer A [(in mM) 96 NaCl, 27 sodium citrate, 1.5 KCl, 8 KH2PO4, 5.6 Na2HPO4, and 40 pg/mL PMSF (pH 7.4)]. The ends were clamped with micro-clips and the intestine was filled to a pressure of 50 cmH2O. The filled intestine was submerged in oxygenated 0.15 M NaCl at 37°C for 40 min., drained and the solution was discarded. The intestine was then filled with buffer B [(in mM) 109 NaCl, 2.4 KCl, 1.5 KH2PO4, 4.3 Na2HPO4, 1.5 EDTA, 10 glucose, 5 glutamine, 0.5 DTT, and 40 pg/mL PMSF (pH 7.4)], incubated at 37°C for another 20 min. and the intestinal contents were drained and collected. The cells from 40-60 min. fraction containing intact and isolated crypts were collected by pelleting at 100 g for 5 min. at 4°C and washed once with PBS.
Transfer of wild type IL-17A splenocytes to wild type or IL-17A deficient mice
Spleens from wild-type (C57BL/6) mice were crushed and splenocytes were passed through a nylon cell strainer (BD Biosciences, San Jose, CA) and collected in phosphate-buffered saline. Red blood cells were lysed and single-cell splenocyte suspensions were transferred intravenously (6×106 to 1×107 splenocytes per transfer, 200 μL) to wild type (C57BL/6) or to IL-17A deficient mice 24 hrs before AKI.
Statistical analysis
The data were analyzed with 2-tailed t test when means between two groups were compared or with one-way (e.g., plasma creatinine or ALT) ANOVA plus Tukey post hoc multiple comparison test to compare mean values across multiple treatment groups. In all cases, P< 0.05 was taken to indicate significance. All data are expressed as mean ± SEM.
Results
Paneth cells degranulate after AKI
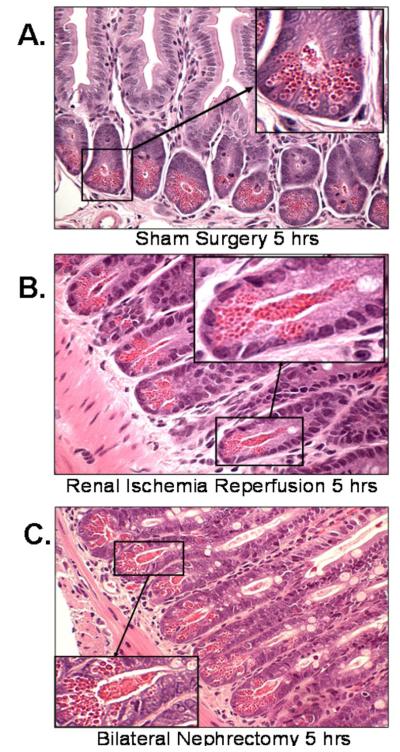
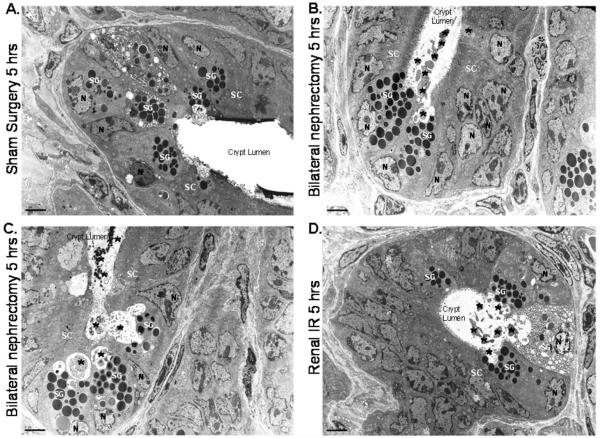
Histological examination of small intestine from sham-operated mice showed Paneth cells containing densely packed eosinophilic secretory granules (Fig. 1A). In contrast, renal IR and bilateral nephrectomy induced extensive Paneth cell degranulation within 5 hrs (Fig. 1B and 1C). Further evidence of Paneth cell degranulation was apparent by electron microscopy of small intestines following bilateral nephrectomy (Fig. 2B and 2C) and renal IR (Fig. 2D and 2E) compared to sham-operated mice (Fig. 2A). The crypt lumen from sham-operated mice was devoid of Paneth cell granules whereas the crypt lumen from mice subjected to AKI showed granules being released into the lumen.
Figure 1. Paneth cell degranulation after acute kidney injury.
Representative H&E staining images of small intestinal (ileum shown) Paneth cells containing dense eosinophilic granules within their apical cytoplasm (400X magnification). Both renal IR and bilateral nephrectomy resulted in small intestinal Paneth cell degranulation (B and C) in 5 hrs compared to sham-operated animals (A). Inserts show enlarged images (2000X magnification) of Paneth cells showing degranulation into the crypt lumen. Representative of 5 independent experiments.
Figure 2. Paneth cell degranulation after acute kidney injury observed with electron microscopy.
Representative electron micrograph images (3000X magnification) of small intestinal Paneth cell degranulation (indicated by *) 5 hrs after bilateral nephrectomy (B and C) or after renal IR (D) compared to sham-operated mice (A). The crypt lumen from sham-operated mice was devoid of Paneth cell granules. Representative of 5 experiments. N = nucleus of Paneth cells. SG = secretory granules of Paneth cells. SC = stem cells located above the Paneth cells.
Portal delivery of Paneth cell granule products by intestinal macrophages after AKI
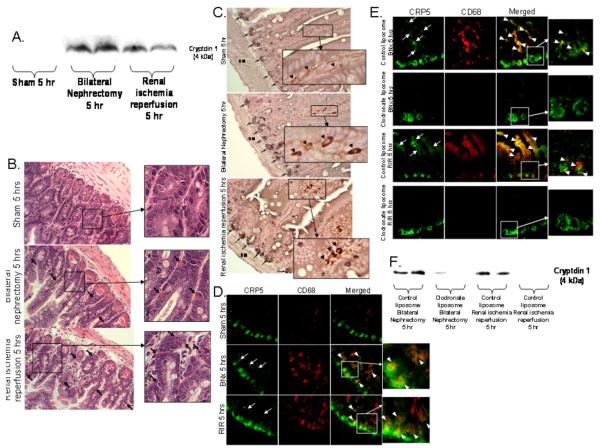
Detection of elevated Paneth cell α-defensin (cryptdin-1) protein levels in portal vein plasma after AKI induced by bilateral nephrectomy or renal IR (Fig. 3A) showed that Paneth cell secretory products were delivered to the liver. Because Paneth cells are the only enteric cell type that expresses α-defensins in mice, this epithelial cell lineage is unambiguously the source for their presence in the portal circulation.
Figure 3. Paneth cell degranulation products are delivered to portal circulation via intestinal macrophage uptake.
A. Acute kidney injury increases portal venous cryptdin-1. Representative (of 3 independent experiments) immunoblotting images for cryptdin-1 (4 kDa) in portal vein plasma from mice subjected to sham-operation, bilateral nephrectomy (BNx) or renal IR (RIR). Portal blood was sampled 5 hrs after surgery and processed for Tricine-SDS PAGE as described in Methods. B-E. Macrophages take up Paneth cells granules after acute kidney injury (400X magnification). B. Representative H&E stained images of small intestine of mice after sham-operation, BNx or RIR 5 hrs prior (400X magnification). Acute kidney injury results in Paneth cell degranulation and Paneth cell granule contents migrating away from the crypt base after taken up by phagocytes (arrows). Inserts show enlarged images (2000X magnification) of eosinophilic granules within phagocytes (arrows). C. Macrophages in small intestine of mice subjected to acute kidney injury (BNx or RIR) stain for both cryptdin-5 (a Paneth cell specific marker, black, arrows) and F4/80 (a macrophage specific marker, brown). Enlarged insert (2000X magnification) shows macrophages also stain for Paneth cell marker in mice subjected to BNx and renal IR (arrow heads). Representative of 5 independent experiments. SM = smooth muscle. D. Representative (of 4 independent experiments, 200X magnification and enlarged insert of 600X magnification) photographs demonstrating immunofluorescence staining for cryptdin-5 (CRP5, a Paneth cell specific marker, green) and CD68 (a macrophage specific marker, red) in small intestine of mice subjected to sham-operation (Sham), BNx or RIR. Acute kidney injury (BNx or RIR) results in Paneth cell degranulation as cryptdin-5 migrates away from the crypt base (arrows). In addition, cryptdin-5 and macrophages co-stain (yellow, arrow heads) demonstrating that degranulated cryptdin-5 is taken up by macrophages. E. Representative (of 4 independent experiments, 200X magnification and enlarged insert of 600X magnification) immunofluorescence staining for cryptdin-5 (CRP5, green) and CD68 (red) in small intestine of mice subjected to BNx or RIR with (control liposome injected) or with (clodronate liposome injected) macrophage depletion. Mice were injected with liposomes i.p. 48 hrs before sham operation or AKI. Note that cryptdin-5 no longer migrates away from the crypt base after macrophage depletion. F. Macrophage depletion with clodronate liposome decreases portal venous cryptdin-1 after AKI. Representative (of 3 independent experiments) immunoblotting images for cryptdin-1 in portal vein plasma from mice subjected to bilateral nephrectomy (BNx) or renal IR (RIR) after control liposome or clodronate liposome injection. Portal blood was sampled 5 hrs after surgery.
Macrophages mediate, at least in part, the removal of Paneth cell secretions from the lumen of small intestinal crypts of mice with AKI. Uptake of eosinophilic Paneth cell granules and granular secretions by phagocytes and their removal from the base of the small intestine was apparent in mice subjected to AKI (arrows, inset Fig. 3B). Co-staining for cryptdin-1 and F4/80 confirmed that macrophages also stain for cryptdin-1 in addition to F4/80 after induction of AKI (inset Fig. 3C). In addition, we performed immunofluorescence co-localization of cryptdin-5 and macrophage CD68 in the small intestine. AKI induced either by renal IR or bilateral nephrectomy, resulted in Paneth cell degranulation as indicated by localization of cryptdin-5 away from the crypt base (Fig. 3D), and co-localization of cryptdin-5 and macrophages demonstrated that secreted cryptdin-5 was taken up by macrophages.
We also show that mice subjected to AKI after macrophage depletion, achieved with clodronate liposome injection 48 hrs prior, showed drastically less detectable cryptdin-5 protein localizing away from the crypt base and marked depletion of cryptdin-5 co-localization with macrophages (Fig. 3E) relative to control mice. Finally, macrophage depleted mice had reduced portal venous cryptdin-1 protein compared to control-liposome injected mice after AKI (Fig. 3F). Taken together, these studies suggest that small intestinal macrophages mediate, at least in part, portal delivery of Paneth cell products after AKI.
Paneth cells release IL-17A to cause multi-organ dysfunction after AKI
To test whether Paneth cells are the small intestinal source of increased IL-17A after AKI, Paneth cell IL-17A mRNA levels were measured in laser capture microdissected crypts (Supplementary Fig. 2A). RNA recoveries from LCM crypts were sufficient for both conventional (Supplementary Fig. 2B) and real time qRT-PCR and showed that IL-17A mRNA levels increased 8+2-fold after bilateral nephrectomy (N=4, P<0.01). IL-17A ELISA performed on isolated crypts (Supplementary Fig. 3A and inset) showed that IL-17A protein levels were increased 8 to 10-fold after bilateral nephrectomy and 30 min. of renal IR (Supplementary Fig. 3B). Measurements of IL-17A mRNA levels in isolated crypts by qRT-PCR were consistent with IL-17A protein levels, increasing approximately 8-fold 5 hrs after bilateral nephrectomy or 30 min. of renal IR (Supplementary Fig. 3C).
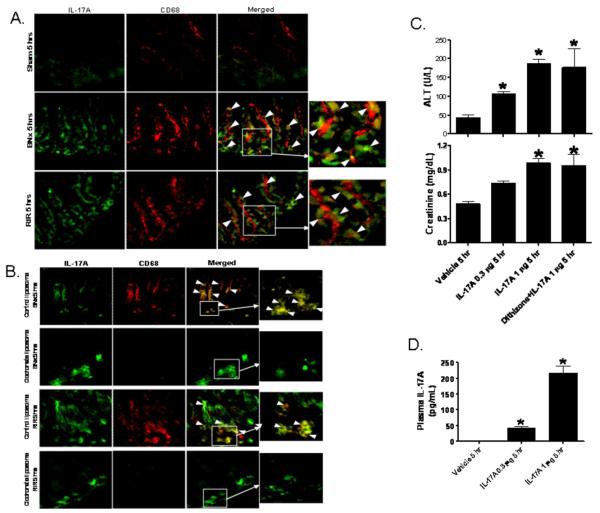
We also performed small intestine immunofluorescence staining with IL-17A (green) and macrophage (CD68, red) antibodies. Five hrs after AKI (renal IR or bilateral nephrectomy), IL-17A immunoreactivity increased in small intestinal crypts and we observed that IL-17A and CD68 co-stain (yellow, arrow heads) demonstrating that macrophages takes up IL-17A released form the Paneth cells (Fig. 4A). Mice subjected to AKI after macrophage depletion (with clodronate liposome injection) showed increased IL-17A expression in intestinal crypts compared to sham-operated mice. However, macrophage depleted mice had markedly less cryptdin-5 protein expression in intestinal epithelia away from base (green) and near complete loss of co-localization of IL-17A and macrophages (yellow, arrowheads, Fig 4B) compared to control liposome injected mice.
Figure 4. Intestinal macrophages take up Paneth cell-derived IL-17A to cause multi-organ dysfunction after AKI.
A. Representative (of 4 independent experiments, 200X magnification and enlarged insert of 600X magnification) photographs of immunofluorescence stain for IL-17A (green) and CD68 (a macrophage specific marker, red) in small intestine of mice subjected to sham-operation (Sham), BNx or renal ischemia reperfusion (RIR). Five hrs after acute kidney injury, IL-17A immunoreactivity increased in small intestinal crypts and we show that IL-17A and CD68 co-stain (yellow, arrow heads) demonstrating that macrophages takes up IL-17A released from the Paneth cells. B. Representative (of 4 independent experiments, 200X magnification and enlarged insert of 600X magnification) immunofluorescence staining for IL-17A (green) and CD68 (red) in small intestine of mice subjected to BNx or RIR with (control liposome injected) or with (clodronate liposome injected) macrophage depletion. Mice were injected with liposomes i.p. 48 hrs before sham operation or AKI. Note that although Paneth cell IL-17A is increased after AKI in crypts after macrophage depletion, IL-17A no longer migrates away from the crypt base. C and D. Recombinant murine IL-17A (0.3 or 1 μg per mouse, iv, N=4 each) recapitulates hepatic, renal and intestinal injury in mice. C. IL-17A injection causes dose-dependent liver (ALT) and renal (creatinine) injury. IL-17A (1 μg per mouse) also caused similar degree of liver and kidney injury in mice treated with dithizone (100 mg/kg, i.v., 6 hrs prior to IL-17A injection). D. IL-17A injection (1 μg) mimics plasma IL-17A levels achieved after acute kidney injury. Plasma samples were analyzed 5 hrs after IL-17A injection. *P<0.05 vs. vehicle-treated mice.
Recombinant murine IL-17A recapitulates multi-organ injury observed after AKI. Administration of recombinant murine IL-17A at intravenous dosages of 0.3 or 1 μg per mouse induced dose-dependent hepatic and renal injury within 5 hrs after injection (Fig. 4C). In mice receiving 1 μg of IL-17A by i.v. injection, plasma IL-17A levels were similar to those induced by AKI (Fig. 4D), showing that circulating IL-17A was the same whether of enteric origin or administered parenterally. In addition, when IL-17A deficient mice were subjected to renal IR or bilateral nephrectomy, the extent of Paneth cell degranulation was similar to wild-type mice, showing that activation of secretion was independent of the cytokine (Supplementary Fig. 4A). Histologically, administration of exogenous recombinant murine IL-17A recapitulated the hepatic, renal, and small intestinal injuries of the bilateral nephrectomy-induced renal failure (Supplementary Fig. 4B-D), thus supporting the conclusion that Paneth cell-derived IL-17A mediates extra-renal remote organ injury after AKI. Furthermore, IL-17A continues to recapitulate hepatic and renal injury in dithizone-treated Paneth cell granule depleted mice (Figure 4C) supporting the conclusion that IL-17A causes liver and kidney injury independently of Paneth cells.
Paneth cell deficient mice are protected from remote organ injury after renal IR or bilateral nephrectomy
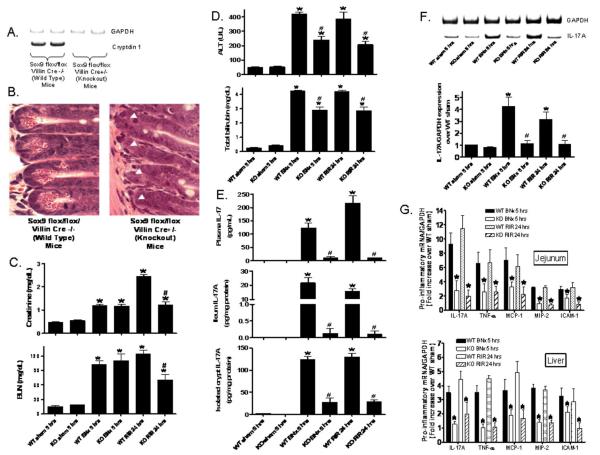
To test whether Paneth cell secretory products are required for hepatic injury induced by AKI, responses in mice genetically deficient in the Paneth cell lineage (14) were investigated. Paneth cell deficiency characteristic of Sox9 flox/flox/ Villin Cre+/− mice is apparent from their lack of cryptdin-1 mRNA (Fig 5A) and from the absence of characteristic Paneth cell secretory granules crypts of these knockout mice (Fig. 5B). Renal IR injury led to a significant and graded rise in serum BUN and creatinine in Sox9 flox/flox/ Villin Cre−/− control mice relative to sham-operated animals at 24 hrs as did bilateral nephrectomy 5 hrs after injury (Fig. 5C). The plasma creatinine 24 hrs after renal IR rose higher than the plasma creatinine w5 hrs after bilateral nephrectomy. Also, acute hepatic dysfunction also developed after renal IR or bilateral nephrectomy injury as shown by significantly elevated plasma ALT and bilirubin levels (Fig. 5D). However, Paneth cell deficient mice were protected against both hepatic and renal injury caused by renal IR (Fig. 5C, D) and from hepatic injury after bilateral nephrectomy as evident from the lower plasma ALT levels (Fig. 5D). Due to the complete absence of renal function in the anephric state, plasma creatinine remained high in the Sox9 deficient state. Sox9 deficient mice had 10-20 fold lower IL-17A protein levels in plasma, ~160-fold lower levels in ileum, and 4-5 fold lower levels in isolated crypts after bilateral nephrectomy or induction of renal IR (Fig. 5E). Also, Paneth cell deficiency in Sox9 null intestine reduced IL-17A mRNA levels in isolated crypts in response to AKI relative to control mice (Fig. 5F). Finally, mice with conditional Sox9 deficiency had lower mRNA levels for TNF-α, IL-17A, MCP-1, MIP-2 and ICAM-1 in jejunum, ileum, and liver (Figure 5G, jejunum and liver shown).
Figure 5. Paneth cell deficiency with intestinal specific Sox9 deletion.
Sox9 flox/flox Villin Cre+/− mice are deficient in Paneth cell marker (cryptdin-1 mRNA, A) and in Paneth cells (B, magnification 1000X) compared to wild type (Sox9 flox/flox Villin Cre−/−) mice. Arrow heads represents complete lack of Paneth cells in Sox9 flox/flox Villin Cre+/− mice. C. Paneth cell in Sox9 flox/flox Villin Cre+/− (KO) mice protects against acute kidney injury (creatinine and blood urea nitrogen) after RIR. D. Paneth cell deficiency also protects against hepatic injury (ALT and total bilirubin) after ischemic acute kidney injury (RIR) or bilateral nephrectomy (BNx) compared to Sox9 flox/flox Villin Cre−/− (WT) mice. Mice were subjected to sham-operation (Sham, N=4), BNx (N=5) or 30 min. RIR (N=5). Plasma was collected 5 hrs after BNx and 24 hrs after RIR. E. Paneth cell deficiency in Sox9 flox/flox Villin Cre+/− (KO) mice reduces plasma (N=4), small intestine (ileum shown, N=4) and isolated crypts (N=4) IL-17A levels in mice subjected to acute kidney injury (BNx and RIR). Plasma, small intestine and isolated crypt samples were analyzed 5 hrs after acute kidney injury. F. Paneth cell deficiency in Sox9 flox/flox Villin Cre+/− (KO) mice reduces IL-17A mRNA levels in isolated crypts after acute kidney injury. Small intestinal crypts were isolated 5 hrs after sham-operation (Sham, N=4), 5 hrs after BNx (N=4) or 24 hrs after 30 min. RIR (N=4). *P<0.05 vs. sham-operated mice. #P<0.05 vs. WT mice subjected to acute kidney injury. G. Paneth cell deficiency in Sox9 flox/flox Villin Cre+/− (KO) mice reduces pro-inflammatory mRNA expression (IL-17A, TNF-α, MCP-1, MIP-2 and ICAM-1) in the liver and jejunum after acute kidney injury. Tissues were extracted 5 hrs after BNx (N=4) or 24 hrs after 30 min. RIR (N=4). *P<0.05 vs. WT mice subjected to acute kidney injury. Error bars represent 1 SEM.
Pharmacological depletion of Paneth cells attenuates remote organ injury after renal IR or bilateral nephrectomy
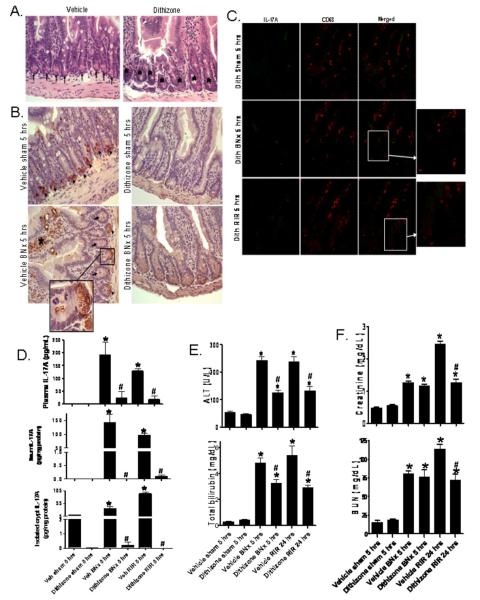
To complement the studies in mice genetically deficient in the Paneth cells, we depleted Paneth cell granules pharmacologically. Zinc depletion with dithizone treatment rapidly depletes mouse Paneth cells of their secretory granules (23,24). Secretory granules are evident and abundant in ileal Paneth cells from vehicle (lithium carbonate)-treated mice (Fig. 6A, left panel, arrows). In contrast, dithizone administration to mice almost completely depleted ileal Paneth cells of their granules within 6 hrs of dithizone exposure (Fig. 6A, right panel, *). Lysozyme immunoreactivity was strong in crypt Paneth cells (arrows, Fig. 6B) of mice treated with Li2CO3 vehicle, and dithizone treatment eliminated lysozyme staining in Paneth cells due to granule depletion (arrow heads). In addition, crypt lysozyme staining was reduced after bilateral nephrectomy compared to sham operated mice. In mice subjected to bilateral nephrectomy after vehicle treatment, lysozyme was released by Paneth cells into the crypt lumen (enlarged insert) and villous lysozyme staining was evident (*). Most importantly, dithizone-induced Paneth cell granule depletion profoundly reduced crypt IL-17A production after AKI (Fig. 6C).
Figure 6. Paneth cell granule depletion with dithizone treatment.
A. Representative H&E (of 6 experiments, magnification 400X) of ileum from mice treated with vehicle (Li2CO3) or with dithizone 6 hrs prior. Note complete depletion of Paneth cell granules (arrows) after dithizone treatment (*). B. Representative (of 5 experiments, 400X) ileum lysozyme immunostaining. Note heavy lysozyme stain in Paneth cells (arrow heads) of mice treated with vehicle (Li2CO3). Paneth cell depletion with dithizone treatment decreased lysozyme staining in Paneth cells. In addition, lysozyme staining in Paneth cells was reduced after bilateral nephrectomy (BNx) compared to sham operated mice (Sham) and we were able to detect lysozyme staining in crypt lumen (arrows and enlarged insert of 2000X magnification). Villous lysozyme staining was also evident (*). C. Representative (of 3 experiments, 200X magnification and enlarged insert, 600X magnification) immunofluorescence stain for IL-17A (green) and CD68 (red) in small intestine of mice treated with dithizone (100 mg/kg, i.v. 6 hrs prior to renal ischemia or nephrectomy) and subjected to sham-operation, BNx or renal ischemia reperfusion (RIR). Paneth cell granule depletion reduces crypt IL-17A production after AKI. D. Dithizone treatment reduces plasma IL-17A levels (analyzed 5 hrs after acute kidney injury) in mice subjected to acute kidney injury (BNx or 30 min. RIR, N=4 each). Dithizone treatment also reduced IL-17A protein upregulation in small intestine (N=4) and in isolated crypts (N=4) 5 hrs after acute kidney injury. *P<0.05 vs. sham-operated mice. #P<0.05 vs. mice subjected to vehicle treated animals subjected to acute kidney injury. Error bars represent 1 SEM. E. Paneth cell granule depletion with dithizone treatment protects against hepatic injury after ischemic acute kidney injury (RIR) or bilateral nephrectomy (BNx). Mice were subjected to sham-operation (vehicle (veh) or dithizone Sham, N=4), BNx (N=6) or 30 min. RIR (N=6). Plasma was collected 5 hrs after BNx and 24 hrs after RIR. F. Paneth cell granule depletion with dithizone treatment also protects against acute kidney injury (creatinine and blood urea nitrogen) after RIR.
Treating mice with dithizone reduced plasma IL-17A levels induced by renal IR or bilateral nephrectomy by approximately 10-fold (Fig. 6D). Furthermore, dithizone granule depletion drastically reduced IL-17A protein levels in the small intestine (ileum) and in isolated crypts 5 hrs after induction of renal IR or bilateral nephrectomy. Depletion of Paneth cell granules with dithizone improved liver function after bilateral nephrectomy or 30 min. of renal IR. Dithizone treatment also improved renal function after renal IR (Fig. 6F).
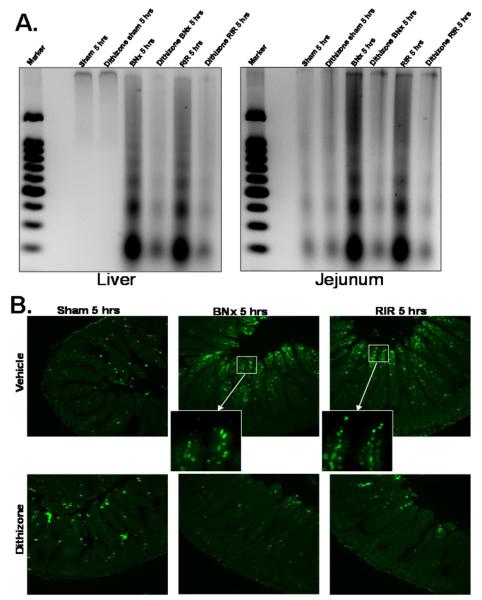
Depletion of Paneth cell granules with dithizone attenuated liver and small intestine apoptosis (reflected in decreased DNA laddering) after bilateral nephrectomy or renal IR (Fig. 7A). This reduced intestinal apoptosis was confirmed by TUNEL assays. Both renal IR and bilateral nephrectomy resulted in rapid intestinal villous apoptosis, predominantly in endothelial cells within 5 hrs and Paneth cell depletion reduced intestinal cell apoptosis as determined by this assay as well (Fig. 7B). We confirmed that the TUNEL positive cells in intestinal vili are endothelial cells by staining jejunum serial sections with TUNEL and CD34 (an endothelial cell marker, Abcam Inc., Cambridge, MA) and confirmed that TUNEL positive cells also stained for CD34 (data not ahown). Furthermore, depletion of Paneth cells with dithizone did not induce Paneth cell or small intestinal crypt apoptosis.
Figure 7. Liver and small intestine (jejunum shown) apoptosis after ischemic acute kidney injury (RIR) or bilateral nephrectomy (BNx).
A. Representative gel images (of 4 experiments) demonstrating DNA laddering as an index of DNA fragmentation in the liver and jejunum from sham-operated mice (sham), mice subjected to BNx or to RIR. Tissues were harvested 5 hrs after surgery. Paneth cell granule depletion with dithizone treatment reduces liver and small intestine (jejunum shown) apoptosis after RIR or BNx. Apoptotic DNA fragments were extracted according to the methods of Herrmann et al. (29). This method of DNA extraction selectively isolates apoptotic, fragmented DNA and leaves behind the intact chromatin. B. Representative photomicrographs of TUNEL staining in small intestine (jejunum, magnification 100X) sections. Six hrs after vehicle (Li2CO3) or dithizone treatment, mice were subjected to sham operation (sham), to BNx or to RIR. Enlarged inserts (600X magnification) show that majority of TUNEL positive cells are villous capillary endothelial cells. Dithizone treatment did not induce Paneth cell or crypt apoptosis. Photographs are representative of 4 independent experiments.
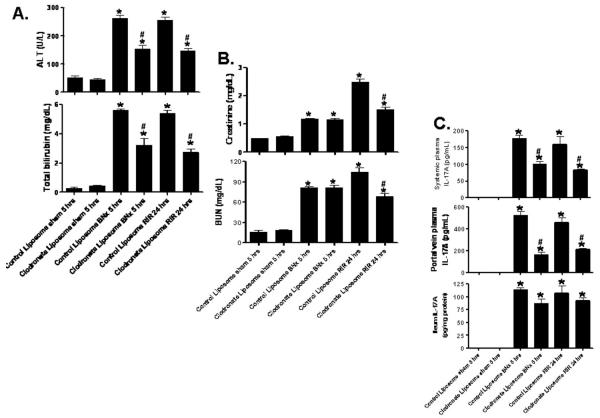
Macrophage depletion attenuates hepatic injury and portal and systemic IL-17A delivery after renal IR or bilateral nephrectomy
Mice injected with clodronate liposome 48 hrs prior and subjected to renal IR or bilateral nephrectomy were protected against hepatic injury after AKI (Fig. 8A). Macrophage depletion also protected against renal IR injury (Fig. 8B). In addition, macrophage depleted mice also had significantly less systemic and portal plasma IL-17A levels compared to control liposome-treated mice after AKI (Fig. 8C). However, IL-17A in the ileum did not change significantly in clodronate liposome-treated mice suggesting that macrophage depletion reduces portal and systemic delivery without affecting the synthesis of Paneth cell-derived IL-17A.
Figure 8. Macrophage depletion with clodronate liposome.
Mice were subjected to sham-operation (N=4), bilateral nephrectomy (BNx, N=5) or ischemic acute kidney injury (RIR, N=5) after control liposome or clodronate liposome injection. Mice were injected with liposomes i.p. 48 hrs before sham operation or AKI. Plasma was collected 5 hrs after BNx and 24 hrs after RIR. A. Macrophage depletion protects against hepatic injury (ALT and bilirubin) after RIR or BNx. B. Macrophage depletion also protects against acute kidney injury (creatinine and blood urea nitrogen) after RIR. C. Macrophage depletion reduces systemic and portal vein plasma IL-17A levels without decreasing ileum IL-17A levels in mice subjected to acute kidney injury (BNx or 30 min. RIR, N=4 each, analyzed 5 hrs after acute kidney injury). *P<0.05 vs. sham-operated mice. #P<0.05 vs. control liposome injected mice subjected to acute kidney injury. Error bars represent 1 SEM.
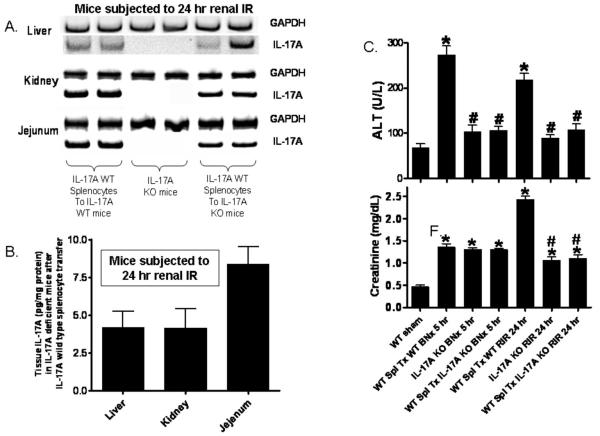
Leukocyte IL-17A is not required extra-renal remote organ injury after AKI
We initially determined whether IL-17A wild type splenocytes injected into IL-17A deficient mice released IL-17A. We were able to detect IL-17A mRNA by RT-PCR in the liver, kidney and small intestines of IL-17A deficient mice after IL-17A wild type splenocyte injection (Fig. 9A). Furthermore, we were able to measure IL-17A protein (with ELISA) in plasma (152+41 pg/mL, N=5) and tissues (Fig. 9B) of IL-17A deficient mice subjected to renal IR after splenocyte injection. Importantly, IL-17A deficient mice transfused with wild type splenocytes 24 hrs before renal IR or bilateral nephrectomy were still protected against hepatic and renal injury after AKI (Fig. 9C). Wild type mice transfused with wild type splenocytes did not alter liver and kidney injury after AKI. These findings suggest that leukocyte IL-17A does not contribute to hepatic and renal injury after AKI.
Figure 9. Successful reconstitution of IL-17A in IL-17A deficient mice.
A. Detection of IL-17A mRNA by RT-PCR in the liver, kidney and small intestines of IL-17A deficient mice after IL-17A wild type splenocyte injection (representative of 4 experiments). Mice were subjected to renal ischemia and 24 hrs reperfusion. B. Detection of IL-17A protein with ELISA in the liver, kidney and small jejunum of IL-17A deficient mice after IL-17A wild type splenocyte injection (N=4). Mice were subjected to renal ischemia and 24 hrs reperfusion. C. Splenocyte IL-17A does not contribute to remote organ injury after AKI. IL-17A deficient mice transfused with wild type splenocytes were still protected against hepatic and renal injury after ischemic AKI (RIR) and bilateral nephrectomy (BNx). Mice were subjected to sham-operation (Sham, N=4), BNx (N=5) or 30 min. RIR (N=5). Plasma was collected 5 hrs after BNx and 24 hrs after RIR. *P<0.05 vs. sham-operated mice. #P<0.05 vs. mice subjected to acute kidney injury after transfused with wild type splenocytes.
Discussion
AKI is a common complication during the peri-operative period and is a strong, independent predictor of mortality (2). Unfortunately, current clinical management of AKI is limited to supportive measures (e.g. hemodialysis). One of the major reasons for difficulties in developing treatments for AKI is that patients with AKI frequently develop extra-renal organ dysfunction (4). For example, patients who develop AKI in the ICU frequently suffer from respiratory, hepatic and intestinal barrier dysfunction (7,32,33). Indeed, initiation or exacerbation of remote organ injury in patients suffering from AKI leads to a vicious cycle of organ injury and contributes significantly to mortality and morbidity (32). Therefore, a better understanding of the mechanisms of remote organ injury due to AKI would lead to improved therapy for patients suffering from AKI.
Our current studies suggest that small intestinal Paneth cells generate pro-inflammatory cytokine IL-17A after ischemic AKI or bilateral nephrectomy. IL-17A release was originally characterized from Th17 CD4+ T cells (34,35). Subsequent studies have demonstrated that other cell types including CD3+ invariant NK T- cells, myeloid cells, neutrophils as well as Paneth cells can produce IL-17A in response to pathogenic stimuli (36). Our previous (10) and current studies enabled us to implicate Paneth cell-derived pro-inflammatory IL-17A to directly cause multi-organ injury after ischemic AKI or bilateral nephrectomy. We previously demonstrated that ischemic AKI or bilateral nephrectomy caused a rapid release of small intestinal IL-17A and led to intestinal injury with subsequent hepatic dysfunction and systemic release of TNF-α and IL-6 (10). We also demonstrated the critical role of IL-17A release in response to AKI as neutralization of IL-17A or deficiency in IL-17A blocked systemic inflammation and remote organ injury after ischemic AKI or bilateral nephrectomy (10). In this study, we show that Paneth cell depletion via pharmacological or genetic approaches significantly reduced induction of IL-17A and attenuated multi-organ injury after AKI.
We ruled out the leukocyte and myeloid source of IL-17A based on the following experimental data. Using a calcium chelation technique, we were able to isolate individual crypts containing Paneth cells to show that these crypts show increased IL-17A mRNA and protein after ischemic AKI and bilateral nephrectomy. These isolated crypts are free of leukocytes as well as cells of myeloid origin. Since isolated crypts also contain stem cells and transition cells in addition to Paneth cells, we performed LCM to specifically capture Paneth cells to confirm increased expression of IL-17A mRNA in these cells.
Taken together, our collective findings suggest that Paneth cell-derived IL-17A is critical for inducing remote organ injury after AKI. We further propose that both ischemic AKI and bilateral nephrectomy induces production of IL-17A in Paneth cells rapidly promoting the increased production of additional (TNF-α and IL-6) cytokine generation, intestinal apoptosis and hepatic injury. The unique position of IL-17A as a regulator of both innate and acquired immunity makes this cytokine a crucial signal for the reinforcement and crosstalk of host defense systems. The mechanisms leading to Paneth cell degranulation and increased Paneth cell-derived IL-17A after renal IR or bilateral nephrectomy remain to be determined. AKI due to IR or nephrectomy causes systemic inflammation, increased oxidant stress and toll like receptor (TLR) signaling (8,32,37). Moreover, TLR-mediated Paneth cell degranulation has been described (38,39).
We were able to deplete small intestinal Paneth cell granules with pharmacological (with dithizone treatment) or genetic (with SOX9/Villin Cre mice) approaches. Dithizone, a zinc chelator, has been shown to deplete Paneth cell granules in adult mice and rats (23,24). Although our TUNEL data demonstrate that dithizone did not induce small intestinal Paneth cell apoptosis, the use of dithizone may be limited by systemic and non-specific side effects (e.g., effects on beta-cells, pulmonary toxicity) especially at high doses and Paneth cell depletion is transient (with complete re-population of Paneth cell granules within 12-24 hrs after injection). Therefore, we complemented the dithizone studies with studies in SOX9/Villin Cre+/− mice. These mice selectively lack the Sox9 transcription factor in intestinal epithelial cells and as a result show absent or significantly reduced number of mature Paneth cells in adult mice (14,40). These approaches of Paneth cell depletion allowed us to conclude that Paneth cells are critical in generating hepatic injury and small intestinal IL-17A generation in mice after AKI. Furthermore, we propose that reduction in Paneth cell IL-17A removed the key cytokine involved in causing multi-organ vascular impairment. Improved vascular integrity would lead to less leukocyte infiltration and cytokine generation and subsequently reduced epithelial necrosis, apoptosis and inflammation.
Paneth cells are critical in regulating the precarious balance between intestinal bacteria and crypt sterility required for the host (41-45). Indeed, small intestinal Paneth cells provide critical mucosal innate immunity against pathogens and can actively secrete several antimicrobial peptides (e.g., lysozyme, α-defensins and cryptdin-related peptides) (46-48). Paneth cells are also known to express transcripts for several pro-inflammatory molecules such as TNF-α, inducible NO synthase, GM-CSF as well as IL-17A (24,49). α-Defensins are important regulators of small intestinal bacterial flora composition and intestinal bacterial flora modulate intestinal Th17 cell differentiation (50,51). Therefore, although the Paneth cells (with ability to kill bacteria and release pro-inflammatory mediators) are essential barriers providing mucosal and innate immunity (44,52), we propose that their dysregulation and overproduction of IL-17A after AKI leads to a systemic inflammatory syndrome and causes extra-renal organ dysfunction. In contrast, previous studies suggest that chronic loss of Paneth cell α-defensin expression could skew mucosal responses towards a pro-inflammatory phenotype (51). Indeed, disruption of Paneth cells homeostasis has been implicated in the pathogenesis of several inflammatory bowl diseases including Crohn’s and necrotizing entericolitis (53). In small intestinal Crohn’s disease, Paneth cell products are significantly reduced and ileal extracts collected from patients with Crohn’s disease have reduced capacity to clear bacteria (54). We propose that acute depletion of Paneth cells (with acute reduction of Paneth cell-derived IL-17A after AKI leading to multi-organ protection) have a very different effect compared to chronic depletion (bacterial floral changes, IL-17A induction and increased chronic intestinal inflammation).
Our studies provide potential a mechanism for systemic absorption of Paneth cell granules released after AKI. We show in this study that intestinal macrophages ingest Paneth cell granules and transport them towards the intestinal epithelial villi promoting portal and systemic absorption of Paneth cell granules. Our studies further expand and are consistent with the findings demonstrated previously (6). Kramer et al. demonstrated that renal IR injury in rats cause lung injury and alter pulmonary vascular permeability via macrophage-derived inflammatory products. Taken together, we propose that macrophages play a major role in mediating remote organ injury after renal IR. Furthermore, we propose that protective effects of macrophage depletion in renal IR (55) most likely involve direct (attenuation of renal inflammation by macrophages) and indirect (reduced intestinal uptake of Paneth cell-derived IL-17A by macrophages) mechanisms. However, our current data cannot rule out other potential mechanisms of portal delivery of Paneth cell products including basolateral paracellular transcytosis.
Previous study by Li et al. proposes that innate immune component of kidney IR requires neutrophil-derived IL-17A activation of IL-12/IFN-γ signaling pathways in mice (56). Their bone marrow chimera studies indicate that myeloid cell-derived IL-17A play a major role in generating renal IR injury. However, we find that IL-17A wild type mouse splenocyte transfusion did not exacerbate renal and hepatic injury after AKI in IL-17A KO mice. Therefore, our studies suggest that remote organ injury to the liver and intestine is dependent on Paneth cell-derived IL-17A production. However, although our studies suggest that splenocytes are not an important source of IL-17A, we cannot exclude a role for neutrophil-derived IL-17A as proposed by Li et al. (56).
Although pharmacological or genetic Paneth cell granule depletion resulted in significant hepatic protection after bilateral nephrectomy and hepatic and renal protection after renal IR, the protective responses observed were partial - Paneth cell depleted or deleted mice still had increased liver and kidney injury compared to sham-operated mice. It is possible that Paneth cell depletion or deletion was incomplete in our study. It is also possible that in addition to the Paneth cell-derived IL-17A, other cell types including leukocytes or epithelial cells may generate IL-17A and additional cytokines in response to AKI (35,36). Our previous study suggests that increased IL-17A after AKI originates in the small intestine with subsequent hepatic and systemic induction of IL-6 and TNF-α (10). We also previously determined that blockade of one cytokine was sufficient to attenuate hepatic and small intestinal injury and circulating levels of other cytokines after AKI. These findings suggest that the generation of cytokines after injury is not a redundant process, but rather individual cytokine (e.g., IL-17A) may generate another cytokine (e.g., TNF-α).
In summary, we propose that the small intestinal Paneth cell generation of IL-17A leads to intestinal and hepatic injury and subsequent generation of TNF-α and IL-6 further potentiating renal injury and systemic inflammatory responses. Small intestinal Paneth cells may initiate the cascade of multi-organ injury by altering enteric innate immunity in response to ischemic AKI or bilateral nephrectomy. Modulation of Paneth cell dysregulation may have important therapeutic implications in reducing systemic complications arising from AKI. Future studies will address the mechanisms of AKI-induced Paneth cell degranulation and Paneth cell IL-17A induction.
Supplementary Material
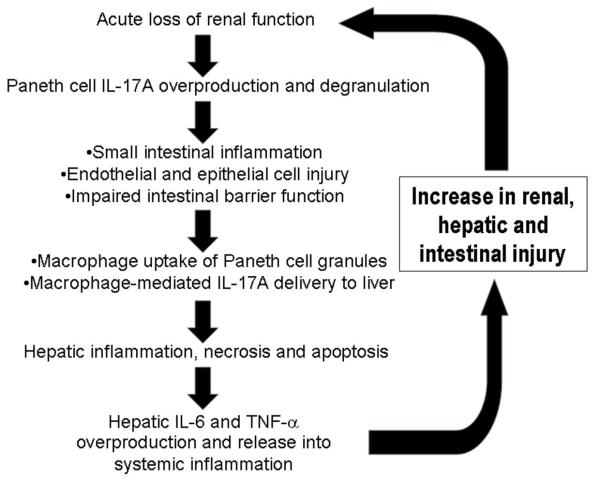
Figure 10. Proposed mechanisms of acute kidney injury induced liver dysfunction and systemic inflammation.
Acute loss of renal function causes small intestinal Paneth cell generation of IL-17A and Paneth cell degranulation. We propos that IL-17A released by Paneth cells directly cause intestinal injury. Our data suggest that intestinal macrophages uptake of released Paneth cell granules promote portal delivery of IL-17A. This leads to hepatic injury (necrosis, inflammation and apoptosis) and increased generation and systemic release of TNF-α and IL-6 propagating multi-organ injury and systemic inflammation. The mechanisms that cause Paneth cells to produce increased IL-17A and degranulate after AKI remain to be determined.
Acknowledgments
Funding: This work was supported by National Institute of Health Grant RO1 DK-58547.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Author Contributions: SWP, MK, AH, JYK and KMB performed experiments and analyzed data. AJO and YMA gave scientific advice and contributed to the manuscript. VDD performed the studies, gave scientific advice and contributed to the manuscript. HTL conceived the project, designed the studies, performed experiments and wrote the manuscript.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005;16:3365. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Jones DR, Lee HT. Perioperative renal protection. Best. Pract. Res. Clin. Anaesthesiol. 2008;22:193. doi: 10.1016/j.bpa.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Elapavaluru S, Kellum JA. Why do patients die of acute kidney injury? Acta Clin. Belg. 2007;(Suppl):326. doi: 10.1179/acb.2007.074. [DOI] [PubMed] [Google Scholar]
- 4.Paladino JD, Hotchkiss JR, Rabb H. Acute kidney injury and lung dysfunction: a paradigm for remote organ effects of kidney disease? Microvasc. Res. 2009;77:8. doi: 10.1016/j.mvr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J. Am. Soc. Nephrol. 2008;19:547. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55:2362. doi: 10.1046/j.1523-1755.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- 7.Faubel S. Pulmonary complications after acute kidney injury. Adv. Chronic. Kidney Dis. 2008;15:284. doi: 10.1053/j.ackd.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Golab F, Kadkhodaee M, Zahmatkesh M, Hedayati M, Arab H, Schuster R, Zahedi K, Lentsch AB, Soleimani M. Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney Int. 2009;75:783. doi: 10.1038/ki.2008.683. [DOI] [PubMed] [Google Scholar]
- 9.Serteser M, Koken T, Kahraman A, Yilmaz K, Akbulut G, Dilek ON. Changes in hepatic TNF-alpha levels, antioxidant status, and oxidation products after renal ischemia/reperfusion injury in mice. J. Surg. Res. 2002;107:234. doi: 10.1006/jsre.2002.6513. [DOI] [PubMed] [Google Scholar]
- 10.Park SW, Chen SW, Kim M, Brown KM, Kolls JK, D’Agati VD, Lee HT. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest. 2011;91:63. doi: 10.1038/labinvest.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi N, Vanlaere I, de RR, Cauwels A, Joosten LA, Lubberts E, van den Berg WB, Libert C. IL-17 produced by Paneth cells drives TNF-induced shock. J. Exp. Med. 2008;205:1755. doi: 10.1084/jem.20080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SW, Kim M, Brown KM, D’Agati VD, Lee HT. Paneth cell-derived IL-17A causes multi-organ dysfunction after hepatic ischemia and reperfusion injury. Hepatology. 2011;53:1662. doi: 10.1002/hep.24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grootjans J, Hodin CM, de Haan JJ, Derikx JP, Rouschop KM, Verheyen FK, van Dam RM, Dejong CH, Buurman WA, Lenaerts K. Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology. 2011;140:529. doi: 10.1053/j.gastro.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Mori-Akiyama Y, van den BM, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de CB. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J. Am. Soc. Nephrol. 2004;15:102. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- 16.Park SW, Chen SW, Kim M, D’Agati VD, Lee HT. Human heat shock protein 27 overexpressing mice are protected against acute kidney injury after hepatic ischemia and reperfusion. Am. J. Physiol Renal Physiol. 2009;297:F885–F894. doi: 10.1152/ajprenal.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SW, Kim M, Chen SW, Brown KM, D’Agati VD, Lee HT. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P(1) receptor activation. Lab Invest. 2010;90:1209. doi: 10.1038/labinvest.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SW, Kim M, Chen SW, D’Agati VD, Lee HT. Sphinganine-1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock. 2010;33:31. doi: 10.1097/SHK.0b013e3181c02c1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van RN, Lawton MT, Young WL, Liang EI, Nuki Y, Hashimoto T. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173. doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nuki Y, Matsumoto MM, Tsang E, Young WL, van RN, Kurihara C, Hashimoto T. Roles of macrophages in flow-induced outward vascular remodeling. J. Cereb. Blood Flow Metab. 2009;29:495. doi: 10.1038/jcbfm.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HT, Park SW, Kim M, D’Agati VD. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab Invest. 2009;89:196. doi: 10.1038/labinvest.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HT, Kim M, Jan M, Penn RB, Emala CW. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int. 2007;71(12):1249. doi: 10.1038/sj.ki.5002227. [DOI] [PubMed] [Google Scholar]
- 23.Sawada M, Takahashi K, Sawada S, Midorikawa O. Selective killing of Paneth cells by intravenous administration of dithizone in rats. Int. J. Exp. Pathol. 1991;72:407. [PMC free article] [PubMed] [Google Scholar]
- 24.Seno H, Sawada M, Fukuzawa H, Morita-Fujisawa Y, Takaishi S, Hiai H, Chiba T. Involvement of tumor necrosis factor alpha in intestinal epithelial cell proliferation following Paneth cell destruction. Scand. J. Gastroenterol. 2002;37:154. doi: 10.1080/003655202753416803. [DOI] [PubMed] [Google Scholar]
- 25.Ouellette AJ, Miller SI, Henschen AH, Selsted ME. Purification and primary structure of murine cryptdin-1, a Paneth cell defensin. FEBS Lett. 1992;304:146. doi: 10.1016/0014-5793(92)80606-h. [DOI] [PubMed] [Google Scholar]
- 26.Chen SW, Kim M, Kim M, Song JH, Park SW, Wells D, Brown K, Belleroche JD, D’Agati VD, Lee HT. Mice that overexpress human heat shock protein 27 have increased renal injury following ischemia reperfusion. Kidney Int. 2008;75:499. doi: 10.1038/ki.2008.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SW, Chen SW, Kim M, D’Agati VD, Lee HT. Human activated protein C attenuates both hepatic and renal injury caused by hepatic ischemia and reperfusion injury in mice. Kidney Int. 2009;76:739. doi: 10.1038/ki.2009.255. [DOI] [PubMed] [Google Scholar]
- 28.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr., Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am. J. Physiol Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann M, Lorenz HM, Voll R, Grunke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanji N, Ross MD, Cara A, Markowitz GS, Klotman PE, D’Agati VD. Effect of tissue processing on the ability to recover nucleic acid from specific renal tissue compartments by laser capture microdissection. Exp. Nephrol. 2001;9:229. doi: 10.1159/000052616. [DOI] [PubMed] [Google Scholar]
- 31.Traber PG, Gumucio DL, Wang W. Isolation of intestinal epithelial cells for the study of differential gene expression along the crypt-villus axis. Am. J. Physiol. 1991;260:G895–G903. doi: 10.1152/ajpgi.1991.260.6.G895. [DOI] [PubMed] [Google Scholar]
- 32.Faubel S. Acute kidney injury and multiple organ dysfunction sindrome. Minerva Urol. Nefrol. 2009;61:171. [PubMed] [Google Scholar]
- 33.Hassoun H, Grigoryev DN, Lie M, Liu M, Cheadle C, Tuder RM, Rabb H. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am. J. Physiol Renal Physiol. 2007;293:F30–F40. doi: 10.1152/ajprenal.00023.2007. [DOI] [PubMed] [Google Scholar]
- 34.Chang SH, Dong C. IL-17F: regulation, signaling and function in inflammation. Cytokine. 2009;46:7. doi: 10.1016/j.cyto.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaffen SL, Kramer JM, Yu JJ, Shen F. The IL-17 cytokine family. Vitam. Horm. 2006;74:255. doi: 10.1016/S0083-6729(06)74010-9. [DOI] [PubMed] [Google Scholar]
- 36.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 37.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008;74:901. doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 38.Foureau DM, Mielcarz DW, Menard LC, Schulthess J, Werts C, Vasseur V, Ryffel B, Kasper LH, Buzoni-Gatel D. TLR9-dependent induction of intestinal alpha-defensins by Toxoplasma gondii. J. Immunol. 2010;184:7022. doi: 10.4049/jimmunol.0901642. [DOI] [PubMed] [Google Scholar]
- 39.Rumio C, Besusso D, Palazzo M, Selleri S, Sfondrini L, Dubini F, Menard S, Balsari A. Degranulation of paneth cells via toll-like receptor 9. Am. J. Pathol. 2004;165:373. doi: 10.1016/S0002-9440(10)63304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J. Cell Biol. 2007;178:635. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke LL, Gawenis LR, Bradford EM, Judd LM, Boyle KT, Simpson JE, Shull GE, Tanabe H, Ouellette AJ, Franklin CL, Walker NM. Abnormal Paneth cell granule dissolution and compromised resistance to bacterial colonization in the intestine of CF mice. Am. J. Physiol Gastrointest. Liver Physiol. 2004;286:G1050–G1058. doi: 10.1152/ajpgi.00393.2003. [DOI] [PubMed] [Google Scholar]
- 42.Eisenhauer PB, Harwig SS, Lehrer RI. Cryptdins: antimicrobial defensins of the murine small intestine. Infect. Immun. 1992;60:3556. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koslowski MJ, Beisner J, Stange EF, Wehkamp J. Innate antimicrobial host defense in small intestinal Crohn’s disease. Int. J. Med. Microbiol. 2010;300:34. doi: 10.1016/j.ijmm.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Ouellette AJ. Defensin-mediated innate immunity in the small intestine. Best. Pract. Res. Clin. Gastroenterol. 2004;18:405. doi: 10.1016/j.bpg.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Ouellette AJ, Bevins CL. Paneth cell defensins and innate immunity of the small bowel. Inflamm. Bowel. Dis. 2001;7:43. doi: 10.1097/00054725-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Huttner KM, Selsted ME, Ouellette AJ. Structure and diversity of the murine cryptdin gene family. Genomics. 1994;19:448. doi: 10.1006/geno.1994.1093. [DOI] [PubMed] [Google Scholar]
- 47.Ouellette AJ, Hsieh MM, Nosek MT, Cano-Gauci DF, Huttner KM, Buick RN, Selsted ME. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect. Immun. 1994;62:5040. doi: 10.1128/iai.62.11.5040-5047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouellette AJ. Paneth cell alpha-defensin synthesis and function. Curr. Top. Microbiol. Immunol. 2006;306:1. doi: 10.1007/3-540-29916-5_1. [DOI] [PubMed] [Google Scholar]
- 49.Bultinck J, Sips P, Vakaet L, Brouckaert P, Cauwels A. Systemic NO production during (septic) shock depends on parenchymal and not on hematopoietic cells: in vivo iNOS expression pattern in (septic) shock. FASEB J. 2006;20:2363. doi: 10.1096/fj.06-5798fje. [DOI] [PubMed] [Google Scholar]
- 50.Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, Yu Y, Artis D. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J. Exp. Med. 2008;205:2191. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 2010;11:76. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouellette AJ. Paneth cell alpha-defensins: peptide mediators of innate immunity in the small intestine. Springer Semin. Immunopathol. 2005;27:133. doi: 10.1007/s00281-005-0202-x. [DOI] [PubMed] [Google Scholar]
- 53.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin. Immunol. 2007;19:70. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Wehkamp J, Wang G, Kubler I, Nuding S, Gregorieff A, Schnabel A, Kays RJ, Fellermann K, Burk O, Schwab M, Clevers H, Bevins CL, Stange EF. The Paneth cell alpha-defensin deficiency of ileal Crohn’s disease is linked to Wnt/Tcf-4. J. Immunol. 2007;179:3109. doi: 10.4049/jimmunol.179.5.3109. [DOI] [PubMed] [Google Scholar]
- 55.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 2011;22:317. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 2010;120:331. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.