Abstract
We have recently identified I602S as a frequent single nucleotide polymorphism of human TLR1 which greatly inhibits cell surface trafficking, confers hyporesponsiveness to TLR1 agonists, and protects against the mycobacterial diseases leprosy and tuberculosis. Since mycobacteria are known to manipulate the TLR system to their advantage, we hypothesize that the hyporesponsive 602S variant may confer protection by enabling the host to overcome this immune subversion. We report that primary human monocytes and macrophages from homozygous TLR1 602S individuals are resistant to mycobacterial-induced downregulation of macrophage MHCII, CD64, and IFNγ responses compared to individuals who harbor the TLR1 602I variant. Additionally, when challenged with mycobacterial agonists, macrophages from TLR1 602S/S individuals resist induction of host arginase-1; an enzyme that depletes cellular arginine stores required for production of antimicrobial reactive nitrogen intermediates. The differences in cell activation mediated by TLR1 602S and TLR1 602I are observed upon stimulation with soluble mycobacterial-derived agonists but not with whole mycobacterial cells. Taken together, these results suggest that the TLR1 602S variant protects against mycobacterial disease by preventing soluble mycobacterial products, perhaps released from granulomas, from disarming myeloid cells prior to their encounter with whole mycobacteria.
Keywords: TLR, Toll, I602S, mycobacteria, IFNγ
Introduction
Over the past several decades numerous mechanisms by which mycobacteria neutralize and even subvert host defenses have been uncovered (1). Mycobacteria employ strategies which counteract a large majority of not only innate host defenses, such as killing by macrophage phagocytosis and oxidative burst, but also adaptive responses including T cell activation and maturation of antigen presenting cells (2). One third of the world’s population is estimated to be infected with Mycobacterium tuberculosis. Non-tuberculous mycobacterial infections, such as those caused by M. leprae and M. avium complex species, are also important worldwide health concerns, with more than 200,000 new cases reported annually worldwide (3).
Pattern recognition receptors (PRRs) are essential to the production of proinflammatory cytokines and chemokines required for effective containment or clearance of invading mycobacteria (4, 5). Key PRRs include the ten-member family of Toll-like receptors (TLR) which serve as innate sensors of conserved microbial components, including nucleic acids and bacterial cell wall constituents (6). Although mycobacteria possess agonists for several members of the TLR family, TLR2, as a heterodimer with either TLR1 or TLR6, is the primary sensor by which immune cells recognize mycobacterial cell wall and membrane components. Mycobacterial derived agonists for the TLR1/2 heterodimer include lipomannan, lipoarabinomannan, phosphatidylinositol dimannoside, and various lipoproteins (7–14).
A number of TLR1 and TLR2 single nucleotide polymorphisms have been associated with mycobacterial disease (reviewed in 15, 16). We and others identified TLR1 I602S as a common single nucleotide polymorphism in TLR1 which markedly reduces primary monocyte/macrophage responses to soluble TLR1 agonists, such as synthetic triacylated lipopeptides and bacterial membrane components (17, 18). Surprisingly, the TLR1 I602S polymorphism has been shown to be a key protective allele for mycobacterial diseases, including tuberculosis, leprosy, and leprosy reversal reaction (17, 19–21). Most significantly, TLR1 602S has been identified as a major source of protection against leprosy in a genome-wide association study among 258 leprosy patients and 300 controls in New Delhi (P = 5.7×10−8, OR = 0.31, 95% CI = 0.20–0.48) (19). We discovered that an inability to traffic to the plasma membrane underlies the inability of the TLR1 602S variant to mediate responses to TLR1 agonists (17, 22).
Although deficient recognition of a pathogen would be expected to have detrimental effects on immune responses to infection, increasing evidence suggests that subversion of TLR1/2 signaling in macrophages represents an important immunoevasive strategy used by mycobacteria to establish and maintain chronic disease. Prolonged stimulation of macrophages with mycobacterial components are known to inhibit various aspects of macrophage antimicrobial functions in a TLR2-dependent manner (23–30). Stimulation of macrophages with mycobacterial components reduces IFNγ-induced surface levels of both MHCII (31–38) and FcγRI (CD64) (25, 26, 36, 39), receptors essential for antigen presentation and antibody-dependent phagocytosis, respectively. In addition, mycobacterial activation through TLR2 has been shown to upregulate host arginase-1; a metabolic enzyme that depletes macrophages of intracellular arginine, which is a substrate required to produce microbicidal nitric oxide (40, 41). The subversion of TLR1/2 signaling by mycobacteria leading to reduced cell surface MHCII, FcγRI, and oxidative burst is consistent with their well-established ability to dampen local T-cell mediated immunity to infection and create highly suitable intracellular niches.
We hypothesize that individuals lacking surface TLR1, and thus exhibiting hyporesponsiveness to TLR1/2 agonists, might resist the aforementioned TLR1/2-dependent immunosuppressive mechanisms employed by pathogenic mycobacteria. Our studies have revealed that compared to TLR1 602I, myeloid cells from individuals homozygous for TLR1 602S resist down regulation of MHCII and CD64, and also fail to upregulate arginase-1 when stimulated with mycobacterial membrane components. However, both TLR1 602S and 602I expressing cells drive similar protective responses to whole mycobacteria which may reflect the recruitment of both receptor variants, along with other pattern recognition receptors, to phagosomal compartments. Together, these results provide important insights into the mechanism by which an apparently defective TLR1 polymorphic variant provides protection against mycobacterial diseases.
Materials and Methods
Reagents
Cells used in ELISA and flow cytometric analyses were stimulated with the following TLR agonists for 24 hours: PAM3CSK4 (50 ng/ml, EMC Microcollections), zymosan (1×108 particles/ml, Invitrogen), PAM3CSK4-coated polystyrene beads (1×107 particles/ml), H37Rv M. tuberculosis membrane fraction (500 ng/ml, BEI Resources), and gamma-irradiated H37Rv M. tuberculosis (500 ng/ml, BEI Resources).
Cell Culture
Primary human monocytes and macrophages were grown in RPMI-1640 media (Cellgro) supplemented with 10% fetal bovine serum (FBS, Thermo Scientific), penicillin/streptomycin (Cellgro), and 20 mM L-glutamine (Cellgro). Primary human peripheral blood mononuclear cells were isolated by ficoll paque (GE Healthcare) gradient centrifugation in 50 ml Leucosep tubes (Greiner Bio One). For flow cytometry and Western blotting, monocytes were purified by plate adherence (described previously, 22) or using a MACS monocyte isolation kit II (Miltenyi) according to the manufacturer’s protocol. Primary human monocyte-derived macrophages were generated by stimulating monocytes with 50 ng/ml M-CSF (R&D Systems) for at least seven days. Mycobacterium avium strain 104 pMH109 (a kind gift from Jerry Cangelosi and Trude Flo, Trondheim Norwegian University of Science and Technology, Trondheim, Norway) was cultured in 7H9 Middlebrook broth (BD Difco) supplemented with ADC enrichment (BD BBL) and 20 µg/ml kanamycin, or on 7H10 Middlebrook agar (BD Difco) plates supplemented with OADC enrichment (BD BBL) and 20 µg/ml kanamycin.
Flow Cytometric Analysis
Surface expression of macrophage activation markers was measured using a BD FACS Canto flow cytometer. To stain for surface CD64 or MHCII, cells were blocked with flow buffer (10% rabbit serum and 0.3% NaN3 in PBS), followed by incubation with 10 µg/ml FITC-conjugated mouse anti-human CD64 (Caltag Laboratories, BD Pharmingen) or PE mouse anti-human HLA-DR (Invitrogen) on ice for 30 minutes. Cells were subsequently fixed in 4% paraformaldehyde. Flow data were gated on the appropriate monocyte/macrophage population and analyzed using FCS Express V3 (De Novo Software). Statistical analyses using a minimum of triplicate measurements were performed using the Student’s t-test.
ELISA
Quantification of TNFα and IL-6 from primary monocyte and macrophage supernatants was performed using a human TNFα or IL-6 CytoSet kit (Invitrogen), according to the manufacturer’s protocol. Cells were stimulated with TLR agonists (at above concentrations) or co-incubated with cytochalasin D (10 µM, Sigma Aldrich) for 12 hours.
Immunoblotting
Lysates were prepared by detaching monocytes using ice-cold 10mM EDTA, followed by lysis in RIPA buffer. Protein samples were run in 10% SDS PAGE gels. Primary rabbit anti-human arginase-1 (Santa Cruz Biotechnologies) and rabbit anti-human actin (Thermo Scientific) antibodies were incubated 1:1000 in 5% milk-TBST. Secondary HRP-conjugated anti-rabbit antibodies were incubated 1:10,000. Proteins were detected by chemiluminescence (Pierce).
Microscopy
Primary human macrophages were seeded onto chambered slides (Lab-Tek). M. avium cells were grown to log phase (OD~0.6), stained with auramine-O (Sigma Aldrich) for 30 minutes, and subsequently opsonized with FBS for 30 minutes at 37°C. Macrophages were incubated with mycobacteria (10:1 MOI) for 30 minutes at 4°C to allow binding. Following incubation at 37°C for 15 minutes, macrophages were washed with PBS, fixed with 4% paraformaldehyde at 4°C for one hour, and permeabilized with acetone for four minutes at −20°C. Phagosomal TLR1 was detected using a triple-step staining protocol. Cells were blocked with flow buffer (see flow cytometry methods), then stained using a primary mouse anti-human TLR1 antibody (GD2.F4, eBioscience), followed by a biotinylated anti-mouse Fab fragment (Jackson ImmunoResearch), and finally, streptavidin-conjugated Alexa Fluor-647 (Invitrogen). Staining steps were performed for 30 minutes on ice, followed by a wash with flow buffer. Images were acquired using a Carl Zeiss LSM510 laser-scanning confocal microscope using appropriate filter sets.
Results
TLR1 602S/S macrophages are activated by whole bacteria but not soluble agonists
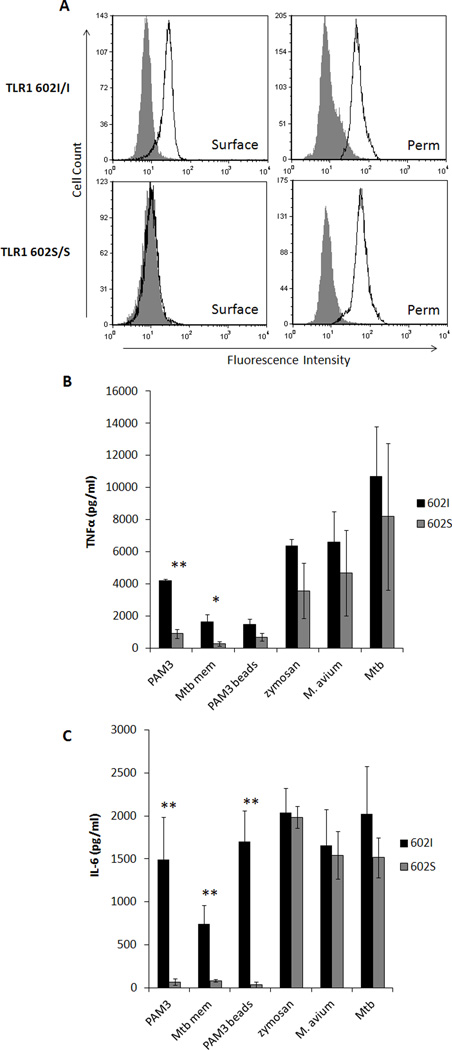
We previously showed that peripheral blood monocytes derived from individuals who are homozygous for the 602S allele lack cell surface expression of TLR1 and exhibit blunted responses to TLR1/2 agonists compared to individuals who are either heterozygous or homozygous for the TLR1 602I allele (17, 22). Importantly, monocyte-derived macrophages retained the same expression phenotype (Fig. 1A, left). This result appears to be due to a receptor trafficking defect, as equivalent levels of TLR1 were observed in permeabilized cells regardless of genotype (Fig 1A, right). As expected, individuals possessing the surface-expressed TLR1 602I allele responded to soluble TLR1 agonists PAM3 as measured by secretion of TNFα and IL-6, while TLR1 602S/S macrophages did not (Fig. 1 B, C). Similar results were obtained with a complex outer membrane fraction of Mycobacterium tuberculosis (Mtb mem). However, upon stimulation with the complex particulate agonists zymosan, M. avium, or whole M. tuberculosis, comparable levels of proinflammatory cytokines were produced regardless of TLR1 602 genotype. Interestingly, PAM3-coated beads were unable to elicit responses from TLR1 602S/S macrophages. Taken together, these results show that the ability of the TLR1 602S/S macrophages to mediate cell activation depends upon both the molecular complexity and the particulate nature of the agonist.
Figure 1. TLR1 602S/S macrophages are activated by whole bacteria but not soluble agonists.
A. Representative surface (left column) and permeabilized (right column, perm) staining of TLR1 in primary human monocyte-derived macrophages from a TLR1 602I (top row) and TLR1 602S (bottom row) homozygous blood donor is shown (filled histogram; isotype control, black histogram; TLR1). Primary human monocyte-derived macrophages from venous blood donors of the various TLR1 602 genotypes were stimulated with agonists, as indicated, for 24 hours and secretion of TNFα (B) and IL-6 (C) was measured from culture supernatants by ELISA (black bars; TLR1 602I/I or TLR1 602I/S donors, gray bars; TLR1 602S/S donors). Error bars represent the standard deviation of at least three donors. Asterisks denote significant differences between TLR1 602I-expressing cells versus TLR1 602S/S cells (*p<0.05, **p<0.005). (PAM3; PAM3CSK4Mtb mem; M. tuberculosis membrane fraction, PAM3 beads; PAM3CSK4-coated polystyrene beads, Mtb; gamma-irradiated M. tuberculosis)
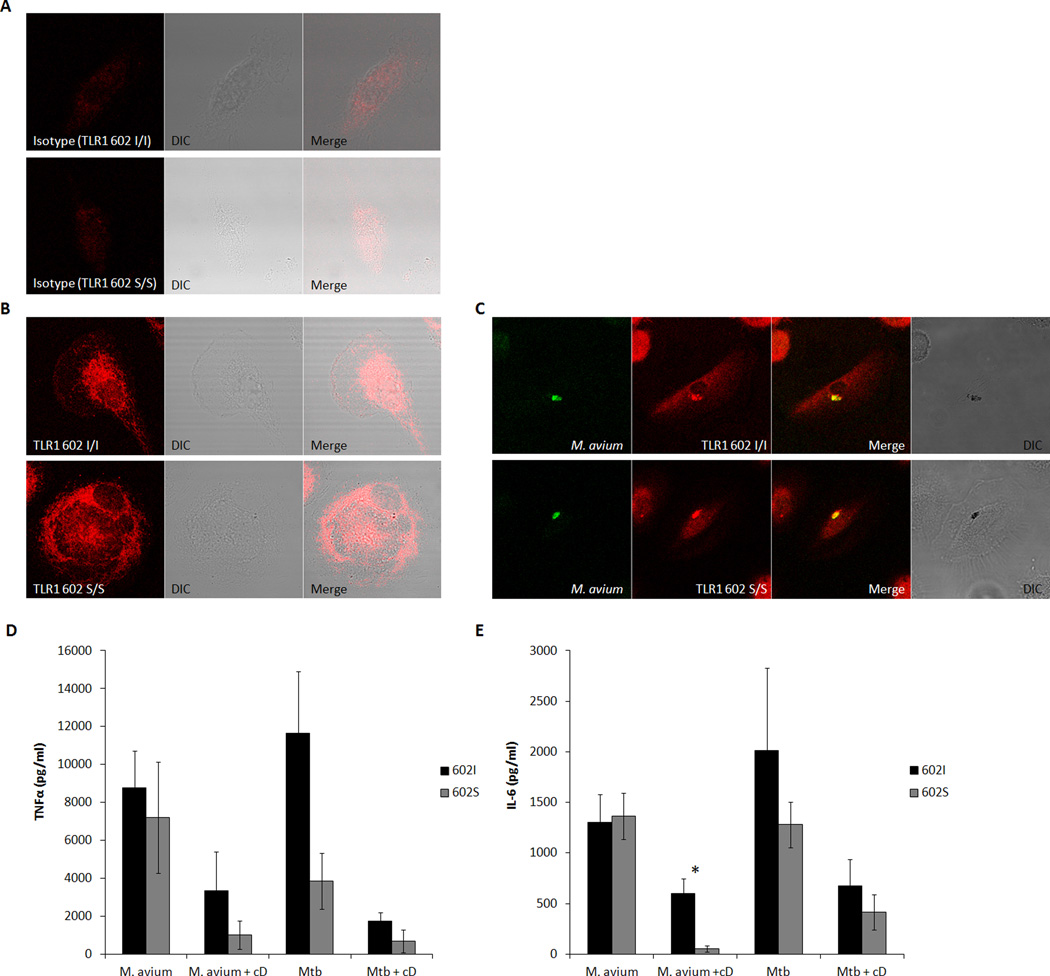
Given the fact that TLR1 602S/S cells are activated in response to whole mycobacteria, we next examined the ability of TLR1 to be recruited to phagosomes containing Mycobacterium avium. To this end, the localization of TLR1 in primary human macrophages from individuals either homozygous for TLR1 602S or the TLR1 602I allele was followed in relation to serum-opsonized M. avium cells stained with fluorescent auramine-O. In resting macrophages, both receptor variants of TLR1 (stained red) concentrate intracellularly in a perinuclear compartment previously identified as the endoplasmic reticulum (Fig. 2B) (22). As shown in Figure 2C, upon phagocytosis of live M. avium, both variants appear to localize to the phagosome (stained green). Taken together with the cytokine release data, these findings suggest that both TLR1 602I and TLR1 602S variants are capable of contributing to macrophage activation from mycobacteria containing phagosomes. This result is consistent with our published finding that both variants activate macrophages and traffic to the phagosome following ingestion of yeast zymosan particles (22). The fact that PAM3-coated beads activated macrophages through TLR1 602I but not TLR1 602S suggests that recognition of complex ligands by additional phagocytic receptors are required for the full cellular activation observed.
Figure 2. Intracellular TLR1 602S gains access to phagocytized mycobacteria in endosomal compartments.
A. Isotype control staining (red) of permeabilized resting macrophages from a TLR1 602I/I (top) or TLR1 602S/S (bottom) blood donor. B. Total TLR1 staining (red) of permeabilized resting macrophages from a TLR1 602I/I (top) or TLR1 602S/S (bottom) blood donor. C. Monocyte-derived macrophages were stimulated with auramine-O labeled M. avium (green) for 15 minutes. Cells were subsequently fixed, permeabilized, and stained for TLR1 (red). Primary human monocyte-derived macrophages from venous blood donors of the indicated TLR1 602 genotypes were stimulated for 12 hrs with Mycobacterium avium or Mycobacterium tuberculosis in the presence or absence of cytochalasin D (cD), followed by ELISA quantification of secreted TNFα (D) or IL-6 (E). Error bars represent the standard deviation of at least three donors. Asterisks denote significant differences between TLR1 602I-expressing cells versus TLR1 602S/S cells (*p<0.05) (black bars; TLR1 602I/I or TLR1 602I/S donors, grey bars; TLR1 602S/S donors). (DIC; differential interference contrast, Mtb; Mycobacterium tuberculosis, cD; cytochalasin D
Phagocytosis and endosomal trafficking of mycobacteria affect activation of TLR1 602S/S cells
To verify that bacterial internalization is necessary for macrophage TLR1 602S recruitment and activation, cytokine release in response to whole mycobacteria was measured in the presence or absence of the cytochalasin D; an inhibitor of actin polymerization and endocytosis. Cytochalasin D decreased the cytokine release mediated by both TLR1 602I and TLR1 602S (Fig. 2D, E). Interestingly, cell activation through TLR1 602I was more resistant to cytochalasin D inhibition than TLR1 602S. This increased resistance is likely due to some initial activation of TLR1 602I at the cell surface prior to endocytosis. Conversely, since TLR1 602S is not surface expressed, there is more absolute recruitment for this variant to gain access to endosomal compartments containing mycobacteria. Additionally, co-incubation of cells with the phagosome acidification inhibitor, bafilomycin A, or the microtubule polymerization/early endosome trafficking disruptor, nocodazole, gave similar results to that observed with cytochalasin D (data not shown). Together, these results confirm that internalization and trafficking of TLR1 602S to mycobacteria containing phagosomes is necessary for macrophage activation.
TLR1 602S/S macrophages resist downregulation of MHCII in response to solubilized, but not whole mycobacterial agonists
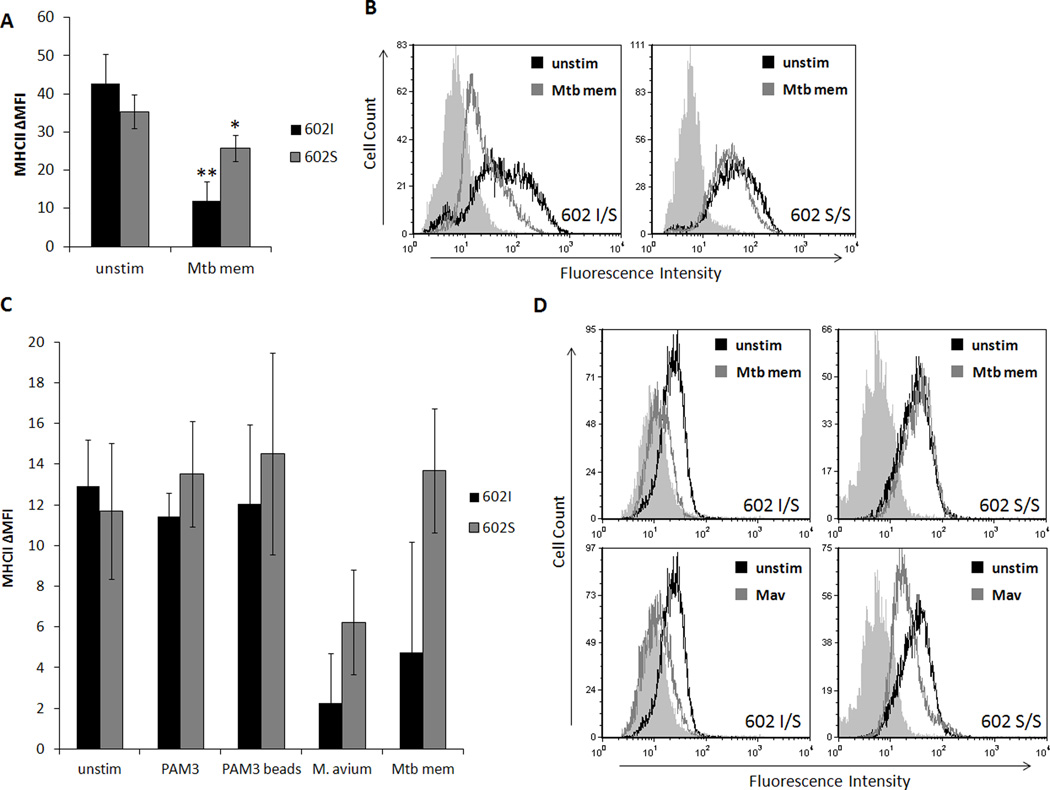
Several studies have demonstrated that pathogenic mycobacteria inhibit surface expression of class II major histocompatibility complex (MHCII), a vital component of antigen presentation and activation of CD4+ T helper cells (reviewed in 15). The suppression of surface MHCII was shown to be dependent on TLR2 recognition of a 19 kDa triacylated lipoprotein of mycobacteria, LpqH, resulting in inhibition of CIITA, a transcriptional activator of the MHCII gene (31, 34, 35, 36, 37, 42). We hypothesized that if TLR1/2 signaling is mediating this immunosuppression by mycobacteria, a deficiency in TLR1 activity, such as that of TLR1 602S, could confer protection. To this end, we initially examined primary human monocytes from blood donors of various TLR1 602 genotypes for changes in MHCII (HLA-DR) expression after challenge with M. tuberculosis membrane fraction (Mtb mem). Flow cytometric analysis verified that there was no significant difference in baseline surface MHCII based on TLR1 genotype (Fig. 3A). Following stimulation with Mtb mem for 24 hours, monocytes possessing a surface- expressed TLR1 602I allele exhibited an over 3-fold reduction in surface MHCII, whereas cells lacking surface-expressed TLR1 (TLR1 602S/S) displayed only marginal suppression (Fig. 3A, B).
Figure 3. TLR1 602S/S macrophages resist downregulation of MHCII in response to soluble, but not whole mycobacterial agonists.
A. Primary human monocytes of different TLR1 602 genotypes were incubated with or without M. tuberculosis membrane fraction (Mtb mem) for 24 hours. Surface levels of MHCII were assessed by flow cytometry (black bars; TLR1 602I/I or I/S, gray bars; TLR1 602S/S). Error bars represent the standard deviation from at least three different blood donors. Asterisks denote significant differences versus unstimulated cells (*p<0.05, **p<0.005). B. Representative histograms of monocyte MHCII expression (filled histograms; isotype, black histograms; unstimulated cells, gray histograms; stimulated with Mtb membrane fraction). C. Primary human monocyte-derived macrophages were stimulated with soluble and particulate TLR1/2 agonists for 24 hours and surface levels of MHCII were assessed by flow cytometry (black bars; TLR1 602I/I or I/S, gray bars; TLR1 602S/S). Error bars represent the standard deviation from at least three blood donors. Asterisks denote significant differences versus unstimulated cells (*p<0.05). D. Representative histograms from indicated genotypes (filled histograms; isotype, black histograms; unstimulated cells, gray histograms; stimulated cells). (Mtb mem; mycobacterial membrane fraction, Mav; Mycobacterium avium)
To further characterize this resistance phenotype, primary macrophages were similarly examined for MHCII expression following stimulation with other TLR1/2 agonists for 24 hours. Macrophages from blood donors of the three TLR1 602 genotypes were stimulated with PAM3CSK4 (PAM3), PAM3CSK4-coated beads, M. avium, or Mtb mem, and surface expression of HLA-DR was measured by flow cytometry. Surface levels of MHCII did not significantly differ between either groups when macrophages were left unstimulated or were incubated with PAM3 or PAM3-coated beads for 24 hours. However upon stimulation with live M. avium, macrophage surface MHCII decreased regardless of genotype (Fig. 3C, D). Stimulation of macrophages expressing TLR1 602I with Mtb mem also lead to a dramatic drop in detectable MHCII, while TLR1 602S/S cells largely resisted this reduction. Together, these results suggest that the absence of surface TLR1 602S may be abrogating mycobacterial-dependent suppression of MHCII surface expression. This loss of protection during stimulation with whole bacteria may be due to the fact that similarly to TLR1 602I, TLR1 602S is recruited to the M. avium phagosome, an event which would activate the inhibitory TLR1/2 signal independently of TLR1 genotype.
TLR1 602S/S monocytes and macrophages resist mycobacterial inhibition of IFNγ-induced CD64
CD64 (FcγRI), the phagocytic Fc receptor for IgG, is another marker of macrophage activation whose levels increase following stimulation with bacterial products or proinflammatory IFNγ. Like MHCII, CD64 has also been previously identified as a target of mycobacterial subversion of macrophage function, as expression is negatively modulated in cells exposed to mycobacterial products, even following IFNγ stimulation (25, 26, 36, 39). Similarly to MHCII, we hypothesized that TLR1 602S/S cells would be able to resist these inhibitory effects.
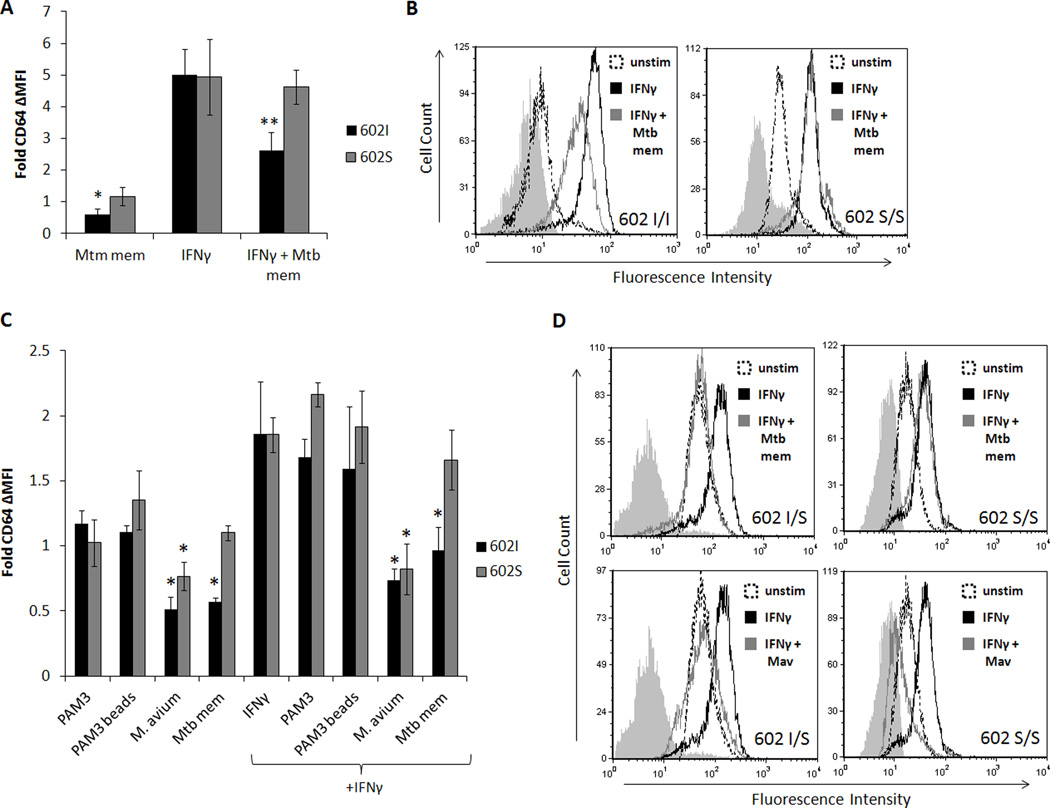
To test this, primary human monocytes from individuals of different TLR1 602 genotypes were treated with Mtb mem alone, IFNγ alone, or the two combined. Changes in surface expression of CD64 were subsequently examined by flow cytometry. As seen in Figure 4A, Mtb mem stimulation of TLR1 602I monocytes for 24 hours resulted in a 50% reduction of surface CD64, whereas TLR1 602S/S cells retained levels comparable to that of unstimulated monocytes. Upon addition of IFNγ, both groups displayed a five-fold increase in plasma membrane-localized FcγRI (Fig. 4A). However, co-incubation of TLR1 602I macrophages with Mtb membrane markedly inhibited the IFNγ-mediated upregulation of CD64, while TLR1 602S/S homozygotes were unaffected (Fig. 4A, B).
Figure 4. TLR1 602S/S monocytes and macrophages resist mycobacterial inhibition of IFNγ-induced CD64.
A. Primary human monocytes of different TLR1 602 genotypes were incubated with or without M. tuberculosis membrane fraction (Mtb mem) in the presence or absence of IFNγ for 24 hours. Surface levels of monocyte CD64 were assessed by flow cytometry. Bars represent the fold change in CD64 expression versus unstimulated cells (black bars; TLR1 602I/I or I/S donors, gray bars; TLR1 602S/S donors). Error bars represent the standard deviation from at least three different blood donors. Asterisks denote significant differences versus unstimulated values (*p<0.05, **p<0.005). B. Representative histograms of CD64 expression (filled histograms; isotype, dashed histograms; unstimulated cells, black histograms; IFNγ-stimulated cells, gray histograms; co-stimulated with IFNγ and Mtb membrane fraction). C. Primary human monocyte-derived macrophages were stimulated with soluble and particulate TLR1/2 agonists in the presence or absence of IFNγ for 24 hours. Surface levels of CD64 were assessed by flow cytometry. Bars represent the fold change in CD64 expression versus unstimulated cells (black bars; TLR1 602I/I or I/S donors, gray bars; TLR1 602S/S donors). Error bars represent the standard deviation from at least three different blood donors. Asterisks denote significant differences versus unstimulated values (*p<0.05). D. Representative histograms from indicated genotypes: top row (filled histograms; isotype, black histograms; IFNγ-stimulated cells, gray histograms; co-stimulated with IFNγ and Mtb membrane fraction) and bottom row (filled histograms; isotype, black histograms; IFNγ-stimulated cells, gray histograms; co-stimulated with IFNγ and M. avium- Mav).
To further examine the influence of other TLR1/2 agonists on CD64 expression, primary human macrophages were stimulated with PAM3, PAM3-coated beads, M. avium, or Mtb mem, and surface expression of CD64 was measured by flow cytometric analysis. As with monocytic cells, TLR1 602I expressing macrophages lost half of baseline CD64 surface expression when stimulated with Mtb membrane fraction, an effect largely resisted in TLR1 602S/S cells (Fig. 4C). Treatment with IFNγ induced CD64 expressed by two-fold in both groups, however, co-treatment with Mtb mem completely blocked this induction in TLR1 602I macrophages. Alternatively, as seen with primary monocytes, Mtb mem had no effect on IFNγ stimulation of CD64 in TLR1 602S/S macrophages. While soluble PAM3 or PAM3 bead stimulation had marginal effects on surface CD64, challenge with whole M. avium resulted in a significant reduction in surface CD64 in macrophages of all TLR1 602 genotypes, which could not be rescued by IFNγ treatment (Fig. 4C, D). Taken together, these results suggest that the lack of surface TLR1 602S protects against negative modulation of CD64 by mycobacterial membrane stimulation. However, as with MHCII, stimulation of macrophages with whole mycobacteria promotes subversive effects regardless of TLR1 602 genotype.
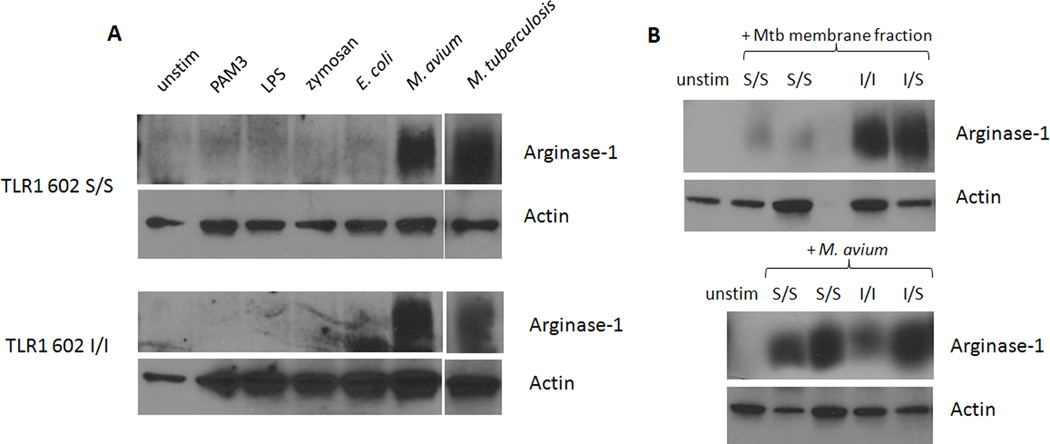
TLR1 602S/S monocytes resist induction of host arginase-1 when stimulated with mycobacterial membrane but not whole mycobacteria
Nitric oxide has been shown to be essential for the restriction of growth and killing of intracellular mycobacteria (41, 43–47). Inducible nitric oxide synthase (iNOS) and the metabolic enzyme Arginase-1 compete for cytoplasmic pools of arginine as an enzyme substrate. A study by El Kasmi et al. revealed that TLR2 stimulation by mycobacteria was capable of upregulating arginase-1 in mice, leading to both a reduction in nitric oxide intermediates and an enhancement of mycobacterial survival (40). Since subversion of TLR2 signaling by mycobacteria can promote pathways inhibitory to iNOS, we hypothesized that individuals lacking surface TLR1 602S may resist mycobacterial induction of host arginase-1.
To determine if arginase-1 could be induced in human cells, primary monocytes from individuals of the various TLR1 602 genotypes were stimulated with TLR agonists for 24 hours, followed by immunoblotting of cell lysates for Arginase-1. Stimulation of monocytes with TLR1/2 agonists, PAM3 and zymosan, or TLR4 agonists, lipopolysaccharide (LPS) and live Escherichia coli, failed to induce host arginase-1 (Fig. 5A). Conversely, exposure to live M. avium or gamma-irradiated M. tuberculosis greatly enhanced protein levels of this catabolic enzyme in monocytes of all TLR1 genotypes (Fig. 5A, B). Similarly to the experiments involving MHCII and CD64, TLR1 602 S/S cells were unable to resist the immunomodulatory activities of whole mycobacteria. We further examined whether TLR1 602S homozygotes could prevent upregulation of arginase-1 when exposed to Mtb mem, which would only activate TLR1 602I. Strikingly, cell lysates from TLR1 602S/S monocytes stimulated with Mtb mem contained very low levels of Arginase-1, while enzyme expression was strongly induced in TLR1 602I individuals (Fig. 5B). This resistance was observed even under prolonged Mtb mem challenge for 48 hours (Supp. Fig. 1). These results are consistent with the previous observations that differential trafficking of the TLR1 602S variant contributes to protection against the subversive effects mediated by mycobacterial membrane stimulation.
Figure 5. TLR1 602S/S monocytes resist induction of host arginase-1 when stimulated with mycobacterial membrane, but not whole mycobacteria.
A. Blood monocytes from donors of the indicated TLR1 602 genotypes were stimulated with various TLR agonists for 24 hrs followed by detection of Arginase-1 in cell lysates via Western blot. Loading controls were performed by detection of actin. B. Primary human monocytes from the indicated donors were stimulated with M. tuberculosis Mtb) membrane fraction (top), or live M. avium (bottom) for 24 hours. Levels of Arginase-1 were determined by Western blot. Relative protein loading is denoted by actin blots.
Discussion
Dynamic trafficking and differential subcellular distribution of TLRs ensures that a diverse array of microbial products is sensed by the innate immune system. Surface displayed TLRs, including TLR1, 2, 4, 5, 6, and 10, recognize a wide range of molecules shed from or present in bacterial outer walls and membranes. Ligands for these TLRs are present at appreciable concentrations in the extracellular environment of an infection site, where bacteria are actively replicating and physically engaging innate leukocytes. Recognition of pathogens and the establishment of a cytokine profile at the site of infection are essential for microbial clearance and restoration of tissue homeostasis.
We previously discovered I602S as a polymorphism which underlies a trafficking defect in TLR1 (17). Subsequent work in our lab has revealed that position 602 resides within a short 6 amino acid cytoplasmic trafficking motif which, in conjunction with the adjacent transmembrane domain, is sufficient to direct TLR1 to the cell surface (22). A serine at position 602, representing the I602S polymorphism, interrupts this trafficking motif and prevents cell surface expression of TLR1. Importantly, monocytes derived from individuals homozygous for the 602S variant which lack cell surface TLR1 exhibit greatly attenuated responses to a variety of soluble TLR1 agonists including various mycobacterial cell wall components (17, 18).
TLR1 requires TLR2 as a heterodimeric partner in the recognition of mycobacterial cell wall components and this heterodimer initiates intracellular signaling which drives antimicrobial, inflammatory, and adaptive immune responses. The TLR1/2 heterodimer serves as a key sensor by which innate cells recognize mycobacteria and plays a critical role in host defense as evidenced by the fact that numerous functionally deleterious polymorphisms in human TLR1 and TLR2 associate with leprosy and tuberculosis (48, reviewed in 15). These polymorphisms, which include insertion/deletions within the promoter as well as nonsynonymous single nucleotide changes in the receptor itself, result in decreased TLR expression or function and are associated with either an increased incidence or dissemination of mycobacterial infection (reviewed in 15). Surprisingly, we discovered that instead of being deleterious, the TLR1 cell surface trafficking defect of the 602S/S genotype associates with protection against leprosy (17). Importantly, TLR1 602S was also identified in an extensive genome-wide array as one of two alleles in humans that afford the greatest protection against leprosy (21). An independent study also revealed that the 602S variant associated with a decreased incidence of extrapulmonary tuberculosis infection, while TLR1 602I predisposed individuals to infection (19). The fact that a defective trafficking variant of TLR1 protects against leprosy and tuberculosis appears to contradict the important role of the TLR2 subfamily in host protection against pathogenic mycobacteria.
In this paper, we attempted to address the question as to how a deficient TLR1 polymorphism provides protection against mycobacterial disease. Here we report that TLR1 602S/S macrophages are unresponsive to soluble TLR1 agonists, such as PAM3 and M. tuberculosis membrane fraction. However, stimulation with either whole mycobacterial or fungal particles leads to co-localization of the TLR1 602S variant with the endosome along with prominent secretion of proinflammatory cytokines. As expected, pharmacologic inhibitors of vesicular trafficking prevent TLR1 endosomal localization and secretion of proinflammatory cytokines. These data suggest that although TLR1 602S is confined internally in resting cells, this receptor is a fully functional TLR1 variant in the context of whole pathogens, including mycobacteria. Interestingly, PAM3-coated beads do not activate proinflammatory cytokine secretion, indicating that stimulation of co-receptors which engage more complex agonists, such as whole bacteria, is required for induction. In this regard, it has been previously observed that phagocytic co-receptors, such as CD36 or CD14, regulate the subcellular distribution of TLRs in response to complex agonists (49, 50). Indeed, engagement of CD36 is required for TLR2 responses to whole bacteria (51). Additionally, internalization of TLR2 by inflammatory monocytes has been shown to be a prerequisite for driving cytokine responses to both Francisella and viral particles (52, 53).
Many studies have shown that mycobacteria subvert the TLR1/2 system to their advantage. For example, prolonged stimulation of TLR1/2 by the triacylated lipoprotein LpqH induces several immunosuppressive states at both the innate and adaptive levels in myeloid cells, including resistance to IFNγ, inhibition of CIITA transactivation, reduction of surface levels of MHCII, B7.2, and FcγRI, abrogation of antigen presentation and the oxidative burst, and even induction of apoptosis (23–39). We hypothesized that resistance to mycobacterial-mediated subversion of TLR1/2, conferred by lack of TLR1 surface expression, underlies the associated resistance of homozygous TLR1 602S individuals to mycobacterial disease. In support of this hypothesis we found that primary human monocytes and macrophages from TLR1 602I blood donors exhibited dramatic reductions of surface MHCII and CD64 when stimulated with M. tuberculosis membrane fraction, while TLR1 602S/S cells retained significantly higher surface levels of both markers.
Arginine-dependent NO synthesis is known to be important for anti-mycobacterial defense (44, 54). The fact that mycobacterial activation of TLR2 induces host arginase-1 provides another example of subversion of this receptor system, as this enzyme depletes arginine which is required as a substrate in the production of nitric oxide (39). When stimulated with M. tuberculosis membrane fraction, TLR1 602S/S cells did not significantly induce arginase-1, whereas enzyme levels were potently upregulated in monocytes possessing the TLR1 602I allele. Additional examples of mycobacterial subversion of the TLR2 system include the finding that lipoarabinomannan, a major component of mycobacterial cell walls and TLR1/2 agonist, decreases macrophage microbial killing induced by IFN-γ (55, 56). Furthermore, early secreted antigenic target (ESAT-6), a small protein released by M. tuberculosis, inhibits MyD88-dependent signaling following engagement of TLR2 (27, 33). While these additional subversion mechanisms in the context of the I602S polymorphism were not explored in this paper, it would not be surprising to find protection conferred by the TLR1 602S/S genotype.
While several mechanisms of TLR2 subversion by mycobacteria exist, this receptor complex nevertheless plays a key role in host defense against this bacterium. Perhaps most importantly, through induction of a vitamin D pathway, TLR2 activation has been shown to drive upregulation of antimicrobial peptides and induction of autophagy in response to mycobacteria (57, 58). As pointed out above, the TLR1 602S variant retains the ability to traffic and signal from endosomal compartments following uptake of whole microbial particles including mycobacteria. Therefore, in the context of whole mycobacteria, these critical functions would be retained in individuals of the TLR1 602S/S genotype. In fact, in the context of whole mycobacteria we have been unable to identify a macrophage phenotype, either beneficial or detrimental, in association with the I602S polymorphism.
Since granulomas provide the primary means of containment of mycobacterial infection, it is perhaps instructive to consider their protective role in the context of the TLR1 I602S polymorphism. Granulomas consist of a core of surviving mycobacteria that is walled off by other leukocytes, primarily monocytes and effector T cells (59). As monocytes infiltrate a granuloma they are likely to be exposed to soluble mycobacterial components released by actively replicating mycobacteria and infected macrophages (38, 60). Indeed, it has been observed that mycobacteria release membrane vesicles and that those released from virulent strains contain TLR2 lipoprotein agonists (35). Similarly, the more virulent rough morphotypes of Mycobacterium abscessus are associated with increased lipoprotein production and subversive TLR2 engagement (61). Additionally, macrophages infected with M. tuberculosis release exosomes containing degraded mycobacterial components which have been shown to inhibit macrophage activation by IFNγ in a TLR2-dependent fashion (38). We hypothesize that TLR1 602S/S cells resist these effects through the absence of surface TLR1, and that subsequent lack of functional TLR1/2 heterodimers prevent subversion of TLR signals. Thus, due to exposure to soluble mycobacterial components, TLR1 602I monocytes could respond more poorly to whole mycobacteria present in the core of the granuloma compared to TLR1 602S/S cells, leading to reduced clearance and containment of the infection.
The TLR1 I602S polymorphism exhibits vastly different geographic and racial distributions. For example, the 602S allele is far more prevalent among individuals of European versus African descent with frequencies of 75 and 25 percent, respectively. This differential distribution of the TLR1 602S allele could underlie the observation that among 25,000 nursing home patients, African Americans were found to have twice the risk of developing tuberculosis than Caucasians (62). It is interesting to note that in areas of the world with the highest incidences of endemic mycobacterial disease, such as India and Asia, the protective TLR1 602S variant exhibits the lowest allele frequencies at less than 1 percent. Studies focused on additional genome-wide association and ex vivo analyses are necessary to fully elucidate the role TLR1 I602S plays in the complex host-pathogen interaction that occurs following mycobacterial infection.
Supplementary Material
Acknowledgements
We wish to thank our many blood donors in addition to the Flow Cytometry and MCB Microscopy Core facilities of the University of Illinois.
The abbreviations used in this paper are
- TLR
Toll-like receptor
- PRR
pattern recognition receptor
- Mtb
Mycobacterium tuberculosis
- IFNγ
interferon-gamma
- IL-6
interleukin-6
- TNFα
tumor necrosis factor alpha
- MOI
multiplicity of infection
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
Footnotes
This work was supported by the National Institutes of Health grant AI052344 (to RIT).
References
- 1.Gupta A, Kaul A, Tsolaki AG, Kishore U, Bhakta S. Mycobacterium tuberculosis: immune evasion, latency and reactivation. Immunobiology. 2012;217:363–374. doi: 10.1016/j.imbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Baena A, Porcelli SA. Evasion and subversion of antigen presentation by Mycobacterium tuberculosis. Tissue Antigens. 2009;74:189–204. doi: 10.1111/j.1399-0039.2009.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Leprosy update 2011. WER. 2011;86:389–400. [Google Scholar]
- 4.Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R. Innate immune recognition of Mycobacterium tuberculosis. Clin. Dev. Immunol. 2011;2011:405310. doi: 10.1155/2011/405310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsolaki AG. Innate immune recognition in tuberculosis infection. Adv. Exp. Med. Biol. 2009;653:185–197. doi: 10.1007/978-1-4419-0901-5_13. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 8.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 9.Drage MG, Pecora ND, Hise AG, Febbraio M, Silverstein RL, Golenbock DT, Boom WH, Harding CV. TLR2 and its co-receptors determine responses of 27 macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell. Immunol. 2009;258:29–37. doi: 10.1016/j.cellimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, Liu PT, Cole ST, Godowski PJ, Maeda Y, Sarno EN, Norgard MV, Brennan PJ, Akira S, Rea TH, Modlin RL. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat. Med. 2003;9:525–532. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 11.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 12.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 13.Tapping RI, Tobias PS. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J. Endotoxin Res. 2003;9:264–268. doi: 10.1179/096805103225001477. [DOI] [PubMed] [Google Scholar]
- 14.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 15.Hart BE, Tapping RI. Genetic Diversity of Toll-Like Receptors and Immunity to M. leprae Infection. J. Trop. Med. 2012;2012:415057. doi: 10.1155/2012/415057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schumann RR, Tapping RI. Genomic variants of TLR1--it takes (TLR-)two to tango. Eur. J. Immunol. 2007;37:2059–2062. doi: 10.1002/eji.200737604. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CM, Lyle EA, Omueti KO, Stepensky VA, Yegin O, Alpsoy E, Hamann L, Schumann RR, Tapping RI. Cutting edge: A common polymorphism 28 impairs cell surface trafficking and functional responses of TLR1 but protects against leprosy. J. Immunol. 2007;178:7520–7524. doi: 10.4049/jimmunol.178.12.7520. [DOI] [PubMed] [Google Scholar]
- 18.Hawn TR, Misch EA, Dunstan SJ, Thwaites GE, Lan NT, Quy HT, Chau TT, Rodrigues S, Nachman A, Janer M, Hien TT, Farrar JJ, Aderem A. A common human TLR1 polymorphism regulates the innate immune response to lipopeptides. Eur. J. Immunol. 2007;37:2280–2289. doi: 10.1002/eji.200737034. [DOI] [PubMed] [Google Scholar]
- 19.Ma X, Liu Y, Gowen BB, Graviss EA, Clark AG, Musser JM. Full-exon resequencing reveals toll-like receptor variants contribute to human susceptibility to tuberculosis disease. PLoS One. 2007;2:e1318. doi: 10.1371/journal.pone.0001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misch EA, Macdonald M, Ranjit C, Sapkota BR, Wells RD, Siddiqui MR, Kaplan G, Hawn TR. Human TLR1 deficiency is associated with impaired mycobacterial signaling and protection from leprosy reversal reaction. PLoS Negl Trop. Dis. 2008;2:e231. doi: 10.1371/journal.pntd.0000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong SH, Gochhait S, Malhotra D, Pettersson FH, Teo YY, Khor CC, Rautanen A, Chapman SJ, Mills TC, Srivastava A, Rudko A, Freidin MB, Puzyrev VP, Ali S, Aggarwal S, Chopra R, Reddy BS, Garg VK, Roy S, Meisner S, Hazra SK, Saha B, Floyd S, Keating BJ, Kim C, Fairfax BP, Knight JC, Hill PC, Adegbola RA, Hakonarson H, Fine PE, Pitchappan RM, Bamezai RN, Hill AV, Vannberg FO. Leprosy and the adaptation of human toll-like receptor 1. PLoS Pathog. 2010;6:e1000979. doi: 10.1371/journal.ppat.1000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart BE, Tapping RI. Cell surface trafficking of TLR1 is differentially regulated by the ER chaperones PRAT4A and PRAT4B. J. Biol. Chem. 2012 doi: 10.1074/jbc.M112.342717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banaiee N, Kincaid EZ, Buchwald U, Jacobs WR, Jr, Ernst JD. Potent inhibition of macrophage responses to IFN-gamma by live virulent Mycobacterium tuberculosis 29 is independent of mature mycobacterial lipoproteins but dependent on TLR2. J. Immunol. 2006;176:3019–3027. doi: 10.4049/jimmunol.176.5.3019. [DOI] [PubMed] [Google Scholar]
- 24.Fortune SM, Solache A, Jaeger A, Hill PJ, Belisle JT, Bloom BR, Rubin EJ, Ernst JD. Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. J. Immunol. 2004;172:6272–6280. doi: 10.4049/jimmunol.172.10.6272. [DOI] [PubMed] [Google Scholar]
- 25.Gehring AJ, Rojas RE, Canaday DH, Lakey DL, Harding CV, Boom WH. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infect. Immun. 2003;71:4487–4497. doi: 10.1128/IAI.71.8.4487-4497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kincaid EZ, Wolf AJ, Desvignes L, Mahapatra S, Crick DC, Brennan PJ, Pavelka MS, Jr, Ernst JD. Codominance of TLR2-dependent and TLR2-independent modulation of MHC class II in Mycobacterium tuberculosis infection in vivo. J. Immunol. 2007;179:3187–3195. doi: 10.4049/jimmunol.179.5.3187. [DOI] [PubMed] [Google Scholar]
- 27.Kumar P, Agarwal R, Siddiqui I, Vora H, Das G, Sharma P. ESAT6 differentially inhibits IFN-gamma-inducible class II transactivator isoforms in both a TLR2- dependent and -independent manner. Immunol. Cell Biol. 2011 doi: 10.1038/icb.2011.54. [DOI] [PubMed] [Google Scholar]
- 28.Lafuse WP, Alvarez GR, Curry HM, Zwilling BS. Mycobacterium tuberculosis and Mycobacterium avium inhibit IFN- gamma -induced gene expression by TLR2- dependent and independent pathways. J. Interferon Cytokine Res. 2006;26:548–561. doi: 10.1089/jir.2006.26.548. [DOI] [PubMed] [Google Scholar]
- 29.Lopez M, Sly LM, Luu Y, Young D, Cooper H, Reiner NE. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J. Immunol. 2003;170:2409–2416. doi: 10.4049/jimmunol.170.5.2409. [DOI] [PubMed] [Google Scholar]
- 30.Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 2001;167:910–918. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 31.Pai RK, Convery M, Hamilton TA, Boom WH, Harding CV. Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J. Immunol. 2003;171:175–184. doi: 10.4049/jimmunol.171.1.175. [DOI] [PubMed] [Google Scholar]
- 32.Pai RK, Pennini ME, Tobian AA, Canaday DH, Boom WH, Harding CV. Prolonged toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect. Immun. 2004;72:6603–6614. doi: 10.1128/IAI.72.11.6603-6614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A, Kaisho T, Kundu M, Basu J. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat. Immunol. 2007;8:610–618. doi: 10.1038/ni1468. [DOI] [PubMed] [Google Scholar]
- 34.Pennini ME, Pai RK, Schultz DC, Boom WH, Harding CV. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-gamma-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signaling. J. Immunol. 2006;176:4323–4330. doi: 10.4049/jimmunol.176.7.4323. [DOI] [PubMed] [Google Scholar]
- 35.Prados-Rosales R, Baena A, Martinez LR, Luque-Garcia J, Kalscheuer R, Veeraraghavan U, Camara C, Nosanchuk JD, Besra GS, Chen B, Jimenez J, Glatman- Freedman A, Jacobs WR, Jr, Porcelli SA, Casadevall A. Mycobacteria release active 31 membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Invest. 2011;121:1471–1483. doi: 10.1172/JCI44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ting LM, Kim AC, Cattamanchi A, Ernst JD. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J. Immunol. 1999;163:3898–3906. [PubMed] [Google Scholar]
- 37.Wang Y, Curry HM, Zwilling BS, Lafuse WP. Mycobacteria inhibition of IFN-gamma induced HLA-DR gene expression by up-regulating histone deacetylation at the promoter region in human THP-1 monocytic cells. J. Immunol. 2005;174:5687–5694. doi: 10.4049/jimmunol.174.9.5687. [DOI] [PubMed] [Google Scholar]
- 38.Simmons DP, Canaday DH, Liu Y, Li Q, Huang A, Boom WH, Harding CV. Mycobacterium tuberculosis and TLR2 agonists inhibit induction of type I IFN and class I MHC antigen cross processing by TLR9. J. Immunol. 2010;185:2405–2415. doi: 10.4049/jimmunol.0904005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh PP, LeMaire C, Tan JC, Zeng E, Schorey JS. Exosomes released from M. tuberculosis infected cells can suppress IFN-gamma mediated activation of naive macrophages. PLoS One. 2011;6:e18564. doi: 10.1371/journal.pone.0018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, Basaraba RJ, Konig T, Schleicher U, Koo MS, Kaplan G, Fitzgerald KA, Tuomanen EI, Orme IM, Kanneganti TD, Bogdan C, Wynn TA, Murray PJ. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat. Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das P, Lahiri A, Lahiri A, Chakravortty D. Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Pathog. 2010;6:e1000899. doi: 10.1371/journal.ppat.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arko-Mensah J, Julian E, Singh M, Fernandez C. TLR2 but not TLR4 signalling is critically involved in the inhibition of IFN-gamma-induced killing of mycobacteria by murine macrophages. Scand. J. Immunol. 2007;65:148–157. doi: 10.1111/j.1365-3083.2006.01888.x. [DOI] [PubMed] [Google Scholar]
- 43.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell. Immunol. 1991;132:150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- 45.Herbst S, Schaible UE, Schneider BE. Interferon gamma activated macrophages kill mycobacteria by nitric oxide induced apoptosis. PLoS One. 2011;6:e19105. doi: 10.1371/journal.pone.0019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris SM., Jr Arginine: master and commander in innate immune responses. Sci. Signal. 2010;3:pe27. doi: 10.1126/scisignal.3135pe27. [DOI] [PubMed] [Google Scholar]
- 47.Nozaki Y, Hasegawa Y, Ichiyama S, Nakashima I, Shimokata K. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect. Immun. 1997;65:3644–3647. doi: 10.1128/iai.65.9.3644-3647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tjarnlund A, Guirado E, Julian E, Cardona PJ, Fernandez C. Determinant role for Toll-like receptor signalling in acute mycobacterial infection in the respiratory tract. Microbes Infect. 2006;8:1790–1800. doi: 10.1016/j.micinf.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 50.Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J. Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cole LE, Shirey KA, Barry E, Santiago A, Rallabhandi P, Elkins KL, Puche AC, Michalek SM, Vogel SN. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect. Immun. 2007;75:4127–4137. doi: 10.1128/IAI.01868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qualls JE, Neale G, Smith AM, Koo MS, DeFreitas AA, Zhang H, Kaplan G, Watowich SS, Murray PJ. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci. Signal. 2010;3:ra62. doi: 10.1126/scisignal.2000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan J, Fan XD, Hunter SW, Brennan PJ, Bloom BR. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect. Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sibley LD, Hunter SW, Brennan PJ, Krahenbuhl JL. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect. Immun. 1988;56:1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fabri M, Modlin RL. A vitamin for autophagy. Cell. Host Microbe. 2009;6:201–203. doi: 10.1016/j.chom.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Tolllike receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 59.Huynh KK, Joshi SA, Brown EJ. A delicate dance: host response to mycobacteria. Curr. Opin. Immunol. 2011;23:464–472. doi: 10.1016/j.coi.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Bouley DM, Ghori N, Mercer KL, Falkow S, Ramakrishnan L. Dynamic nature of host-pathogen interactions in Mycobacterium marinum granulomas. Infect. Immun. 2001;69:7820–7831. doi: 10.1128/IAI.69.12.7820-7831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roux AL, Ray A, Pawlik A, Medjahed H, Etienne G, Rottman M, Catherinot E, Coppee JY, Chaoui K, Monsarrat B, Toubert A, Daffe M, Puzo G, Gaillard JL, Brosch R, Dulphy N, Nigou J, Herrmann JL. Overexpression of proinflammatory TLR-2- signalling lipoproteins in hypervirulent mycobacterial variants. Cell. Microbiol. 2011;13:692–704. doi: 10.1111/j.1462-5822.2010.01565.x. [DOI] [PubMed] [Google Scholar]
- 62.Stead WW, Senner JW, Reddick WT, Lofgren JP. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N. Engl. J. Med. 1990;322:422–427. doi: 10.1056/NEJM199002153220702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.